The Structure of the Atom Chapter 4 Important



















- Slides: 24

+ The Structure of the Atom Chapter 4

+ Important Dates for Chapter 4 n Start: Tuesday October 8 n End: Thursday October 24 n Fall Break: October 14 -15 n Vocabulary Quiz: Thursday October 18 n Test: Thursday October 24 n Lab: Thursday October 18 and Tuesday October 22

+ Essential Vocabulary n Alpha particle n Gamma ray n Alpha radiation n Isotope n Atom n Mass number n Atomic mass n Neutron n Atomic mass unit n Nuclear equation n Atomic number n Nuclear reaction n Nucleus n Proton n Beta particle n Beta radiation n Cathode ray n Dalton’s atomic theory n Radiation n electron n radioactivity

+ Early Theories of Matter Section 4. 1

+ The Philosophers Many Greek philosophers thought matter was formed of air, earth, fire, and water. They also associated properties with each of the 4 basic components of matter. The pairings of opposite properties, such as hot and cold, and wet and dry, mirrored the symmetry and balance the philosophers observed in nature. These early nonscientific and incorrect beliefs were not completely dispelled until the 1800 s. n Democritus (460 -370 B. C. ) n 1 st person to propose the idea that matter was not infinitely divisible. n Believed atoms matter was made up of tiny individual particles called atomos n Believed that atoms could not be created, destroyed, or further divided n Atoms are solid, homogeneous, indestructible, and invisible n Different kinds of atoms have different sizes and shapes n The differing of properties of matter are due to the size, shape, and movement of the atom n Apparent changes in matter result from changes in the groupings of atoms and not from changes in the atoms themselves n Most of his ideas do not agree with modern atomic theory, but his belief in the existence of atoms was amazingly ahead of his time

+ The Philosophers Many Greek philosophers thought matter was formed of air, earth, fire, and water. They also associated properties with each of the 4 basic components of matter. The pairings of opposite properties, such as hot and cold, and wet and dry, mirrored the symmetry and balance the philosophers observed in nature. These early nonscientific and incorrect beliefs were not completely dispelled until the 1800 s. n Aristotle (384 -322 B. C. ) n One of the most influential philosophers n Wrote extensively on many subjects, including politics, ethics, nature, physics, and astronomy n Most of his writings have been lost through the ages n Criticized Democritus’s ideas of atomic theory n He rejected Democritus’s atomic “theory” entirely because it did not agree with his own ideas on nature n Did not believe that the “nothingness” of empty space could exist n Able to gain wide acceptance for his ideas on nature– ideas that denied the existence of atoms– for nearly 2000 years! n Concept of the atom was revived in 18 th century, but it took another 100 years before significant progress was made

+ John Dalton (1766 -1844) n English schoolteacher n Marked the beginning of the development of modern atomic theory n Revived and revised Democritus’s ideas based upon the results of scientific research he conducted n Advancements in science allowed him to perform experiments that allowed him to refine and verify theories n Able to accurately determine mass ratios n Proposed his atomic theory in 1803 n Dalton’s Atomic Theory n All matter is composed of extremely small particles called atoms n All atoms of a given element are identical, having the same size, mass, and chemical properties; atoms of a specific element are different from those of any other element n Atoms cannot be created, divided into smaller particles, or destroyed n Different atoms combine in simple whole number ratios to form compounds n In a chemical reaction, atoms are separated, combined, or rearranged

+ Section 4. 1 Assessment n Why were Democritus’s ideas rejected by other philosophers of his time? n Define an atom using your own words. n Which statements in Dalton’s original atomic theory are now considered to be incorrect? Describe how modern atomic theory differs from these statements. n Democritus and Dalton both proposed the concept of atoms. Describe the method each of them used to reach the conclusion that atoms existed. How did Democritus’s method hamper the acceptance of his ideas? n Compare and contrast the atomic theories proposed by Democritus and John Dalton.

+ Subatomic Particles and the Nuclear Atom Section 4. 2

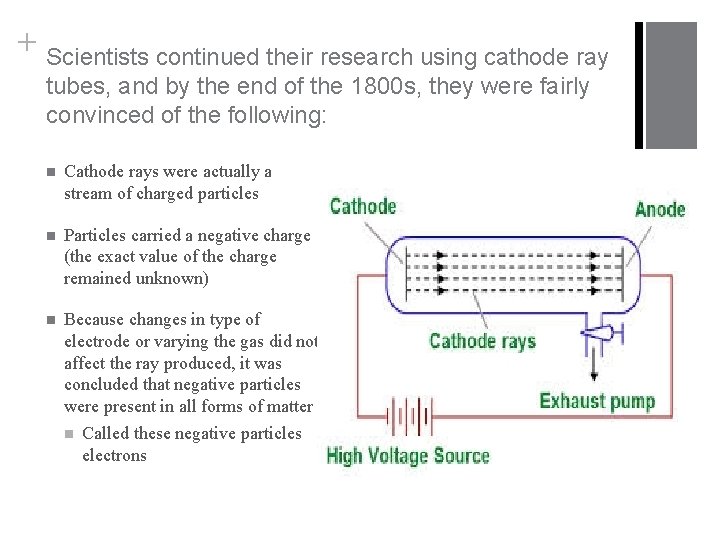
+ Discovering the Electron Has your hair ever clung to your comb? Have you ever gotten shocked by a doorknob after walking across carpet? Observations such as these led scientists in the 1800 s to look for some sort of relationship between matter and electric charge. n Using a recently invented vacuum pump, electricity was passed through glass tubes from which most of the air and matter had been removed n Electrode connected to negative end is called anode; electrode connected to positive end is called cathode n Sir William Crookes n English physicist noticed a flash of light within one of the tubes while working in a darkened laboratory n Flash was produced by some form of radiation striking a light-producing coating that had been applied to the end of the tube n This led to the discovery of radiation traveling from the cathode to the anode within the tube; became known as a cathode ray n Now you have TVs and computer screens

+ Scientists continued their research using cathode ray tubes, and by the end of the 1800 s, they were fairly convinced of the following: n Cathode rays were actually a stream of charged particles n Particles carried a negative charge (the exact value of the charge remained unknown) n Because changes in type of electrode or varying the gas did not affect the ray produced, it was concluded that negative particles were present in all forms of matter n Called these negative particles electrons

+ n In spite of the progress made from all of the cathode ray tube experiments, no one had succeeded in determining the mass of a single cathode ray particle n The next significant discovery came in 1909 when American physicist Robert Millikan determined the charge of an electron to be 9. 1 x 10 -28 g n English physicist J. J. Thomson began a series of experiments in the late 1890 s to determine the ratio of its charge to its mass n Thomson then proposed a model called the plum pudding model which didn’t last long n His conclusion was shocking because it meant that there were particles smaller than an atom; in other words, Dalton was wrong

+ The Nuclear Atom n Ernest Rutherford became interested in studying how positively charged alpha particles interacted with solid matter n Aware of Thomson’s plum pudding model, Rutherford expected only minor deflections through the gold foil n After a few days of testing, Rutherford was amazed to discover that a few of the alpha particles were deflected at very large angles; some even deflected straight back n This meant the plum pudding model was incorrect; Rutherford concluded there must be a nucleus centrally located within the atom that contained the positive charge and most of its mass n Gold Foil Experiment

+ n In 1932, Rutherford’s coworker, English physicist James Chadwick showed that the nucleus also contained another subatomic particle, a neutral particle called the neutron. n Completing the Atom– The Discovery of Protons and Neutrons By 1920, eight years after his revolutionary gold foil experiment, Rutherford had refined the concept of the nucleus. He concluded that the nucleus contained positively charged particles called protons. A proton is a subatomic particle carrying a charge equal but opposite that of an electron. A neutron has a mass nearly equal to that of a proton but carries no electrical charge

+ Section 4. 2 Assessment n Briefly evaluate the experiments that led to the conclusion that electrons were negatively charged particles found in all matter. n Describe the structure of a typical atom. Be sure to identify where each subatomic particle is located. n Make a table comparing the relative charge and mass of each of the subatomic particles. n Compare and contrast Thomson’s plum pudding atomic model with Rutherford’s nuclear atomic model. n Make a timeline of the development of modern atomic theory. Be sure to include the discovery of each subatomic particle.

+ How Atoms Differ Section 4. 3

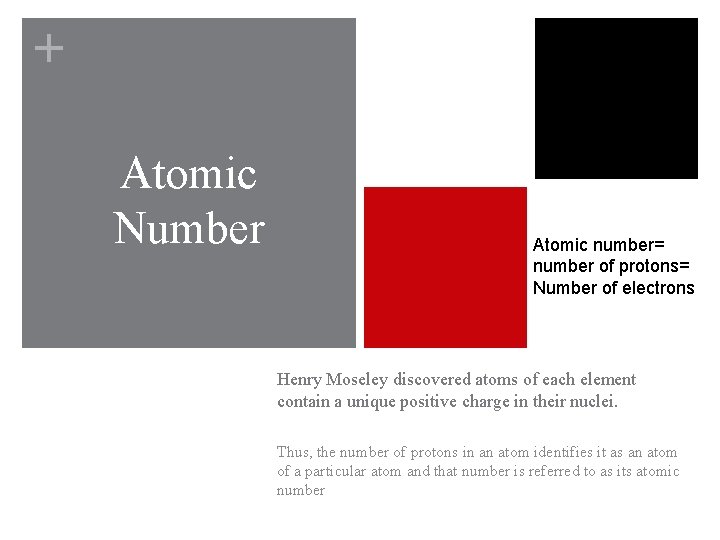
+ Atomic Number Atomic number= number of protons= Number of electrons Henry Moseley discovered atoms of each element contain a unique positive charge in their nuclei. Thus, the number of protons in an atom identifies it as an atom of a particular atom and that number is referred to as its atomic number

+ Isotopes and Mass Number Earlier you learned that Dalton’s atomic theory was wrong about atoms being indivisible. It was also incorrect in stating that all atoms of a particular element are identical. While it is true that all atoms of particular element have the same number of protons and electrons, the number of neutrons on their nuclei may differ. Atoms with the same number of protons but different numbers of neutrons are called isotopes. n Most elements are found as a mixture of isotopes found in a constant relative abundance n Those that contain more neutrons have a greater mass n Have essentially the same chemical behavior regardless of differing number of neutrons n To make it easy to identify each of the various isotopes of an element, chemists add a number after the element’s name n This number is known as the mass number and represents the number of protons and neutrons in the nucleus # of neutrons = mass # - atomic #

+ Mass of Individual Atoms n The masses of protons and neutrons are about 1. 67 x 10 -24 g each. n Electrons are very small– about 1/1840 that of one proton or neutron. n Because these extremely small masses expressed in scientific notation are difficult to work with, chemists developed a method of measuring based on a chosen atomic standard- a carbon-12 atom. n Thus, one atomic mass unit (amu) is defined as 1/12 the mass of a carbon 12 atom. n Because an atom’s mass depends on the number of protons and neutrons it contains, and because protons and neutrons have masses close to 1 amu, you might expect the atomic masses to be very near a whole number. n This does not hold true when the definition of atomic mass is taken into account. n Atomic mass of an element is the weighted average mass of the isotopes of that element.

+ Section 4. 3 Assessment n Which subatomic particle identifies an atom as that of a particular element? How is this particle related to the atom’s atomic number? n What is an isotope? Give an example of an element with isotopes. n Explain how the existence of isotopes is related to atomic masses not being whole numbers. n Nitrogen has two naturally occurring isotopes, N-14 and N-15. The atomic mass of nitrogen is 14. 007 amu. Which isotope is more abundant in nature? Explain. n List the steps in the process of calculating average atomic mass given data about the isotopes of an element.

+ Unstable Nuclei and Radioactive Decay Section 4. 4

+ n Nuclear reactions are reactions that do involve an atom of one element changing into an atom of another element. n In the late 1890 s, scientists noticed that some substances spontaneously emitted radiation in a process they termed radioactivity. n The rays and particles emitted by the radioactive material were called radiation. n Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay. n Instability is caused by either too many or too few neutrons. Radioactivity Recall that chemical reactions involve the change of one more substances into new substances. Although atoms may be rearranged, their identities do not change during the reaction. You may be wondering why atoms of one element do not change into another element during a chemical reaction. The reason lies in the fact that the chemical reaction involves only an atom’s electrons- the nucleus remains unchanged.

+ Types of Radiation Scientists began researching radioactivity in the late 1800 s. By directing radiation from a radioactive source between two electrically charged plates, scientists were able to identify three different types of radiation. Some was deflected toward the negatively charged plate, some was deflected toward the positively charged plate, and some was not deflected at all. n Alpha Radiation n Deflected toward the negatively charged plate n Made up of alpha particles n 2 protons, 2 neutrons, +2 charge n Beta Radiation n Deflected toward the positively charged plate n Made of one fast moving electrons called beta particles n 1 electron, -1 charge n Gamma Radiation or Gamma Rays n High-energy radiation that possess no mass and no charge

+ Section 4. 4 Assessment n Explain how unstable atoms gain stability. What determines whether or not an atom is stable? n Create a table comparing the mass and charge of alpha, beta, and gamma radiation. n In writing a balanced nuclear equation, what must be conserved? n Explain how a nuclear reaction differs from a chemical reaction. n Classify each of the following as a chemical reaction, a nuclear reaction, or neither. n Thorium emits a beta particle. n Two atoms share electrons to form a bond. n A sample of pure sulfur emits heat energy as it slowly cools. n A piece of iron rusts.