Insulin Therapy In Type 1 DM H Delshad


















- Slides: 41


Insulin Therapy In Type 1 DM H. Delshad M. D Endocrinologist Research Institute For Endocrine Sciences

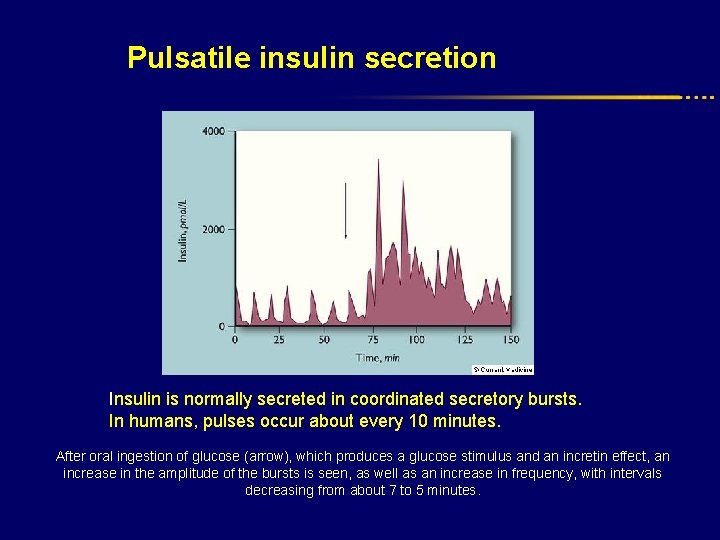
Pulsatile insulin secretion Insulin is normally secreted in coordinated secretory bursts. In humans, pulses occur about every 10 minutes. After oral ingestion of glucose (arrow), which produces a glucose stimulus and an incretin effect, an increase in the amplitude of the bursts is seen, as well as an increase in frequency, with intervals decreasing from about 7 to 5 minutes.

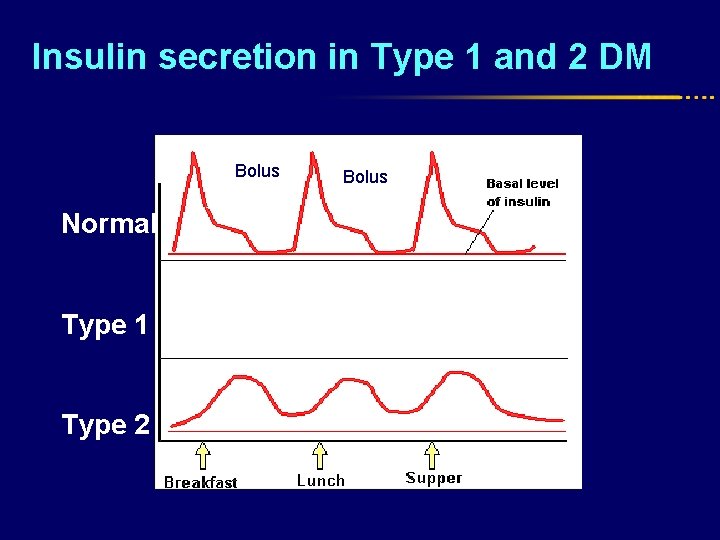
Insulin secretion in Type 1 and 2 DM Prandial Bolus Normal Type 1 Type 2 Bolus

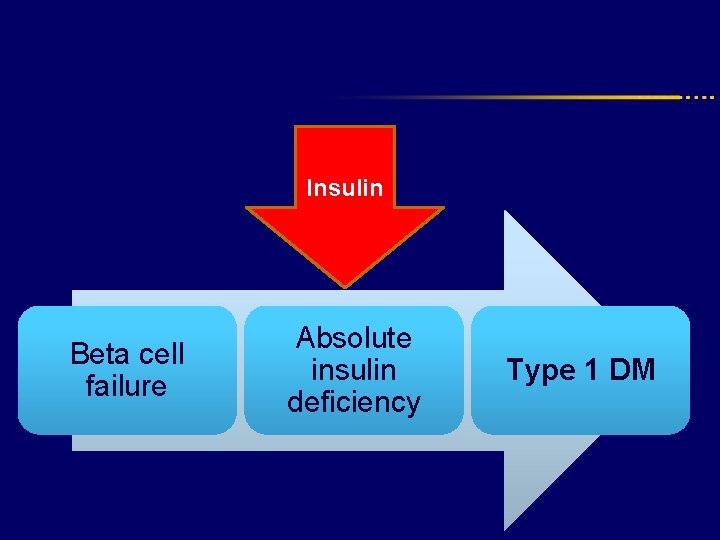
Insulin Beta cell failure Absolute insulin deficiency Type 1 DM

Insulin therapy in T 1 DM: Key points l Discovery of Insulin in 1921 provided a life- sustaining therapy for a previously fatal wasting condition. l Insulin dramatically shifted the prognosis of T 1 DM to that of a chronic disease characterized by devastating longterm micro-and macro vascular complications. l The focus of treatment has changed significantly since 1922, with emphasis now directed toward the avoidance of complications and optimization of patient quality of life. l The DCCT and other studies have provided adequate evidence that there is a direct relationship between Hb. A 1 c and progression of diabetic complications and optimization of glycemic control can significantly reduce the risk of vascular complications.

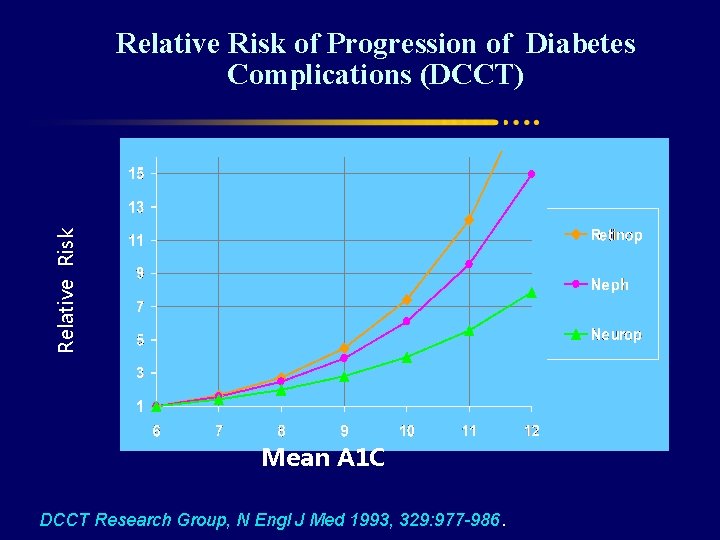
Relative Risk of Progression of Diabetes Complications (DCCT) Mean A 1 C DCCT Research Group, N Engl J Med 1993, 329: 977 -986.

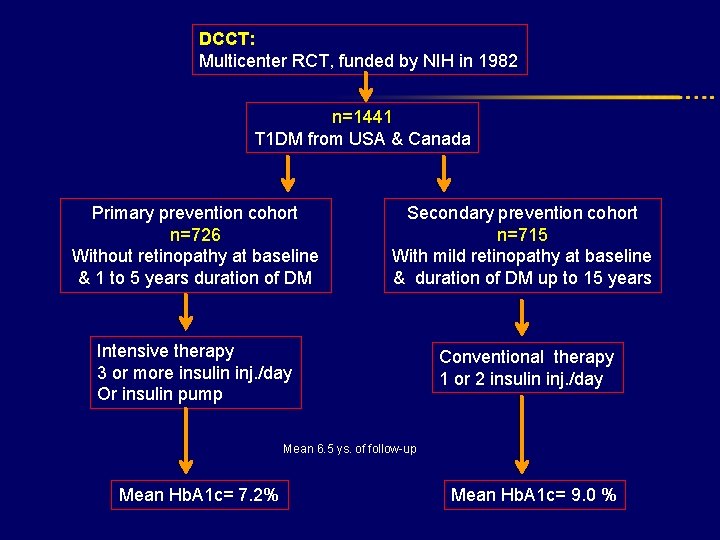
DCCT: Multicenter RCT, funded by NIH in 1982 n=1441 T 1 DM from USA & Canada Primary prevention cohort n=726 Without retinopathy at baseline & 1 to 5 years duration of DM Secondary prevention cohort n=715 With mild retinopathy at baseline & duration of DM up to 15 years Intensive therapy 3 or more insulin inj. /day Or insulin pump Conventional therapy 1 or 2 insulin inj. /day Mean 6. 5 ys. of follow-up Mean Hb. A 1 c= 7. 2% Mean Hb. A 1 c= 9. 0 %

Relative risk reduction Intensive Vs. Conventional therapy Development of retinopathy 76% Progression of retinopathy 54% Development of microalbuminuria 39% Progression of albuminuria 54% (< 300 mg/24 h)

The DCCT: Provided conclusive evidence that Improved glycemic control with intensive therapy: • Can reduce the incidence of micro-vascular complications. Primary prevention • Can slow the progression of established micro-vascular diseases. Secondary prevention

EDIC Study : Epidemiology of Diabetes Interventions and Complications Conventional 95% (n=1375) of the DCCT participants has been followed since the completion of the original trial Intensive Cumulative incidence of further progression of retinopathy during the EDIC study in the former conventional therapy and intensive therapy cohorts from DCCT. Persistent beneficial effects of previous near normal glycemic control.

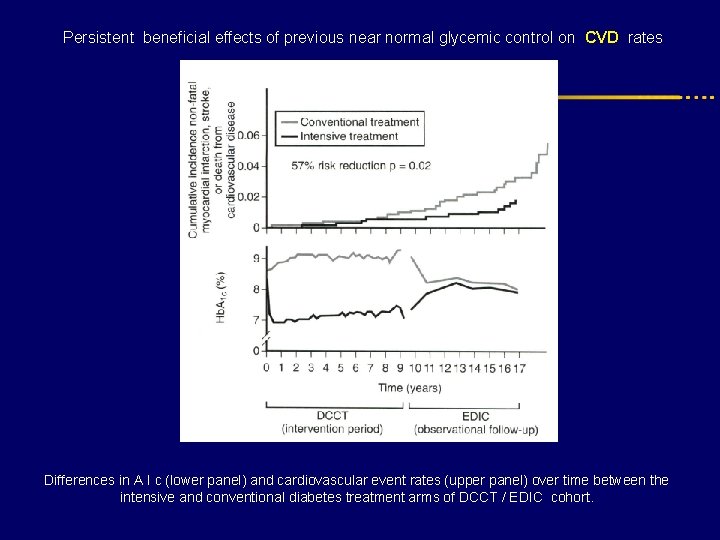
Persistent beneficial effects of previous near normal glycemic control on CVD rates Differences in A I c (lower panel) and cardiovascular event rates (upper panel) over time between the intensive and conventional diabetes treatment arms of DCCT / EDIC cohort.

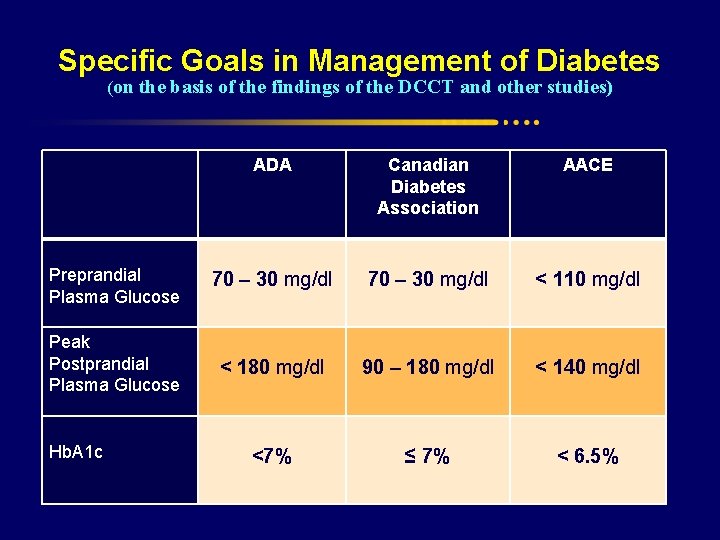
Specific Goals in Management of Diabetes (on the basis of the findings of the DCCT and other studies) ADA Canadian Diabetes Association AACE Preprandial Plasma Glucose 70 – 30 mg/dl < 110 mg/dl Peak Postprandial Plasma Glucose < 180 mg/dl 90 – 180 mg/dl < 140 mg/dl <7% ≤ 7% < 6. 5% Hb. A 1 c

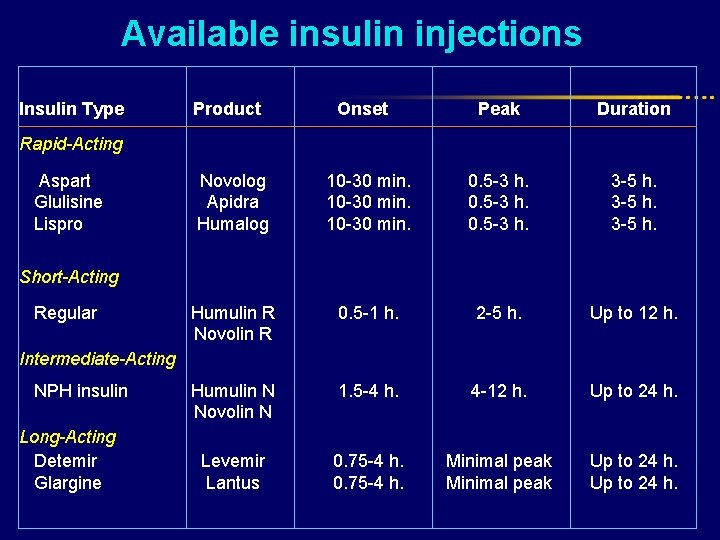
Available insulin injections Insulin Type Product Onset Peak Duration Novolog Apidra Humalog 10 -30 min. 0. 5 -3 h. 3 -5 h. Humulin R Novolin R 0. 5 -1 h. 2 -5 h. Up to 12 h. Humulin N Novolin N 1. 5 -4 h. 4 -12 h. Up to 24 h. Levemir Lantus 0. 75 -4 h. Minimal peak Up to 24 h. Rapid-Acting Aspart Glulisine Lispro Short-Acting Regular Intermediate-Acting NPH insulin Long-Acting Detemir Glargine

Human Insulins l REGULAR : Traditional Bolus insulin since 1921 l NPH : Traditional Basal insulin replacement since 1950 l Several well known limitations : * Absorption Variation : unfavorable plasma profiles * Duration of action * Peak effect * Fasting hyperglycemia * Nocturnal hypoglycemia

Insulin Analogues : 2000 Lispro Aspart Ultrarapid Inhaled Insulin Technosfer Glulisine Glargine Ultralong = Degludec Detemir

Principles of insulin therapy l. Frequently l. Daily used regimens insulin dosage l. Distribution of insulin dosage


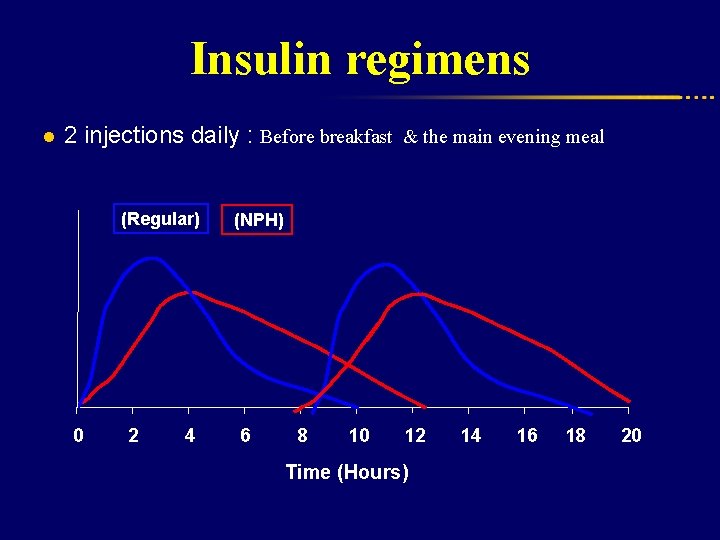
Insulin regimens l 2 injections daily : Before breakfast & the main evening meal (Regular) 0 2 4 (NPH) 6 8 10 12 Time (Hours) 14 16 18 20

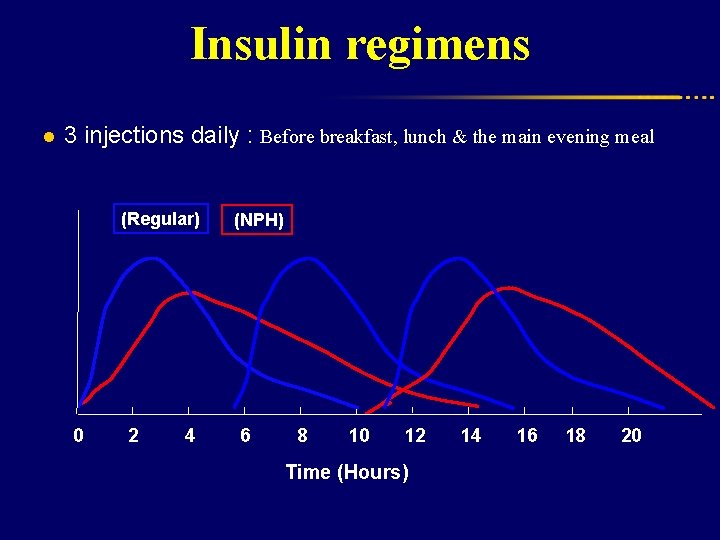
Insulin regimens l 3 injections daily : Before breakfast, lunch & the main evening meal (Regular) 0 2 4 (NPH) 6 8 10 12 Time (Hours) 14 16 18 20

Conventional regimens l Lack 2 or. Problems 3 injections daily of flexibility Inadequate Fasting coverage of post-lunch glycemia hyperglycemia Nocturnal hypoglycemia

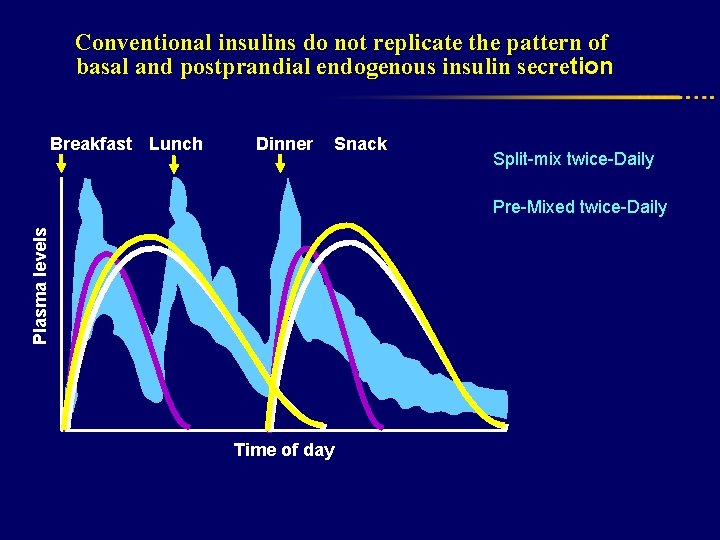
Conventional insulins do not replicate the pattern of basal and postprandial endogenous insulin secretion Breakfast Lunch Dinner Snack Split-mix twice-Daily Plasma levels Pre-Mixed twice-Daily Time of day

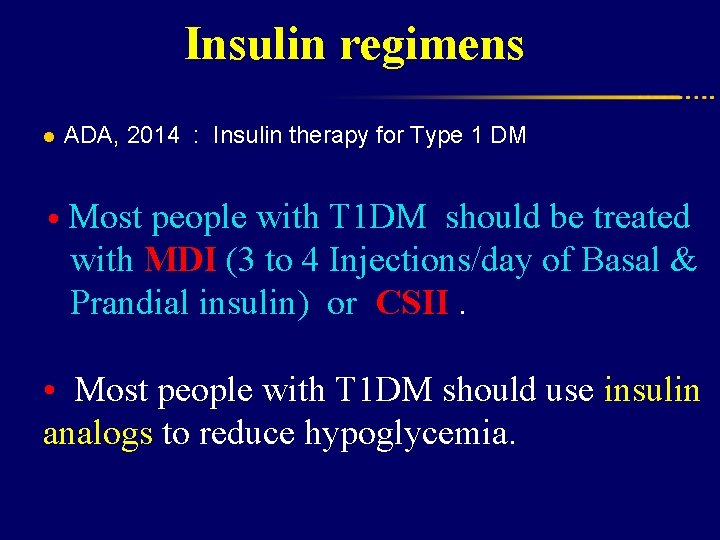
Insulin regimens l ADA, 2014 : Insulin therapy for Type 1 DM • Most people with T 1 DM should be treated with MDI (3 to 4 Injections/day of Basal & Prandial insulin) or CSII. • Most people with T 1 DM should use insulin analogs to reduce hypoglycemia.

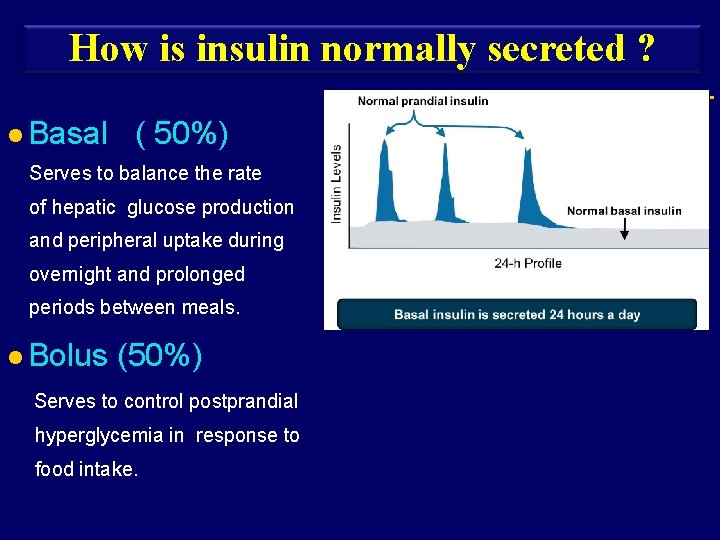
How is insulin normally secreted ? l Basal ( 50%) Serves to balance the rate of hepatic glucose production and peripheral uptake during overnight and prolonged periods between meals. l Bolus (50%) Serves to control postprandial hyperglycemia in response to food intake. Basal

Insulin regimens l Basal and Prandial Insulin regimen Basal Prandial at bedtime 40 -60% TDD Glargine Detemir before meals Aspart Glulisine Lispro

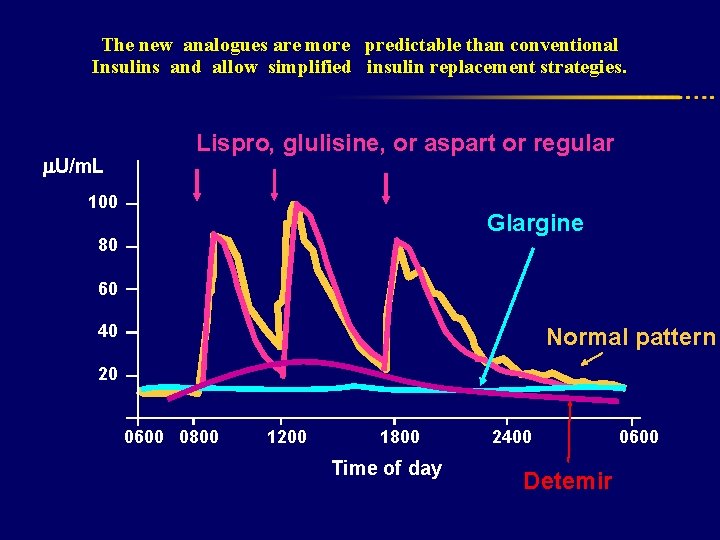
The new analogues are more predictable than conventional Insulins and allow simplified insulin replacement strategies. U/m. L Lispro, glulisine, or aspart or regular 100 Glargine 80 60 40 Normal pattern 20 0600 0800 1200 1800 Time of day 2400 Detemir 0600


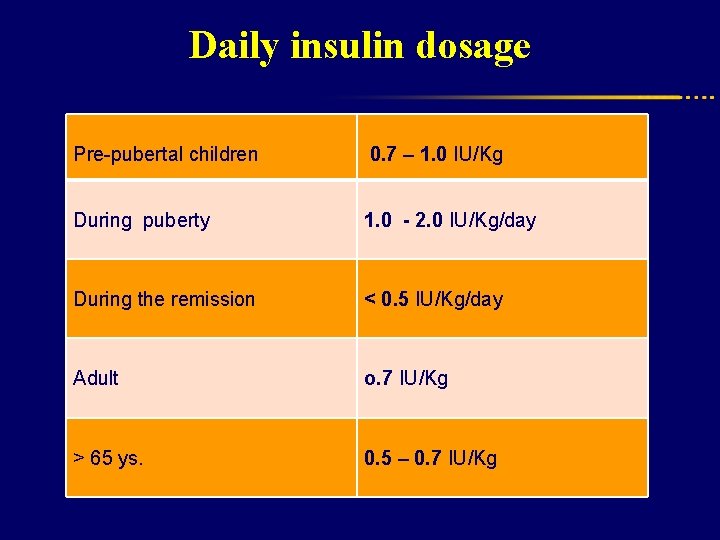
Daily insulin dosage Dosage depends on many factors: l Age l Weight l Stage of puberty l Duration and phase of DM l Nutritional intake and distribution l Exercise patterns l Results of blood glucose monitoring l Intercurrent illness

Daily insulin dosage Pre-pubertal children 0. 7 – 1. 0 IU/Kg During puberty 1. 0 - 2. 0 IU/Kg/day During the remission < 0. 5 IU/Kg/day Adult o. 7 IU/Kg > 65 ys. 0. 5 – 0. 7 IU/Kg


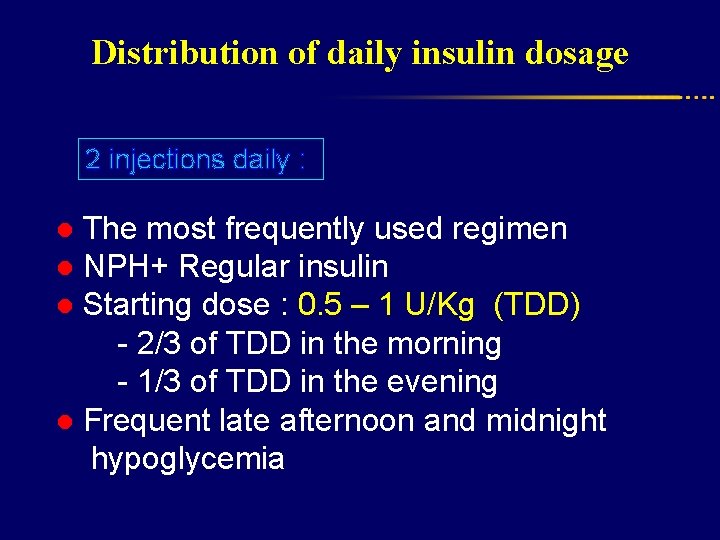
Distribution of daily insulin dosage 2 injections daily : ● The most frequently used regimen ● NPH+ Regular insulin ● Starting dose : 0. 5 – 1 U/Kg (TDD) - 2/3 of TDD in the morning - 1/3 of TDD in the evening ● Frequent late afternoon and midnight hypoglycemia

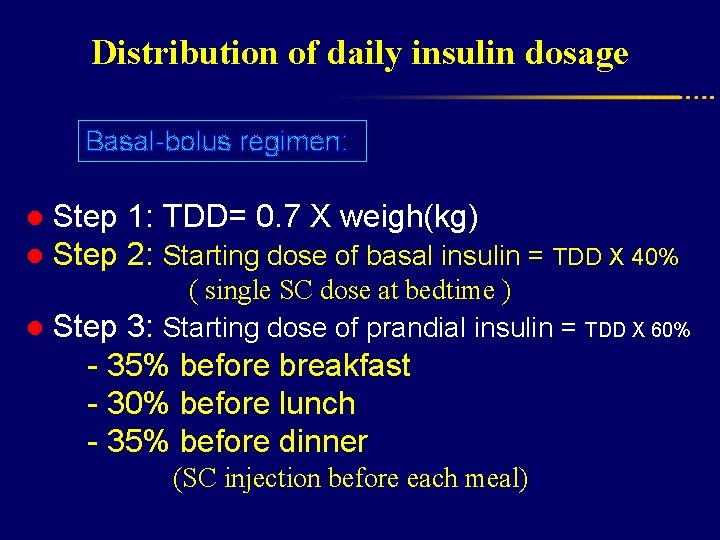
Distribution of daily insulin dosage Basal-bolus regimen: ● Step 1: TDD= 0. 7 X weigh(kg) ● Step 2: Starting dose of basal insulin = TDD X 40% ( single SC dose at bedtime ) ● Step 3: Starting dose of prandial insulin = TDD X 60% - 35% before breakfast - 30% before lunch - 35% before dinner (SC injection before each meal)

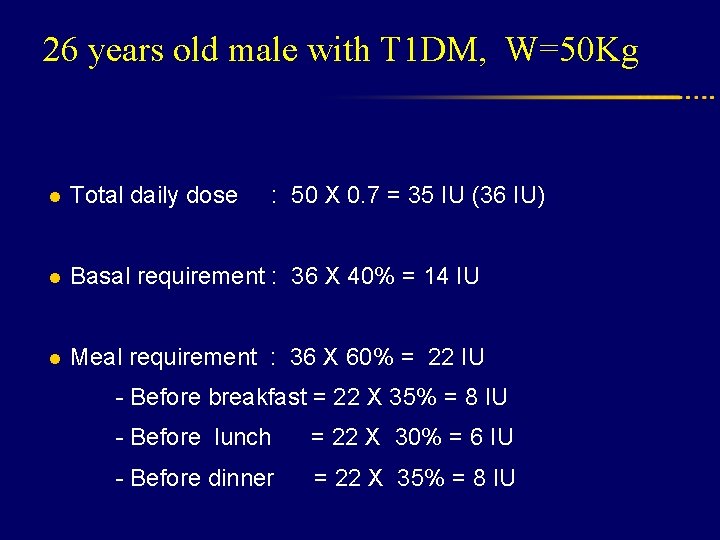
26 years old male with T 1 DM, W=50 Kg l Total daily dose : 50 X 0. 7 = 35 IU (36 IU) l Basal requirement : 36 X 40% = 14 IU l Meal requirement : 36 X 60% = 22 IU - Before breakfast = 22 X 35% = 8 IU - Before lunch = 22 X 30% = 6 IU - Before dinner = 22 X 35% = 8 IU

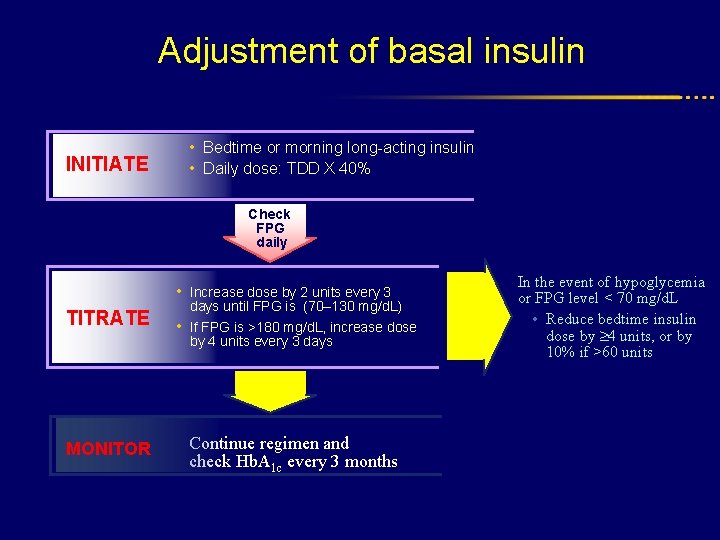
Adjustment of basal insulin INITIATE • Bedtime or morning long-acting insulin • Daily dose: TDD X 40% Check FPG daily • Increase dose by 2 units every 3 TITRATE days until FPG is (70– 130 mg/d. L) • If FPG is >180 mg/d. L, increase dose by 4 units every 3 days MONITOR Continue regimen and check Hb. A 1 c every 3 months In the event of hypoglycemia or FPG level < 70 mg/d. L • Reduce bedtime insulin dose by 4 units, or by 10% if >60 units

Adjustment of meal insulin on the basis of : l Pre-prandial l Anticipated l Upcoming capillary blood glucose carbohydrate intake physical activity

Insulin Sensitivity Factor (ISF) l Calculates how much blood glucose decreases for each unite of bolus insulin l ISF (For human insulin)= 1500 ÷ TDD l ISF (For analogue insulin)= 1800 ÷ TDD l Example: TDD= 50 (human insulin), ISF = 1500 ÷ 50 = 30 It means that each unit of bolus decreases blood glucose by 30 mg/dl

Supplemental Insulin for Correction of meal Hyperglycemia Insulin Regular, Aspart , Glulisine , Lispro can be used to correct for hyperglycemia: • In general, 1 -2 units of insulin will lower the blood glucose by 30 -50 mg/dl. • For every 50 mg/dl above the premeal glucose target (e. g. , 150 mg/dl), add 1 unit of insulin • If premeal glucose is 250 mg/dl, 2 units of insulin should be added to the usual dose of premeal insulin.

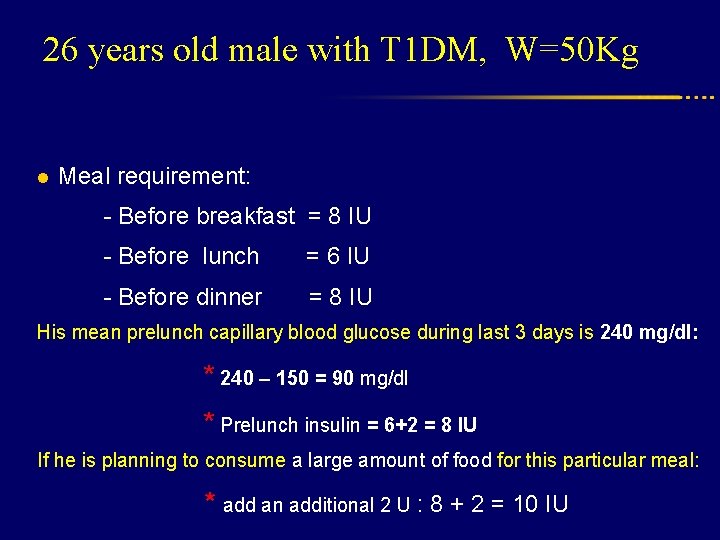
26 years old male with T 1 DM, W=50 Kg l Meal requirement: - Before breakfast = 8 IU - Before lunch = 6 IU - Before dinner = 8 IU His mean prelunch capillary blood glucose during last 3 days is 240 mg/dl: * 240 – 150 = 90 mg/dl * Prelunch insulin = 6+2 = 8 IU If he is planning to consume a large amount of food for this particular meal: * add an additional 2 U : 8 + 2 = 10 IU

Adjustments for Exercise improves insulin sensitivity : • For exercise 1 -3 h. post-prandial : Reduce pre-meal insulin by 50 – 75 % • For exercise > 3 h. post-prandial: Smaller or no change in dose

Place of premixed insulins l Premixed insulins are not recommended § For initiation or during adjustment of doses l If the proportion of rapid- and intermediate-acting insulin is similar to the fixed proportions available ▪ Can be used before breakfast and/or dinner l Easy for some patients
