Lecture 10 Dozimetry of Ionizing Radiations Plan of










- Slides: 47

Lecture # 10 Dozimetry of Ionizing Radiations

Plan of the lecture 1. Interaction of ionizing + radiation (α; βˉ; β ; ɣ; n) with substance 2. The affect of ionizing radiation on biologic objects 3. Dozimetry 4. Medical applications ionizing radiation of

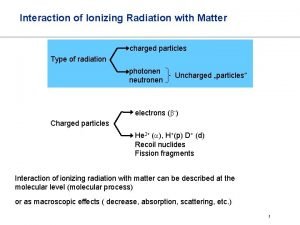
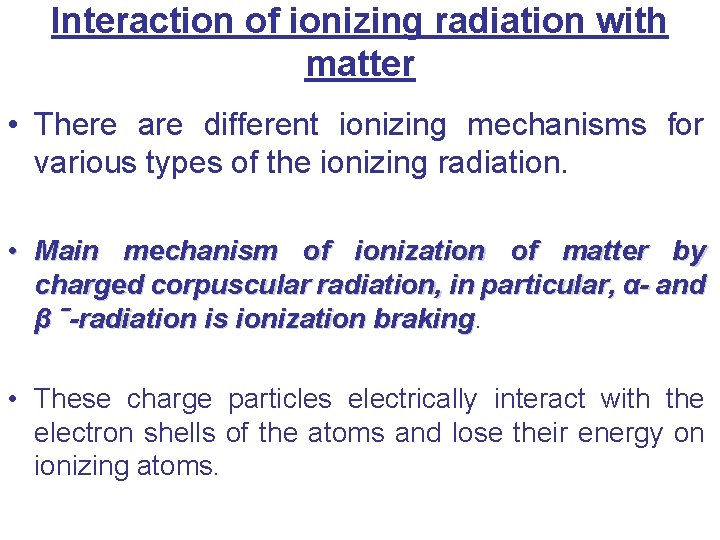
Interaction of ionizing radiation with matter • There are different ionizing mechanisms for various types of the ionizing radiation. • Main mechanism of ionization of matter by charged corpuscular radiation, in particular, α- and β ˉ-radiation is ionization braking • These charge particles electrically interact with the electron shells of the atoms and lose their energy on ionizing atoms.

• Interaction ionizing radiation (IR) with substances leads to (IR) ionization of the atoms of the material. • Ionization is a process of transforming the neutral atom into ions – positive (cations) and negative (anions). ___ Ionizing radiation __ ↓ Corpuscular : • • alpha-radiation beta-radiation neutron flux proton flux ↓ Electromagnetic: • • gamma-radiation X-radiation • In medicine X-radiation is used the most often.

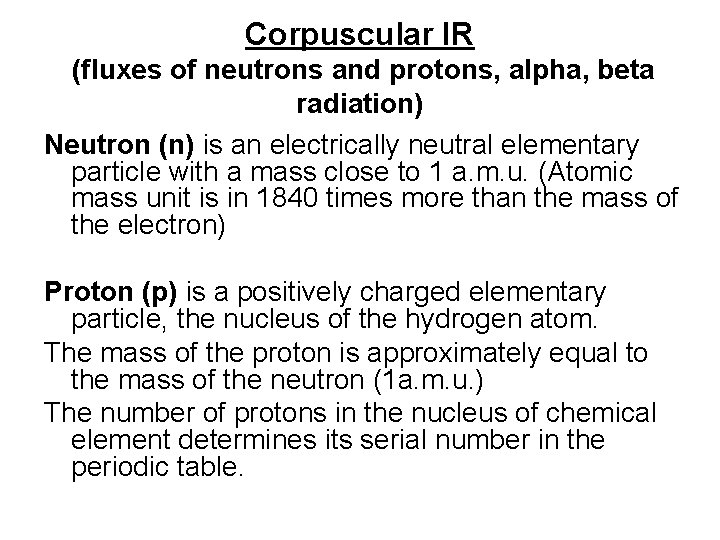
Corpuscular IR (fluxes of neutrons and protons, alpha, beta radiation) Neutron (n) is an electrically neutral elementary particle with a mass close to 1 a. m. u. (Atomic mass unit is in 1840 times more than the mass of the electron) Proton (p) is a positively charged elementary particle, the nucleus of the hydrogen atom. The mass of the proton is approximately equal to the mass of the neutron (1 a. m. u. ) The number of protons in the nucleus of chemical element determines its serial number in the periodic table.

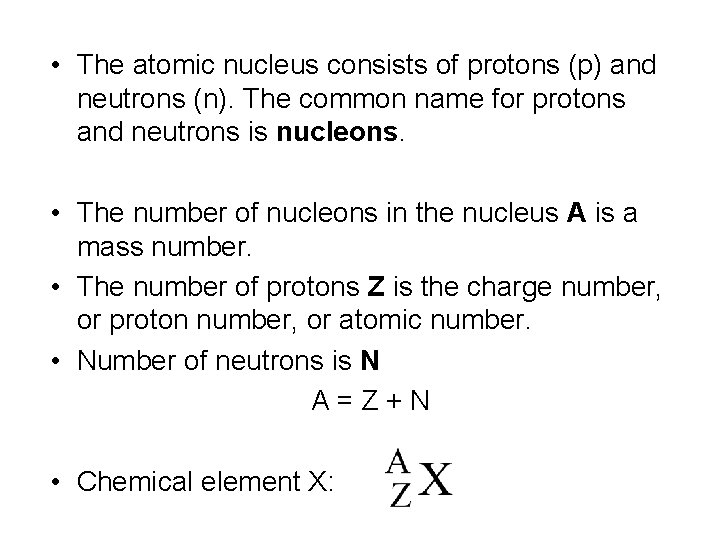
• The atomic nucleus consists of protons (p) and neutrons (n). The common name for protons and neutrons is nucleons. • The number of nucleons in the nucleus A is a mass number. • The number of protons Z is the charge number, or proton number, or atomic number. • Number of neutrons is N A = Z + N • Chemical element X:

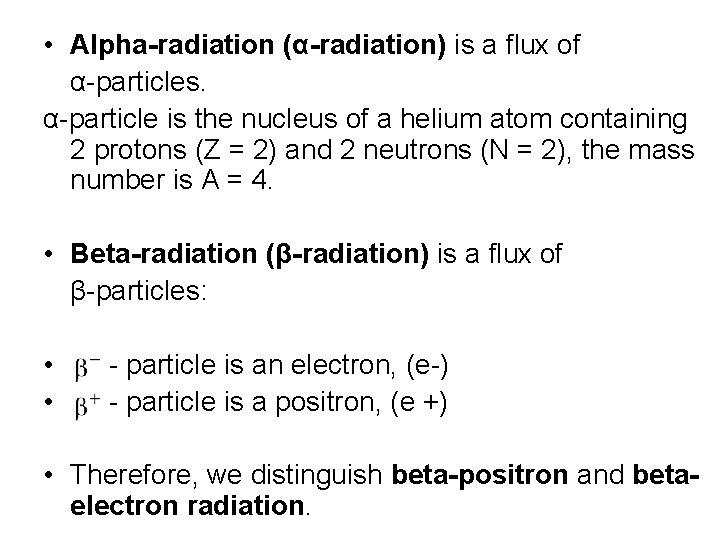
• Alpha-radiation (α-radiation) is a flux of α-particles. α-particle is the nucleus of a helium atom containing 2 protons (Z = 2) and 2 neutrons (N = 2), the mass number is A = 4. • Beta-radiation (β-radiation) is a flux of β-particles: • - particle is an electron, (e-) • - particle is a positron, (e +) • Therefore, we distinguish beta-positron and betaelectron radiation.

• The main mechanism of ionization of substances under the action of charged corpuscular radiation is ionization braking.

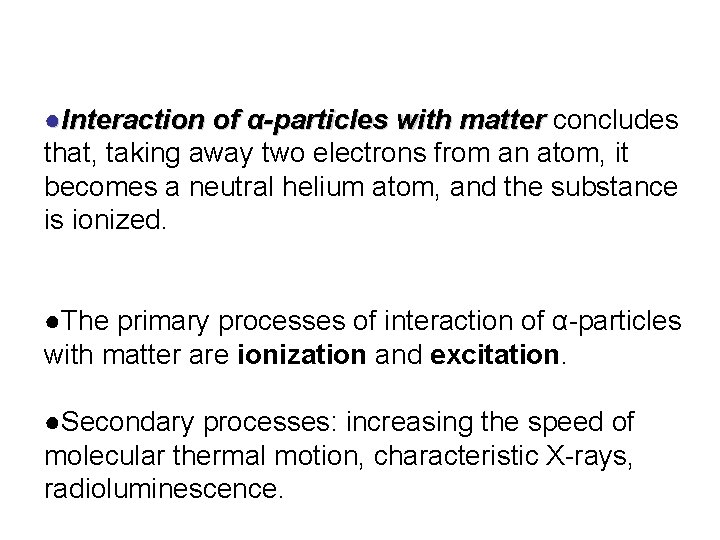
●Interaction of α-particles with matter concludes matter that, taking away two electrons from an atom, it becomes a neutral helium atom, and the substance is ionized. ●The primary processes of interaction of α-particles with matter are ionization and excitation. ●Secondary processes: increasing the speed of molecular thermal motion, characteristic X-rays, radioluminescence.

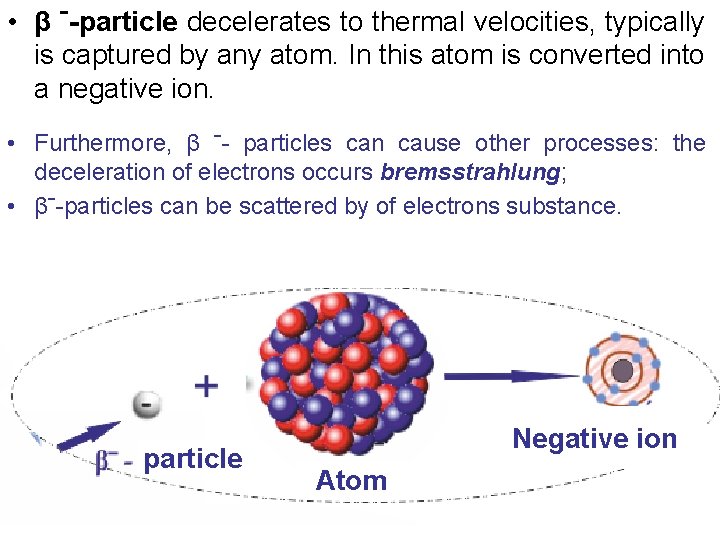
• β ˉ-particle decelerates to thermal velocities, typically is captured by any atom. In this atom is converted into a negative ion. • Furthermore, β ˉ- particles can cause other processes: the deceleration of electrons occurs bremsstrahlung; • βˉ-particles can be scattered by of electrons substance. particle Negative ion Atom

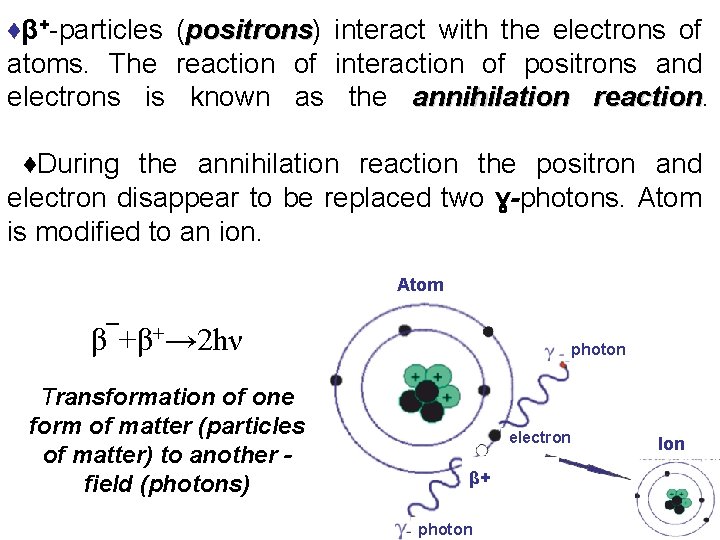
♦β+-particles (positrons) positrons interact with the electrons of atoms. The reaction of interaction of positrons and electrons is known as the annihilation reaction ♦During the annihilation reaction the positron and electron disappear to be replaced two ɣ-photons. Atom is modified to an ion. Atom β¯+β+→ 2 hν Transformation of one form of matter (particles of matter) to another field (photons) photon electron β+ photon Ion

Three basic mechanisms of -radiation interaction with a substance: • photoeffect, • incoherent scattering (Compton effect) and • formation of electron-positron pairs The first two mechanisms are considered early .

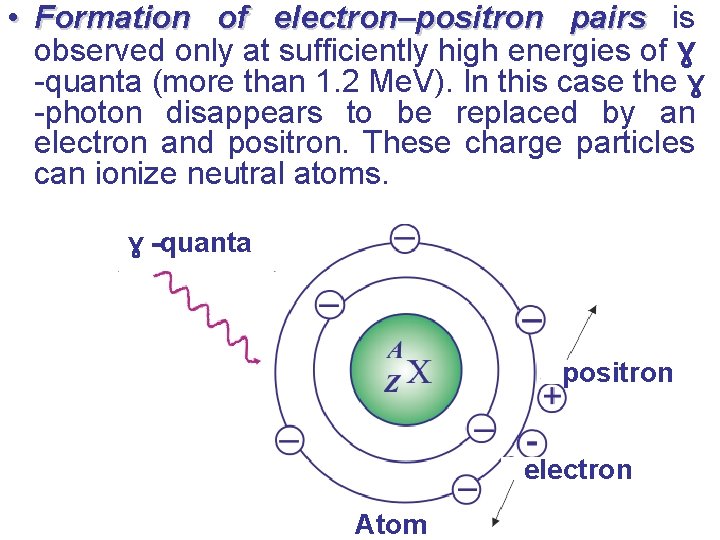
• Formation of electron–positron pairs is observed only at sufficiently high energies of ɣ -quanta (more than 1. 2 Me. V). In this case the ɣ -photon disappears to be replaced by an electron and positron. These charge particles can ionize neutral atoms. ɣ -quanta positron electron Atom

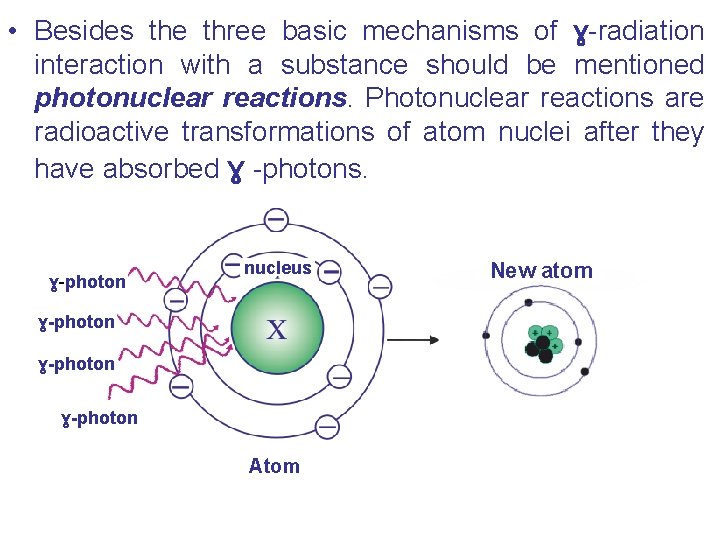
• Besides the three basic mechanisms of ɣ-radiation interaction with a substance should be mentioned photonuclear reactions. Photonuclear reactions are radioactive transformations of atom nuclei after they have absorbed ɣ -photons. ɣ-photon nucleus ɣ-photon Atom New atom

• Neutrons by themselves do not cause ionization of a substance, but in interacting with the nuclei of atoms they generate ionized radiation. Interaction of neutrons with radiation nuclei causes either scattering of neutrons or their capture by the nuclei • During scattering part of the neutron’s kinetic energy is transferred to the nucleus. If the nucleus is a light one, it can acquire a high velocity. Such Compton nuclei have a sufficient kinetic energy for ionizing. • When capturing a neutron the nucleus goes to an excited state, which is often an unstable one. In this case it can be observed βˉ-decay or nuclear fission, though reactions with emission of protons or α-particles can also occur.

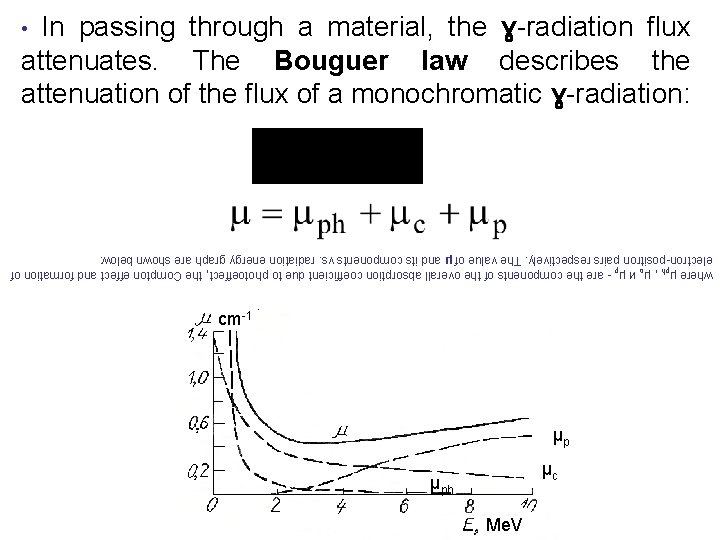
Me. V μph μc μp cm-1 where μph , μc и μp - are the components of the overall absorption coefficient due to photoeffect, the Compton effect and formation of electron-positron pairs respectively. The value of μ and its components vs. radiation energy graph are shown below: attenuates. The Bouguer law describes the attenuation of the flux of a monochromatic ɣ-radiation: • In passing through a material, the ɣ-radiation flux

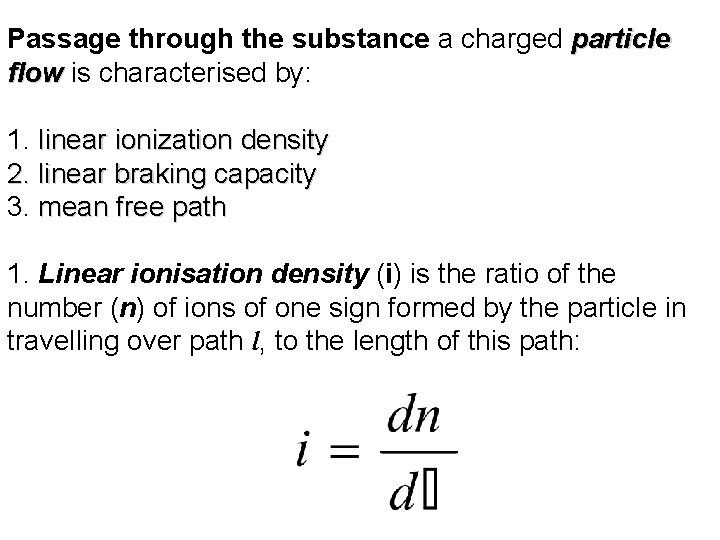
Passage through the substance a charged particle flow is characterised by: flow 1. linear ionization density 2. linear braking capacity 3. mean free path 1. Linear ionisation density (i) is the ratio of the number (n) of ions of one sign formed by the particle in travelling over path l, to the length of this path:

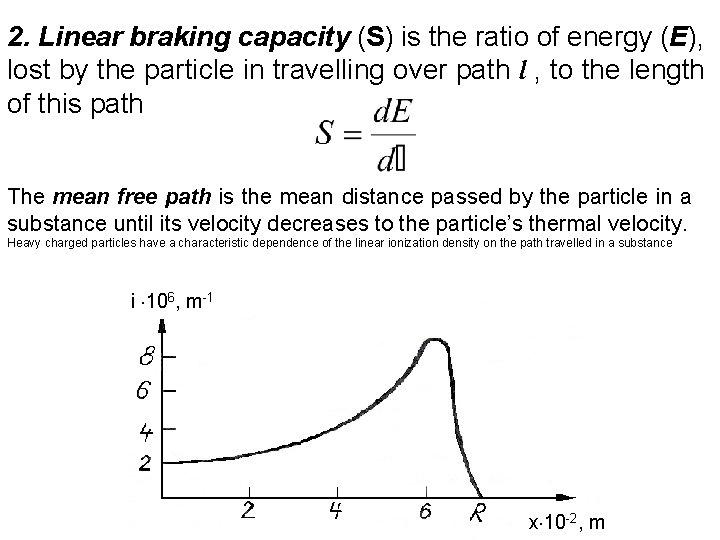
2. Linear braking capacity (S) is the ratio of energy (E), lost by the particle in travelling over path l , to the length of this path The mean free path is the mean distance passed by the particle in a substance until its velocity decreases to the particle’s thermal velocity. Heavy charged particles have a characteristic dependence of the linear ionization density on the path travelled in a substance i 106, m-1 x 10 -2, m

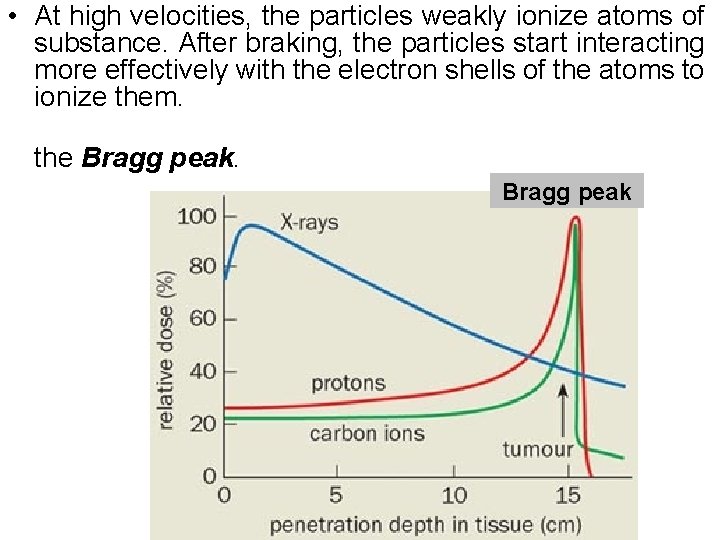
• At high velocities, the particles weakly ionize atoms of substance. After braking, the particles start interacting more effectively with the electron shells of the atoms to ionize them. the Bragg peak

• Flux attenuation of -radiation by the substance: • N 0 is the number of -particles incident on a substance over some time; • N is the number of particles penetrating to depth l ; • is the linear attenuation constant. • Deviation from the exponential character of the N vs. dependence is observed only at small values of N.

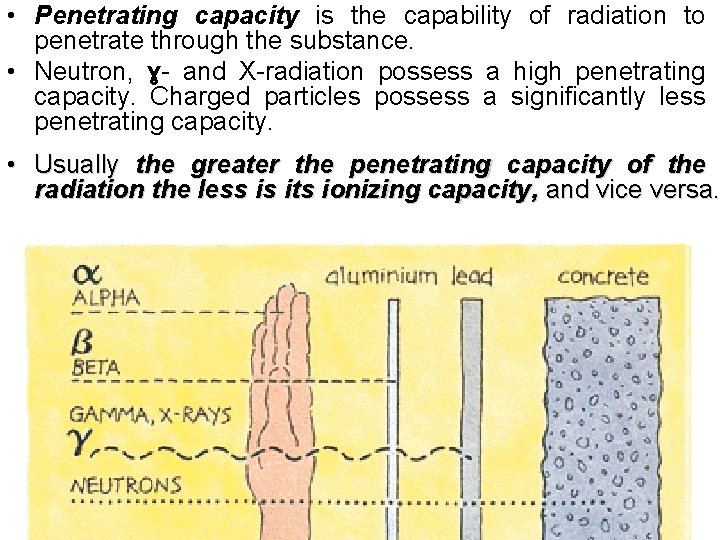
• Penetrating capacity is the capability of radiation to penetrate through the substance. • Neutron, ɣ- and X-radiation possess a high penetrating capacity. Charged particles possess a significantly less penetrating capacity. • Usually the greater the penetrating capacity of the radiation the less is its ionizing capacity, and vice versa.

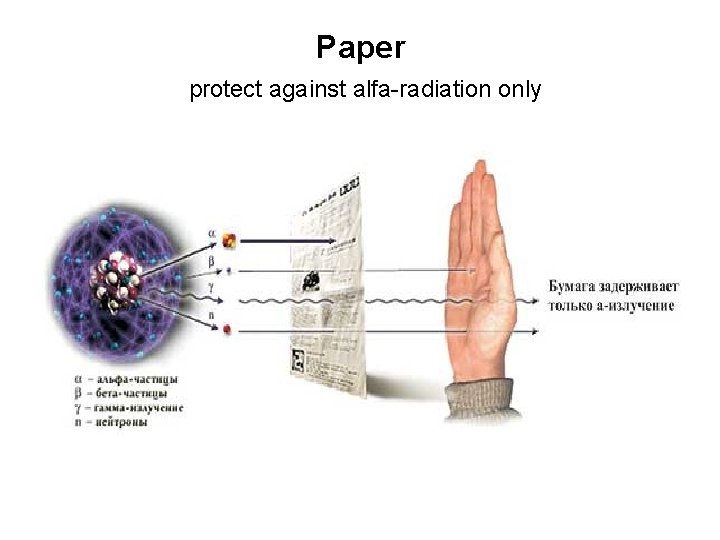
Paper protect against alfa-radiation only

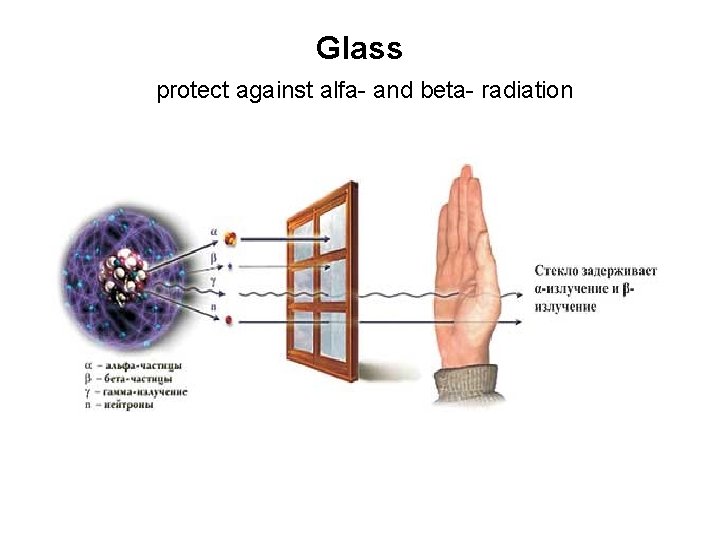
Glass protect against alfa- and beta- radiation

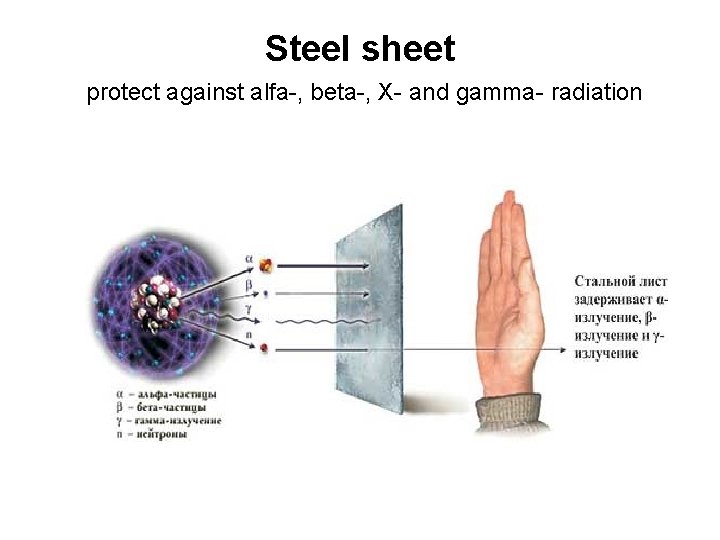
Steel sheet protect against alfa-, beta-, X- and gamma- radiation

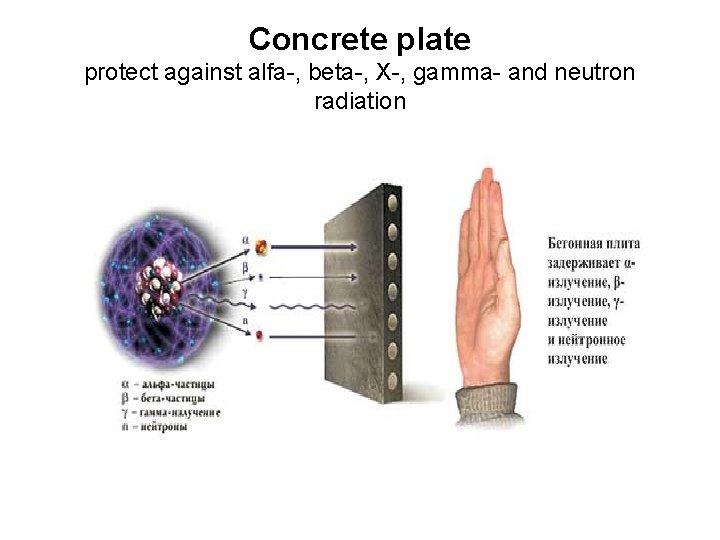
Concrete plate protect against alfa-, beta-, X-, gamma- and neutron radiation


The affect of IR on cells, cells biological tissue and the body is negative and has a number of features: • a significant biological effect is created by negligible doses of IR; • the affect of IR on biological objects has a latent period, i. e. the effect of radiation takes place in some time after effect; • the tissues of a body, the cells, and parts of cells differ as to sensitivity to IR. The most sensitive to radiation are those tissues where cells divide actively.

DOZIMETRY • Dosimetry is an area of science and technology, studying quantitative characteristics effects of ionizing radiation on biological objects (doses), as well as methods and apparatus that allows these characteristics to define. • The radiobiological effect of ionizing radiation is the greater the radiation energy absorbed by the material. Hence, irrespective of the radiation source, its impact on objects can be characterised by the radiation dose.

Types of doses: 1. 2. 3. Radiation dose (D) Exposure dose (X) Equivalent dose (H)

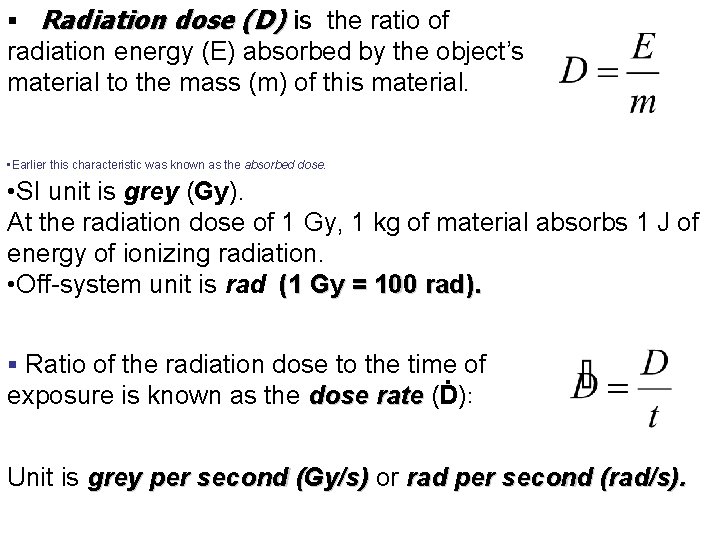
§ Radiation dose (D) is the ratio of radiation energy (E) absorbed by the object’s material to the mass (m) of this material. • Earlier this characteristic was known as the absorbed dose. • SI unit is grey (Gy). At the radiation dose of 1 Gy, 1 kg of material absorbs 1 J of energy of ionizing radiation. • Off-system unit is rad (1 Gy = 100 rad). § Ratio of the radiation dose to the time of exposure is known as the dose rate (Ḋ): rate Unit is grey per second (Gy/s) or rad per second (rad/s).

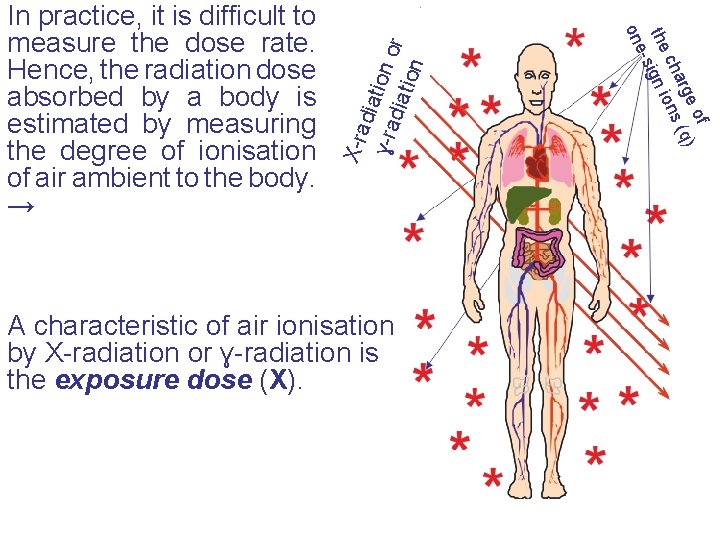
X-ra diat i ɣ-r adia on or tion A characteristic of air ionisation by X-radiation or ɣ-radiation is the exposure dose (X). f e o ) arg (q ch ons the ign i e-s on In practice, it is difficult to measure the dose rate. Hence, the radiation dose absorbed by a body is estimated by measuring the degree of ionisation of air ambient to the body. →

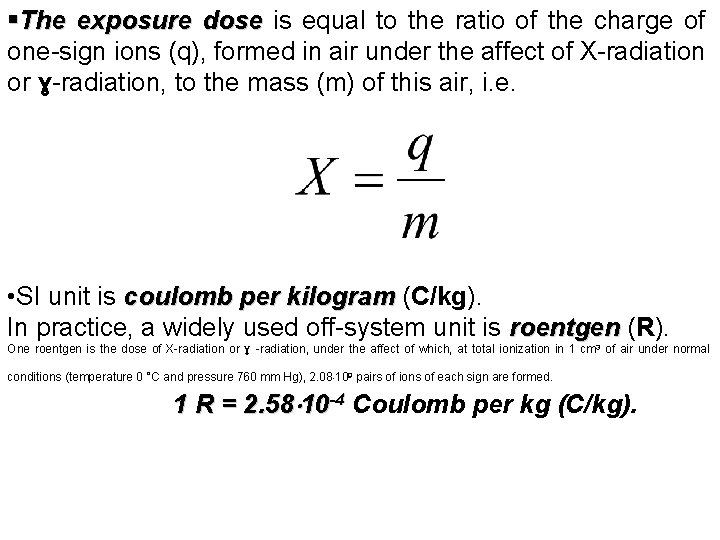
§The exposure dose is equal to the ratio of the charge of one-sign ions (q), formed in air under the affect of X-radiation or ɣ-radiation, to the mass (m) of this air, i. e. • SI unit is coulomb per kilogram (C/kg). kilogram In practice, a widely used off-system unit is roentgen (R). roentgen One roentgen is the dose of X-radiation or ɣ -radiation, under the affect of which, at total ionization in 1 cm 3 of air under normal 1 R = 2. 58 10 -4 Coulomb per kg (C/kg). conditions (temperature 0 °C and pressure 760 mm Hg), 2. 08 109 pairs of ions of each sign are formed.

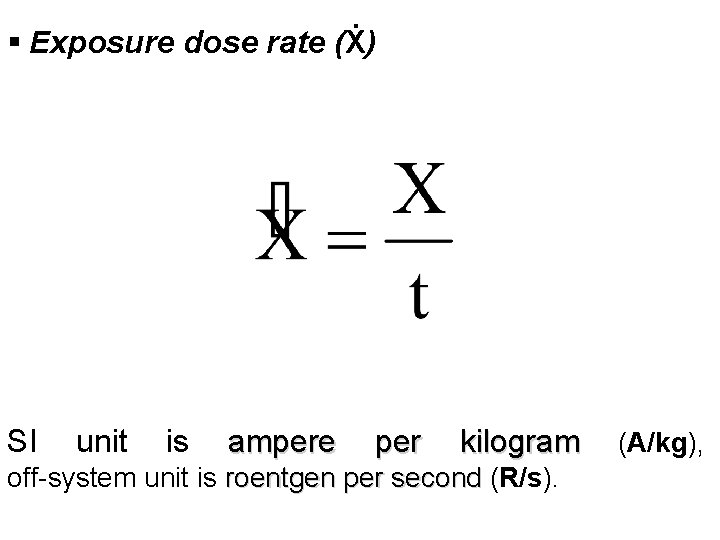
§ Exposure dose rate (Ẋ) SI unit is ampere per kilogram (A/kg), off-system unit is roentgen per second (R/s). roentgen per second

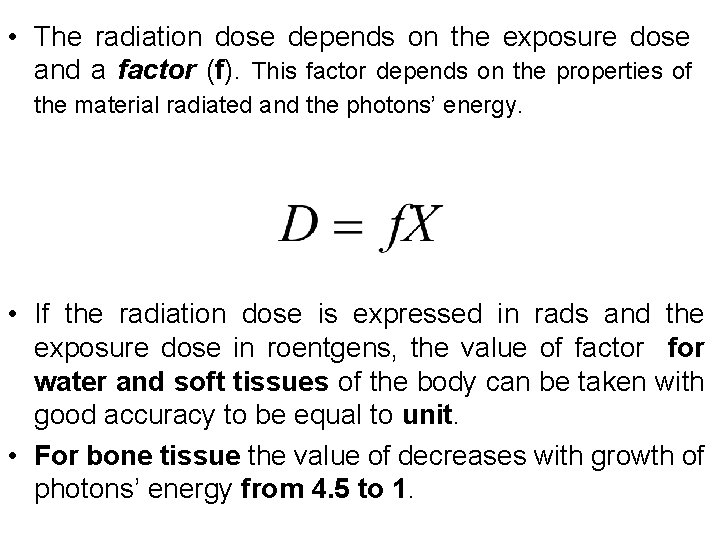
• The radiation dose depends on the exposure dose and a factor (f). This factor depends on the properties of the material radiated and the photons’ energy. • If the radiation dose is expressed in rads and the exposure dose in roentgens, the value of factor for water and soft tissues of the body can be taken with good accuracy to be equal to unit. • For bone tissue the value of decreases with growth of photons’ energy from 4. 5 to 1.

♦The radiobiological effect of IR depends on the type of radiation. Slow (thermal) neutron radiation is more effective than photon (X and gamma) radiation by a factor of three, and -radiation is 20 times more effective. ♦To characterise the radiobiological effect created by different kinds of radiation, the equivalent dose is used. The biological effect created by radiation, at a specified equivalent dose, does not depend on the kind of radiation. where k is the quality factor (relative biological effectiveness) • SI unit is sievert (Sv), • off-system unit is rem (the man roentgen-equivalent).

• Natural sources of ionizing radiation create a certain radiation level that continuously affects all the organisms on Earth. This level is known as the background. The annual equivalent dose related to the natural background is equal to 125 to 200 mrem. • In medicine the maximum permissible dose is used. The maximum permissible dose is the maximum value of an individual annual equivalent dose, which, at uniform exposure during 50 years, shall have no detrimental effect on man’s health. The maximum permissible annual equivalent dose for professional exposure is equal to 5 rem

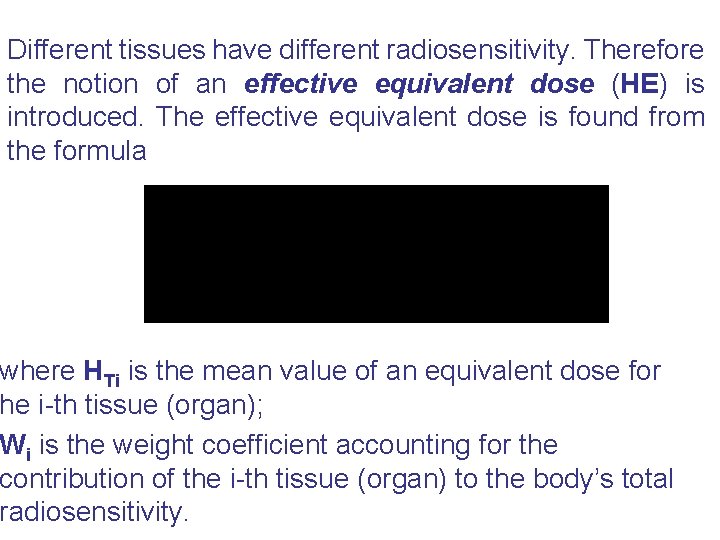
Different tissues have different radiosensitivity. Therefore the notion of an effective equivalent dose (HE) is introduced. The effective equivalent dose is found from the formula where HTi is the mean value of an equivalent dose for he i-th tissue (organ); Wi is the weight coefficient accounting for the contribution of the i-th tissue (organ) to the body’s total radiosensitivity.

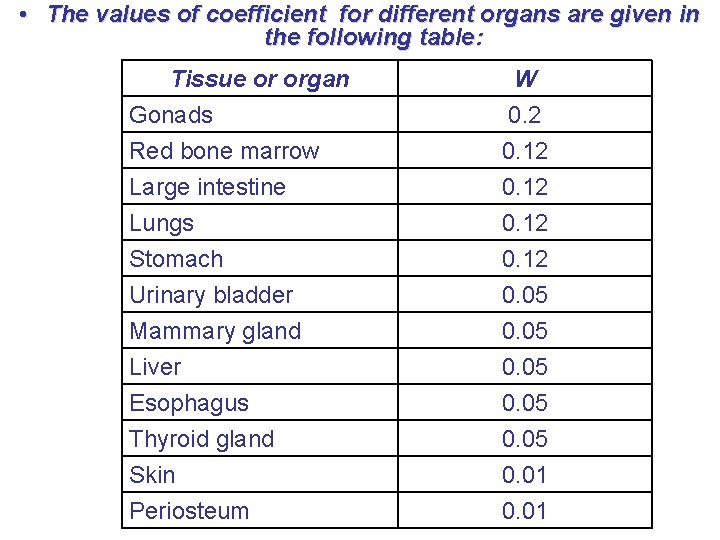
• The values of coefficient for different organs are given in the following table: Tissue or organ W Gonads Red bone marrow Large intestine 0. 2 0. 12 Lungs Stomach Urinary bladder Mammary gland Liver Esophagus Thyroid gland Skin Periosteum 0. 12 0. 05 0. 01

• Internal radiation occurs when sources of ionizing radiation get into the body with aspiration, food or water, or with injury of the skin. Internal radiation has several features: • - the time of body tissue exposure to radiation increases since radiation continues over the entire time during which the radioactive material stays in the body; • - the radiation dose for the internal organs increases due to the small distance between the organs (tissues) and the radiation sources; • - are safe during external radiation become hazardous. For example, - radiation which is completely held back by the skin during external radiation.

• The impact of ionising radiation can lead to death of the organism. To characterise organisms as per the criterion of the probability of a lethal effect, the minimal lethal dose is introduced. • The minimum lethal dose from ɣ-radiation, at radiation of the whole organism, for man is about 600 rem. LD 50 is the radiation dose at which 50 % of radiated organisms die. For man exposed to ɣ-radiation, LD 50 is equal to 2. 5 to 3. 5 Gy.

Dozimetry instruments Different instruments are used for detecting ionising radiation, determining their characteristics, and measuring radiation doses. These instruments can be divided into two groups: detectors of ionising radiation and dosimeters Ionising radiation detectors are intended for registering radiation and measuring some of their characteristics (e. g. , energy and velocity of particles, the ratio of their charge to mass, and others). Detectors include the Wilson chamber, the bubble chamber, the Geiger counter, thick-layer photo plates, and others. The Geiger counter The bubble chamber The Wilson chamber-

Dozimeters are devices for measuring dozes of ionizing radiation, or values related to dozes. Depending on the physical phenomenon determining the dozimeter’s principle of operation is based, the following devices are distinguished: ionization, luminescent, semiconductor and photo dozimeters.

Medical Applications of Ionizing Radiation • Ionizing radiation is widely used in medicine. • Radionuclide diagnostics is based on applying ionizing radiation. Let us remind that the notion of nuclides is equivalent to the notion of the atom nucleus, and radionuclides are radioactive atoms. • Dynamic and static methods of radionuclide diagnostics are distinguished.

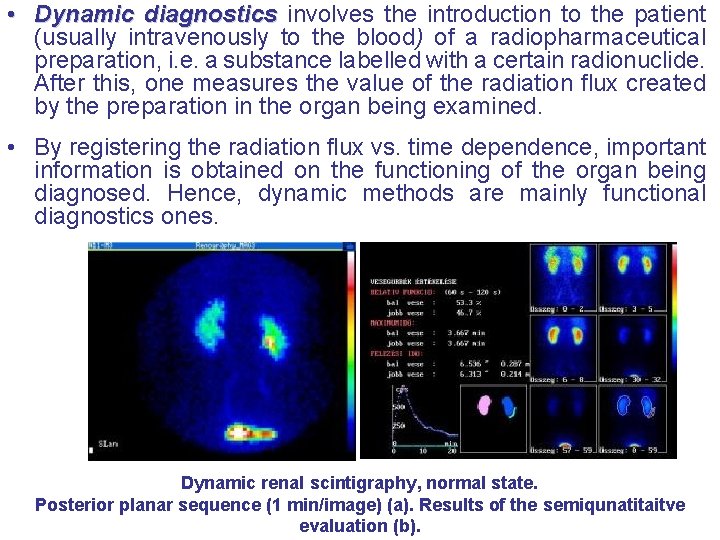
• Dynamic diagnostics involves the introduction to the patient (usually intravenously to the blood) of a radiopharmaceutical preparation, i. e. a substance labelled with a certain radionuclide. After this, one measures the value of the radiation flux created by the preparation in the organ being examined. • By registering the radiation flux vs. time dependence, important information is obtained on the functioning of the organ being diagnosed. Hence, dynamic methods are mainly functional diagnostics ones. Dynamic renal scintigraphy, normal state. Posterior planar sequence (1 min/image) (a). Results of the semiqunatitaitve evaluation (b).

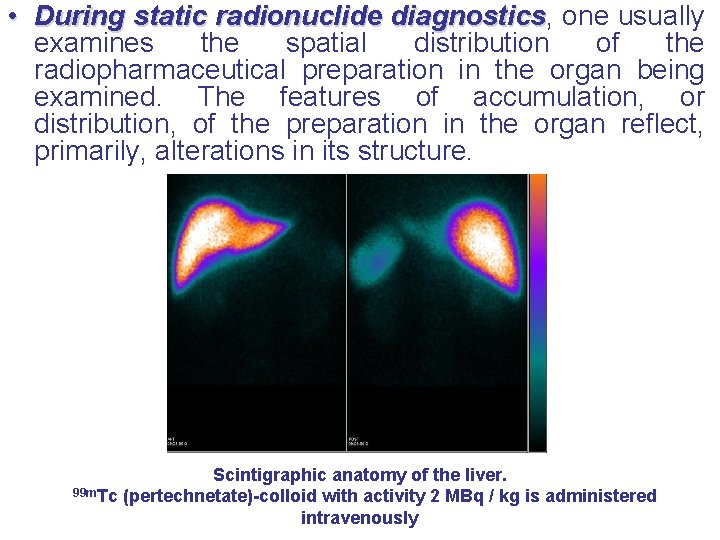
• During static radionuclide diagnostics, one usually diagnostics examines the spatial distribution of the radiopharmaceutical preparation in the organ being examined. The features of accumulation, or distribution, of the preparation in the organ reflect, primarily, alterations in its structure. Scintigraphic anatomy of the liver. 99 m. Tc (pertechnetate)-colloid with activity 2 MBq / kg is administered intravenously

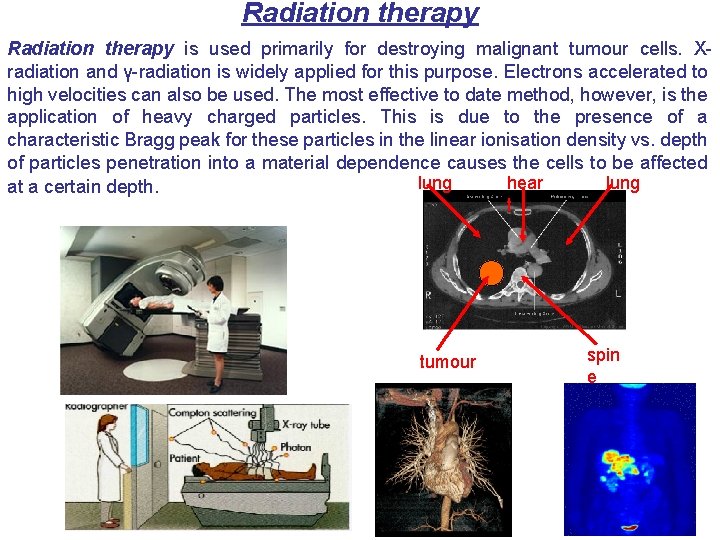
Radiation therapy is used primarily for destroying malignant tumour cells. Xradiation and γ-radiation is widely applied for this purpose. Electrons accelerated to high velocities can also be used. The most effective to date method, however, is the application of heavy charged particles. This is due to the presence of a characteristic Bragg peak for these particles in the linear ionisation density vs. depth of particles penetration into a material dependence causes the cells to be affected hear lung at a certain depth. t tumour spin e

 Sújb
Sújb Ionizing radiation sources
Ionizing radiation sources The osha ionizing radiation standard applies
The osha ionizing radiation standard applies Ionizing radiation
Ionizing radiation Ionizing wet scrubber
Ionizing wet scrubber Ionizing radiation
Ionizing radiation Ionizing radiation
Ionizing radiation Clip art
Clip art Ionizing radiation
Ionizing radiation Ionizing radiation examples
Ionizing radiation examples 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Livesinthebalance alsup
Livesinthebalance alsup Academic field trip plan a plan b
Academic field trip plan a plan b Short, medium and long term planning in education
Short, medium and long term planning in education Problem solving plan (plan b flowchart)
Problem solving plan (plan b flowchart) Micro-teaching lesson plan
Micro-teaching lesson plan Problem solving plan (plan b flowchart)
Problem solving plan (plan b flowchart) New jersey plan vs virginia plan
New jersey plan vs virginia plan Stowage plane
Stowage plane Yakin aja
Yakin aja Man's plan vs god's plan
Man's plan vs god's plan Virginia plan and new jersey plan venn diagram
Virginia plan and new jersey plan venn diagram Constitutional convention compromise
Constitutional convention compromise Dont plan
Dont plan Building owner chapter 6
Building owner chapter 6 Project procurement management lecture notes
Project procurement management lecture notes Lecture about sport
Lecture about sport Lecture on healthy lifestyle
Lecture on healthy lifestyle Existential nihilism
Existential nihilism Meaning of this
Meaning of this Randy pausch last lecture summary
Randy pausch last lecture summary Tensorflow lecture
Tensorflow lecture Theology proper lecture notes
Theology proper lecture notes Strategic management lecture
Strategic management lecture Geology lecture series
Geology lecture series Social psychology lecture
Social psychology lecture In text citation for a lecture
In text citation for a lecture Lecture notes on public sector accounting-ghana pdf
Lecture notes on public sector accounting-ghana pdf 4 p's of software project management
4 p's of software project management Reinforcement lap lengths eurocodes
Reinforcement lap lengths eurocodes Electricity and magnetism lecture notes
Electricity and magnetism lecture notes Classical mechanics
Classical mechanics What is a harmonic wave in physics
What is a harmonic wave in physics Physical science lecture notes
Physical science lecture notes Power system dynamics and stability lecture notes
Power system dynamics and stability lecture notes Natural language processing nlp - theory lecture
Natural language processing nlp - theory lecture Microbial physiology notes
Microbial physiology notes Application of mechatronics ppt
Application of mechatronics ppt