Innovation Investigation Application Chemotherapy Induced Nausea and Vomiting
















- Slides: 38

Innovation ● Investigation ● Application Chemotherapy Induced Nausea and Vomiting (CINV) Causes, Challenges, Evaluation and Optimizing Clinical Management Program Co-Chairman Lee S. Schwartzberg, MD, FACP Medical Director, The West Clinic Supportive Oncology Services President, Accelerated Community Oncology Research Network Memphis, TN

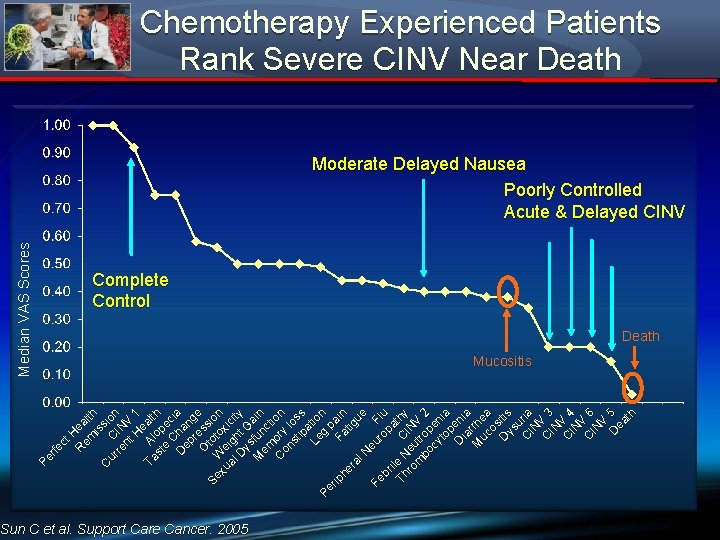
t. H R ea em lth is s C ur C ion re IN nt V H 1 e Ta Al alt s t op h e ec C D h a ia ep n r ge O e ss Se W tot ion xu e ox al igh icit D t. G y ys M fun ai n em c t C ory i on on l st os ip s a L e tio g n Pe pa ri p F he at in ig ra ue l. N e Fe ur Fl op u br a i le Th N C thy ro eu IN m tr V bo op 2 cy en to ia p D eni ia a M rrhe uc a os D itis ys u C ria IN V C 3 IN C V 4 IN V C 6 IN V D 5 ea th fe c Pe r Median VAS Scores Chemotherapy Experienced Patients Rank Severe CINV Near Death Moderate Delayed Nausea Poorly Controlled Acute & Delayed CINV Complete Control Death Mucositis Sun C et al. Support Care Cancer. 2005

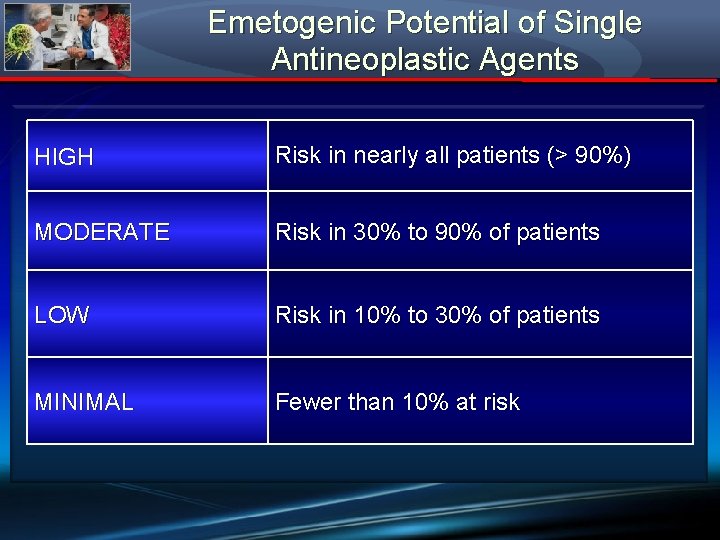
Emetogenic Potential of Single Antineoplastic Agents HIGH Risk in nearly all patients (> 90%) MODERATE Risk in 30% to 90% of patients LOW Risk in 10% to 30% of patients MINIMAL Fewer than 10% at risk



Patient-Specific Risk Factors for CINV ► Age <50 years ► Women > men ► History of light alcohol use ► History of vomiting with prior exposure to chemotherapeutic agents ► Other risks ● History of motion sickness ● History of nausea or vomiting during pregnancy ● History of anxiety ASHP. Am J Health Syst Pharm. 1999: 56: 729 -764; Balfour and Goa. Drugs. 1997: 54: 273 -298.

Types of CINV: Definitions ► Acute (post-treatment) ● ► Delayed ● ● ► Learned or conditioned response from poorly controlled nausea and vomiting associated with previous chemotherapy Breakthrough ● ► CINV that begins after first 24 hours May last for 120 hours Anticipatory ● ► Occurs within first 24 hours after administration of cancer chemotherapy CINV that occurs despite prophylaxis and requires rescue Refractory ● Occurs during subsequent treatment cycles when prophylaxis and/or rescue has failed in previous cycles

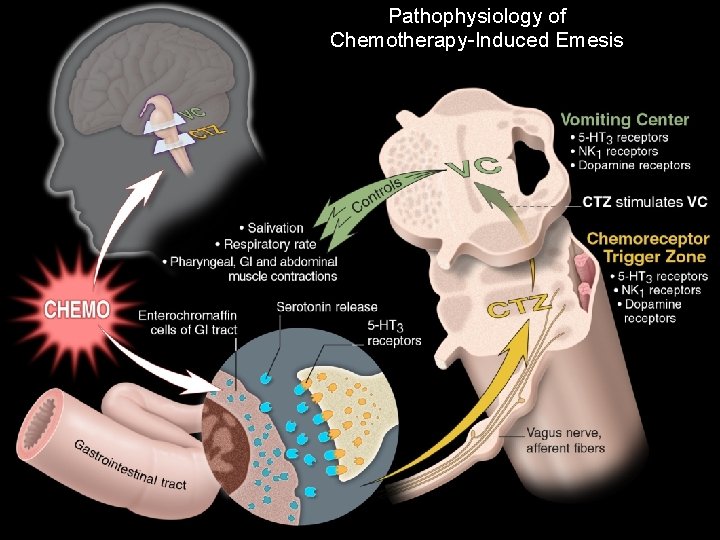
Pathophysiology of Chemotherapy-Induced Emesis

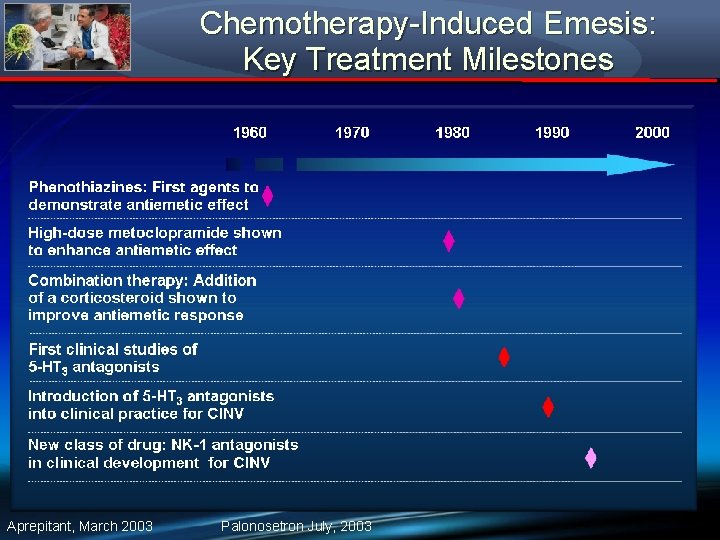
Chemotherapy-Induced Emesis: Key Treatment Milestones Aprepitant, March 2003 Palonosetron July, 2003

Pharmacologic Agents ► Corticosteroids ► Dopamine ► Serotonin ► NK-1 antagonists (5 -HT 3) antagonists receptor antagonists ► Cannabinoids

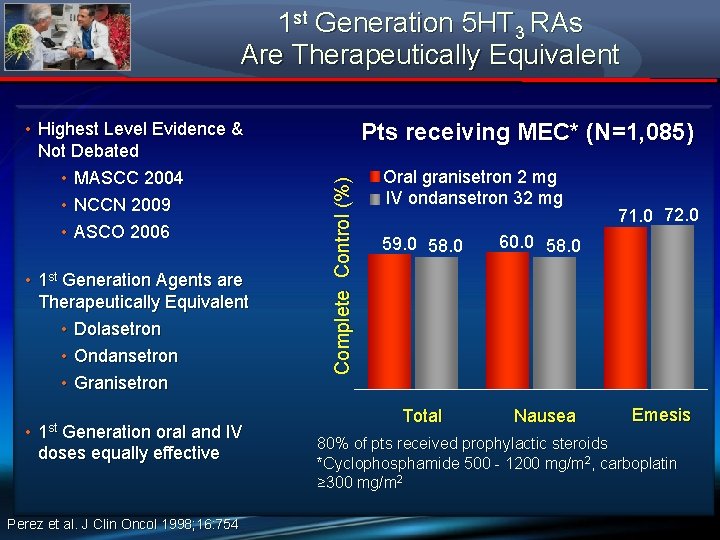
1 st Generation 5 HT 3 RAs Are Therapeutically Equivalent • 1 st Generation Agents are Therapeutically Equivalent • Dolasetron • Ondansetron • Granisetron • 1 st Generation oral and IV doses equally effective Perez et al. J Clin Oncol 1998; 16: 754 Pts receiving MEC* (N=1, 085) Complete Control (%) • Highest Level Evidence & Not Debated • MASCC 2004 • NCCN 2009 • ASCO 2006 Oral granisetron 2 mg IV ondansetron 32 mg 59. 0 58. 0 60. 0 58. 0 Total Nausea 71. 0 72. 0 Emesis 80% of pts received prophylactic steroids *Cyclophosphamide 500 - 1200 mg/m 2, carboplatin ≥ 300 mg/m 2

Palonosetron ► ► Second generation 5 -HT 3 antagonist Pharmacologic differences from older 5 -HT 3 antagonists ● prolonged half-life (~40 hours) ● enhanced receptor binding affinity (30 -fold) FDA approved ● IV formulation July 25, 2003 ● Oral formulation August 22, 2008 Regimens ● IV 0. 25 mg pre chemotherapy acute/delayed HEC/MEC ● PO 0. 50 mg pre chemotherapy acute MEC

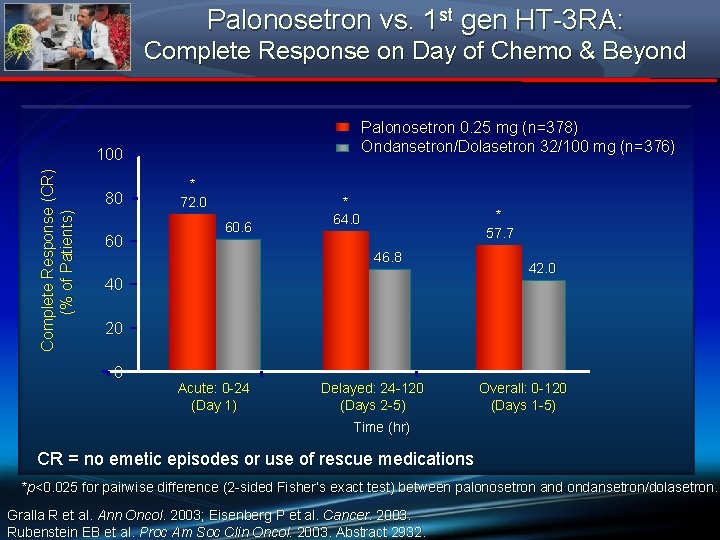
Palonosetron vs. 1 st gen HT-3 RA: Complete Response on Day of Chemo & Beyond Palonosetron 0. 25 mg (n=378) Ondansetron/Dolasetron 32/100 mg (n=376) Complete Response (CR) (% of Patients) 100 80 60 * 72. 0 60. 6 * 64. 0 * 57. 7 46. 8 40 42. 0 20 0 Acute: 0 -24 (Day 1) Delayed: 24 -120 (Days 2 -5) Overall: 0 -120 (Days 1 -5) Time (hr) CR = no emetic episodes or use of rescue medications *p<0. 025 for pairwise difference (2 -sided Fisher’s exact test) between palonosetron and ondansetron/dolasetron. Gralla R et al. Ann Oncol. 2003; Eisenberg P et al. Cancer. 2003. Rubenstein EB et al. Proc Am Soc Clin Oncol. 2003. Abstract 2932.

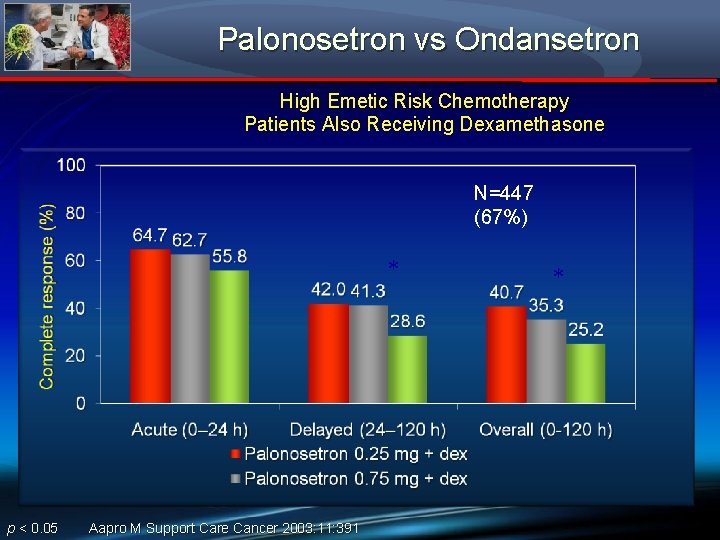
Palonosetron vs Ondansetron High Emetic Risk Chemotherapy Patients Also Receiving Dexamethasone N=447 (67%) * p < 0. 05 Aapro M Support Care Cancer 2003: 11: 391 *

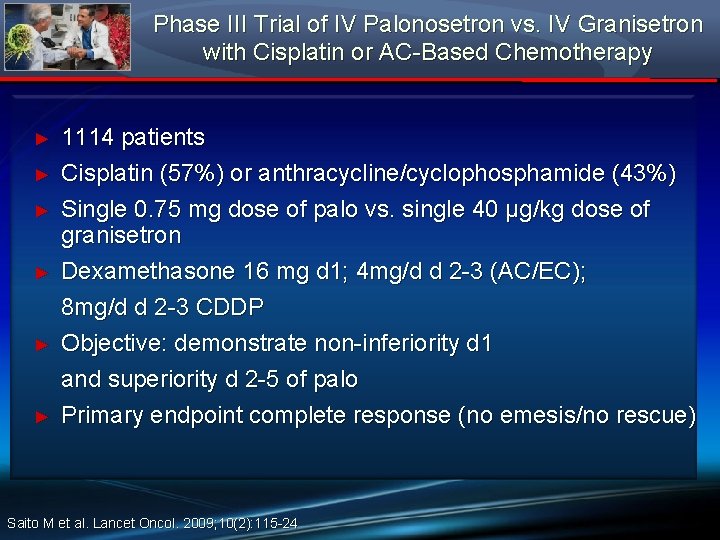
Phase III Trial of IV Palonosetron vs. IV Granisetron with Cisplatin or AC-Based Chemotherapy ► ► ► 1114 patients Cisplatin (57%) or anthracycline/cyclophosphamide (43%) Single 0. 75 mg dose of palo vs. single 40 μg/kg dose of granisetron Dexamethasone 16 mg d 1; 4 mg/d d 2 -3 (AC/EC); 8 mg/d d 2 -3 CDDP Objective: demonstrate non-inferiority d 1 and superiority d 2 -5 of palo Primary endpoint complete response (no emesis/no rescue) Saito M et al. Lancet Oncol. 2009; 10(2): 115 -24

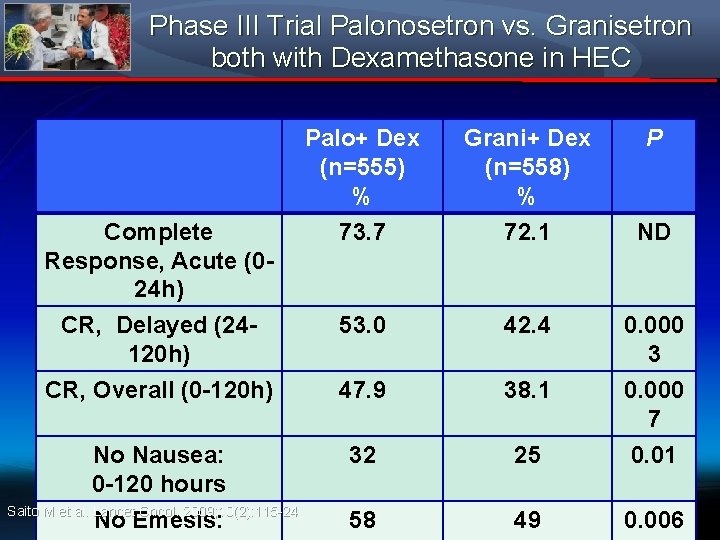
Phase III Trial Palonosetron vs. Granisetron both with Dexamethasone in HEC Palo+ Dex (n=555) % 73. 7 Grani+ Dex (n=558) % 72. 1 53. 0 42. 4 0. 000 3 CR, Overall (0 -120 h) 47. 9 38. 1 No Nausea: 0 -120 hours Saito M et al. Lancet Oncol. 2009; 10(2): 115 -24 No Emesis: 32 25 0. 000 7 0. 01 58 49 0. 006 Complete Response, Acute (024 h) CR, Delayed (24120 h) P ND

Palonosetron: 5 -HT 3 Antagonist of Choice? ► Palonosetron is a 5 -HT 3 antagonist with strong receptor binding affinity and an extended half-life ► In 2 MEC trials, IV palonosetron (single dose) was superior to dolasetron and ondansetron (single dose) in the prevention of acute and delayed emesis in a post-hoc analysis ► In 1 HEC trial, emetic control was comparable between IV palonosetron and ondansetron; better control with palonosetron in the subset receiving dexamethasone ► In large phase III trial with cisplatin or AC, palonosetron was equivalent to granisetron in acute control and superior during the delayed phase ► Comparable tolerability ► Ease of use and trends towards superiority favor palonosetron as the preferred 5 -HT 3 antagonist ► Definitive proof of superiority to first generation 5 -HT 3 antagonists would require trials with control arms utilizing corticosteroids, NK 1 antagonists and repetitive dosing of the first generation agents

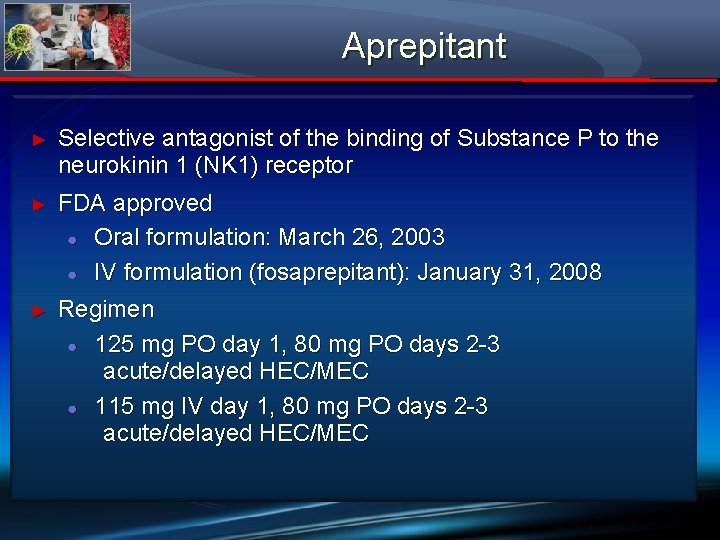
Aprepitant ► Selective antagonist of the binding of Substance P to the neurokinin 1 (NK 1) receptor ► FDA approved ● Oral formulation: March 26, 2003 ● IV formulation (fosaprepitant): January 31, 2008 ► Regimen ● 125 mg PO day 1, 80 mg PO days 2 -3 acute/delayed HEC/MEC ● 115 mg IV day 1, 80 mg PO days 2 -3 acute/delayed HEC/MEC

Aprepitant Randomized Trial: Patients Receiving AC Group Day 1 O D Days 2 -3 A Aprepitant (n = 438) 8 mg BID 12 mg 125 mg Standard (n = 428) 8 mg BID 20 mg P 0 A P 80 mg 8 mg BID O = ondansetron PO A = aprepitant PO D = dexamethasone PO P = placebo PO Warr DG et al. J Clin Oncol 2005; 23: 2822 -2830 P

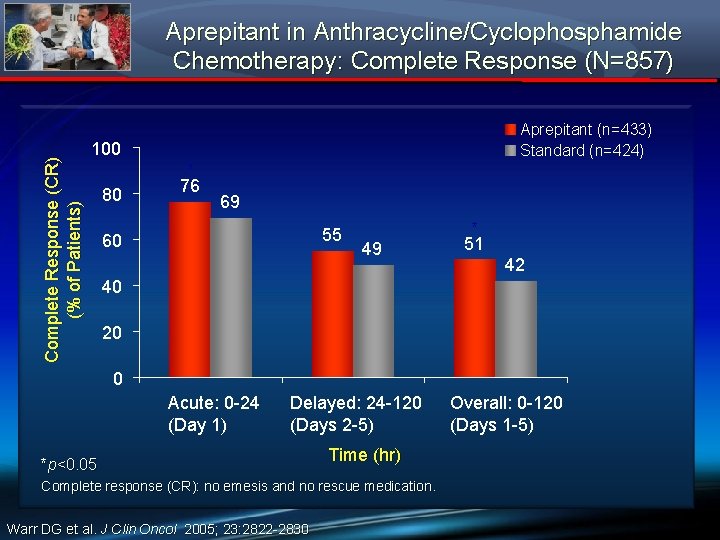
Complete Response (CR) (% of Patients) Aprepitant in Anthracycline/Cyclophosphamide Chemotherapy: Complete Response (N=857) Aprepitant (n=433) Standard (n=424) 100 80 * 76 69 55 60 49 * 51 42 40 20 0 Acute: 0 -24 (Day 1) Delayed: 24 -120 (Days 2 -5) *p<0. 05 Time (hr) Complete response (CR): no emesis and no rescue medication. Warr DG et al. J Clin Oncol 2005; 23: 2822 -2830 Overall: 0 -120 (Days 1 -5)

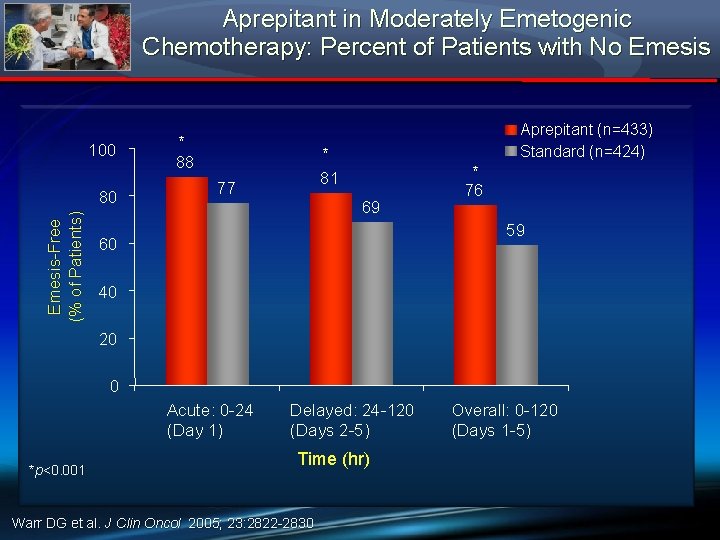
Aprepitant in Moderately Emetogenic Chemotherapy: Percent of Patients with No Emesis 100 Emesis-Free (% of Patients) 80 * Aprepitant (n=433) Standard (n=424) * 88 * 81 77 69 76 59 60 40 20 0 Acute: 0 -24 (Day 1) *p<0. 001 Delayed: 24 -120 (Days 2 -5) Time (hr) Warr DG et al. J Clin Oncol 2005; 23: 2822 -2830 Overall: 0 -120 (Days 1 -5)

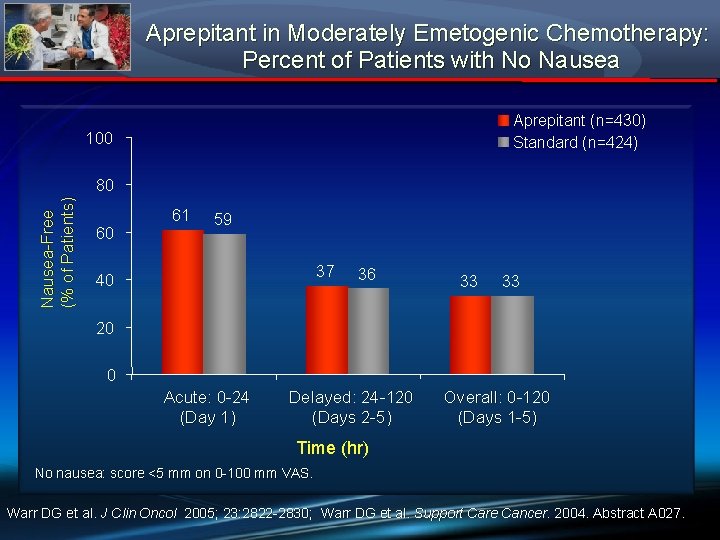
Aprepitant in Moderately Emetogenic Chemotherapy: Percent of Patients with No Nausea Aprepitant (n=430) Standard (n=424) 100 Nausea-Free (% of Patients) 80 60 61 59 37 40 36 33 33 20 0 Acute: 0 -24 (Day 1) Delayed: 24 -120 (Days 2 -5) Overall: 0 -120 (Days 1 -5) Time (hr) No nausea: score <5 mm on 0 -100 mm VAS. Warr DG et al. J Clin Oncol 2005; 23: 2822 -2830; Warr DG et al. Support Care Cancer. 2004. Abstract A 027.

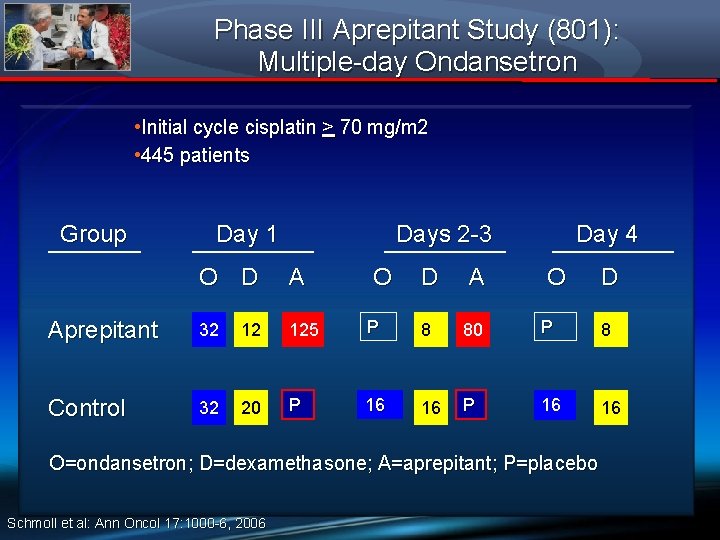
Phase III Aprepitant Study (801): Multiple-day Ondansetron • Initial cycle cisplatin > 70 mg/m 2 • 445 patients Group Day 1 Days 2 -3 O D A Aprepitant 32 12 125 Control 32 20 P O Day 4 D A O P 8 80 P 8 16 16 P 16 16 O=ondansetron; D=dexamethasone; A=aprepitant; P=placebo Schmoll et al: Ann Oncol 17: 1000 -6, 2006 D

Phase III Aprepitant Study (801): Multiple-day Ondansetron ► Identical design to Protocols 052 and 054 except ondansetron dosed days 1 -4 ► Primary endpoint: complete response on days 1 - 5 after cisplatin ► Aprepitant regimen superior to control regimen of protracted ondansetron and dexamethasone dosing, CR 72% vs. 61% respectively Schmoll et al: Ann Oncol 17: 1000 -6, 2006

Palonosetron + Aprepitant + Dexamethasone Phase II Study Design • Multicenter, phase II, open-label study • Naïve and non-naïve patients receiving moderately to moderatelyhighly emetogenic chemotherapy • Treatment: Day 1 PALO D Days 2 -3 A D 0. 25 mg 125 mg PALO = palonosetron IV 8 mg A 80 mg A = aprepitant PO D = dexamethasone PO Grote T et al. Proc Am Soc Clin Oncol. 2004. Abstract 8262.

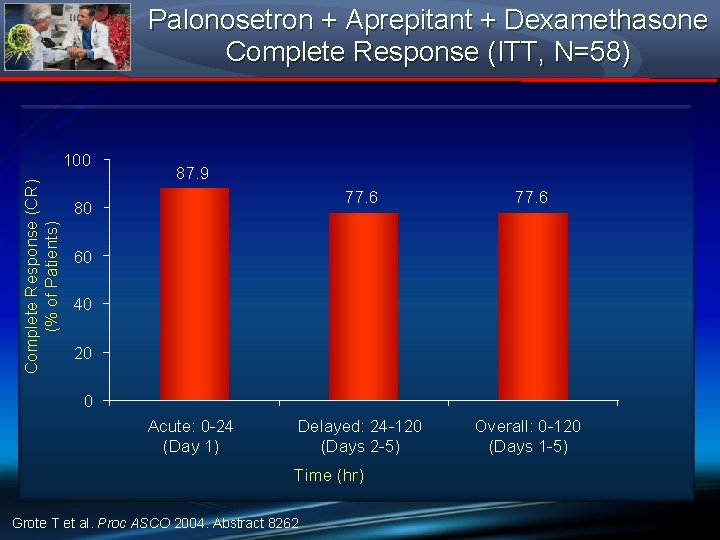
Palonosetron + Aprepitant + Dexamethasone Complete Response (ITT, N=58) Complete Response (CR) (% of Patients) 100 87. 9 77. 6 Delayed: 24 -120 (Days 2 -5) Overall: 0 -120 (Days 1 -5) 80 60 40 20 0 Acute: 0 -24 (Day 1) Time (hr) Grote T et al. Proc ASCO 2004. Abstract 8262

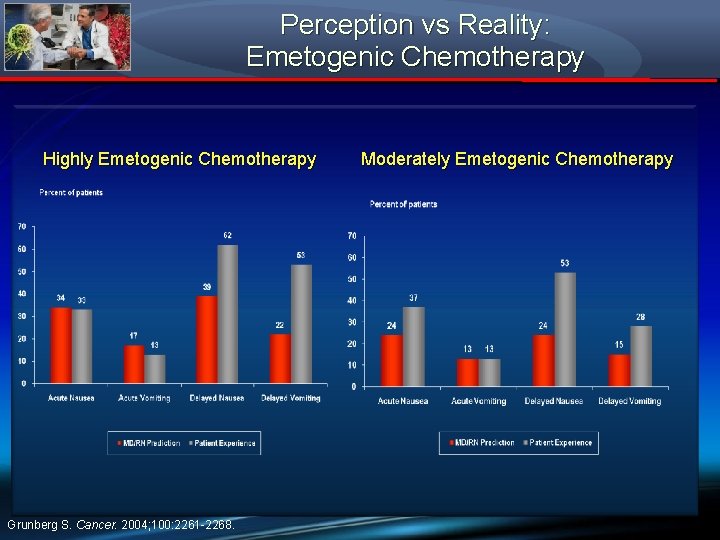
Perception vs Reality: Emetogenic Chemotherapy Highly Emetogenic Chemotherapy Grunberg S. Cancer. 2004; 100: 2261 -2268. Moderately Emetogenic Chemotherapy

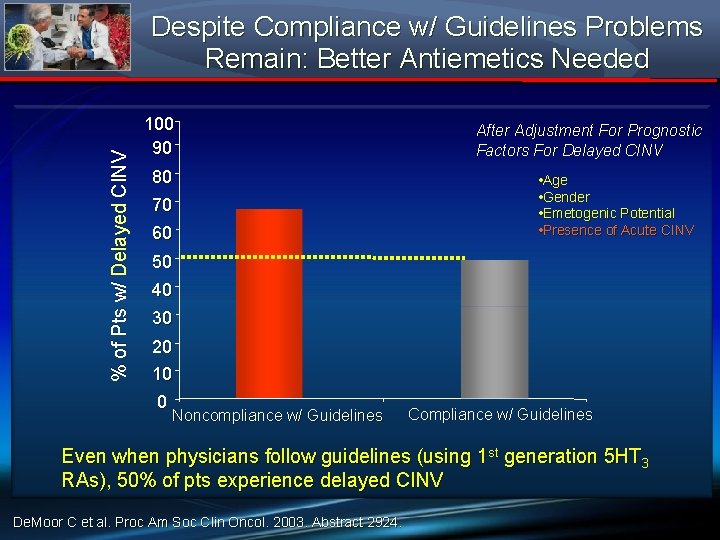
% of Pts w/ Delayed CINV Despite Compliance w/ Guidelines Problems Remain: Better Antiemetics Needed 100 90 80 70 60 After Adjustment For Prognostic Factors For Delayed CINV • Age • Gender • Emetogenic Potential • Presence of Acute CINV 50 40 30 20 10 0 Noncompliance w/ Guidelines Compliance w/ Guidelines Even when physicians follow guidelines (using 1 st generation 5 HT 3 RAs), 50% of pts experience delayed CINV De. Moor C et al. Proc Am Soc Clin Oncol. 2003. Abstract 2924.

Relationship Between Acute CINV and Delayed CINV: Questions ► Is acute CINV a strong predictive factor for delayed CINV in patients receiving moderately emetogenic chemotherapy? ► Is prevention of delayed CINV a carryover effect from prevention of acute CINV or a true pharmacologic effect during the delayed phase? ► What is the difference in the treatment effect of the first-generation 5 -HT 3 receptor antagonists vs palonosetron in preventing delayed CINV after accounting for known prognostic factors, including the carryover effect? Grunberg SM et al. Proc Am Soc Clin Oncol. 2004. Abstract 8051.

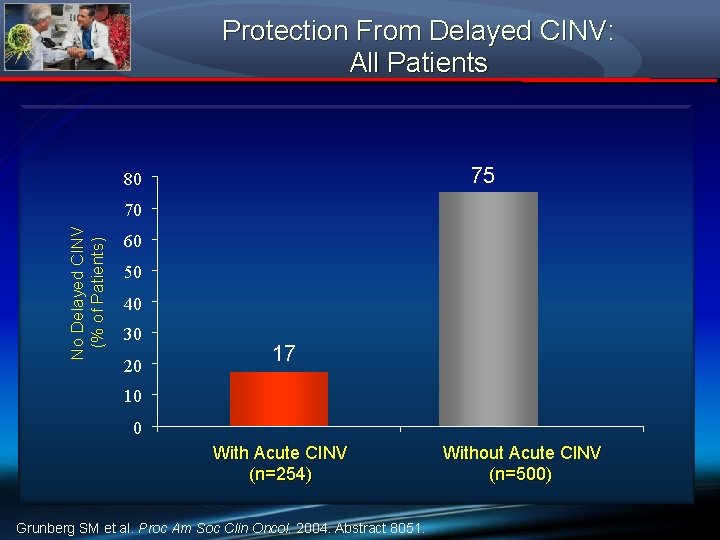
Protection From Delayed CINV: All Patients 75 80 No Delayed CINV (% of Patients) 70 60 50 40 30 20 17 10 0 With Acute CINV (n=254) Grunberg SM et al. Proc Am Soc Clin Oncol. 2004. Abstract 8051. Without Acute CINV (n=500)

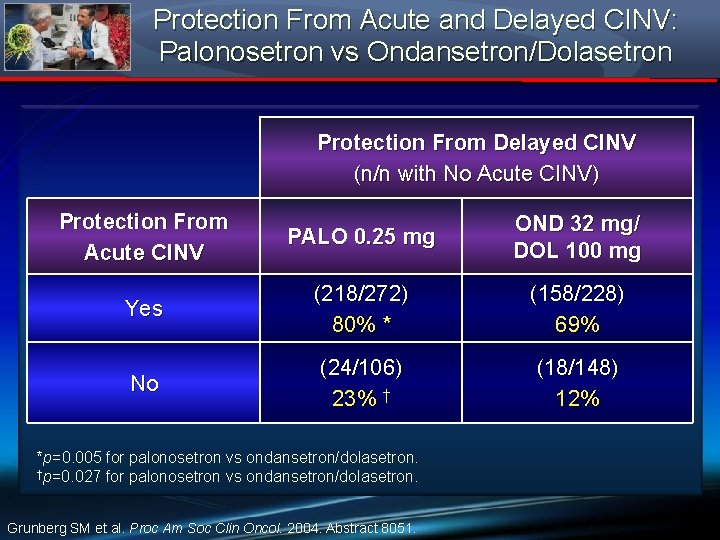
Protection From Acute and Delayed CINV: Palonosetron vs Ondansetron/Dolasetron Protection From Delayed CINV (n/n with No Acute CINV) Protection From Acute CINV PALO 0. 25 mg OND 32 mg/ DOL 100 mg Yes (218/272) 80% * (158/228) 69% No (24/106) 23% † (18/148) 12% *p=0. 005 for palonosetron vs ondansetron/dolasetron. †p=0. 027 for palonosetron vs ondansetron/dolasetron. Grunberg SM et al. Proc Am Soc Clin Oncol. 2004. Abstract 8051.

Are Oral Followup 5 -HT 3 RAs Really Effective for Delayed CINV? ► 671 pts receiving doxorubicin-based chemotherapy ● ► ► all treated w/ 1 st generation 5 HT 3 + Dex on Day 1 of CT Pts then randomized for days 2 and 3: ● Arm 1: Prochlorperazine 10 mg p. o. three times daily (q 8 h) ● Arm 2: Any oral 5 -HT 3 antiemetic, using standard dosing regimens ● Arm 3: Prochlorperazine 10 mg p. o. as needed for nausea Rescue medications for control of symptoms were allowed Hickock et al ASCO 2005 Final Results URCC-CCOP

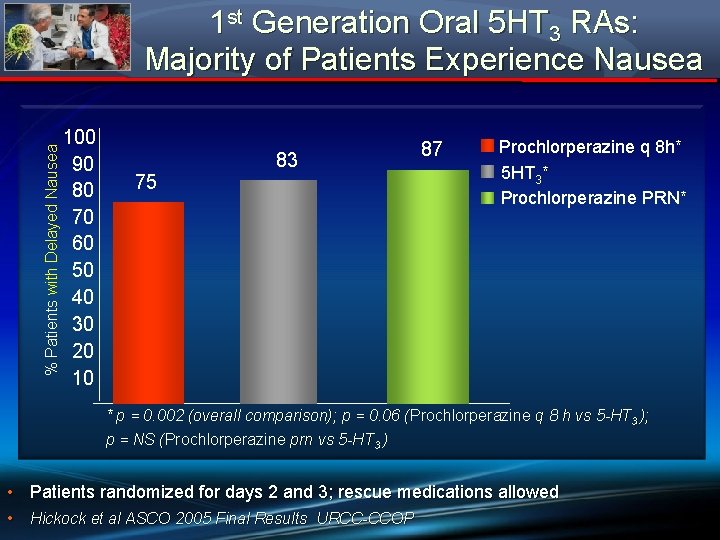
% Patients with Delayed Nausea 1 st Generation Oral 5 HT 3 RAs: Majority of Patients Experience Nausea 100 90 80 70 60 50 40 30 20 10 75 83 87 Prochlorperazine q 8 h* 5 HT 3* Prochlorperazine PRN* * p = 0. 002 (overall comparison); p = 0. 06 (Prochlorperazine q 8 h vs 5 -HT 3 ); ( p = NS (Prochlorperazine prn vs 5 -HT 3 ) ( • Patients randomized for days 2 and 3; rescue medications allowed • Hickock et al ASCO 2005 Final Results URCC-CCOP

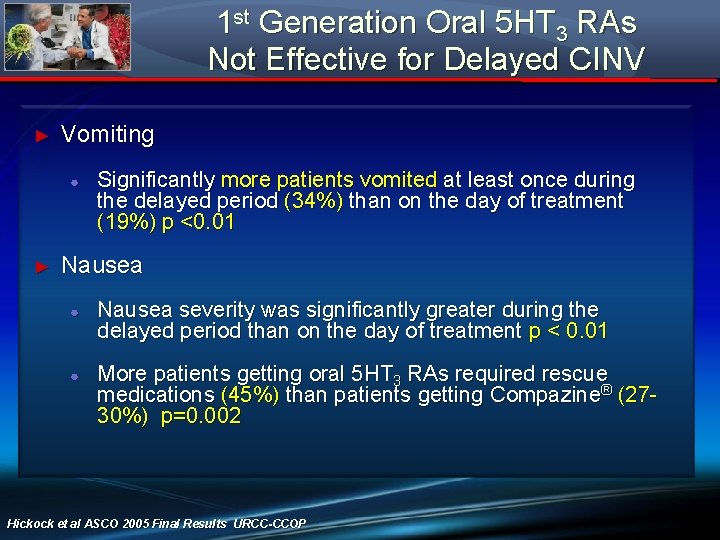
1 st Generation Oral 5 HT 3 RAs Not Effective for Delayed CINV ► Vomiting ● ► Significantly more patients vomited at least once during the delayed period (34%) than on the day of treatment (19%) p <0. 01 Nausea ● Nausea severity was significantly greater during the delayed period than on the day of treatment p < 0. 01 ● More patients getting oral 5 HT 3 RAs required rescue medications (45%) than patients getting Compazine® (2730%) p=0. 002 Hickock et al ASCO 2005 Final Results URCC-CCOP

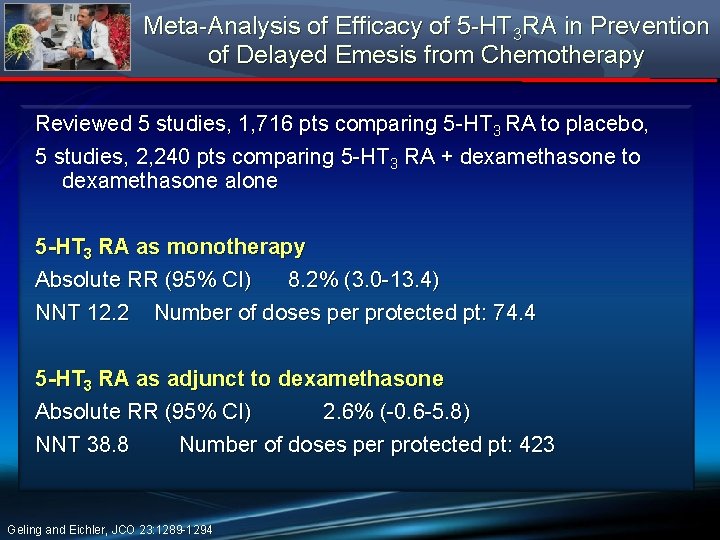
Meta-Analysis of Efficacy of 5 -HT 3 RA in Prevention of Delayed Emesis from Chemotherapy Reviewed 5 studies, 1, 716 pts comparing 5 -HT 3 RA to placebo, 5 studies, 2, 240 pts comparing 5 -HT 3 RA + dexamethasone to dexamethasone alone 5 -HT 3 RA as monotherapy Absolute RR (95% CI) 8. 2% (3. 0 -13. 4) NNT 12. 2 Number of doses per protected pt: 74. 4 5 -HT 3 RA as adjunct to dexamethasone Absolute RR (95% CI) 2. 6% (-0. 6 -5. 8) NNT 38. 8 Number of doses per protected pt: 423 Geling and Eichler, JCO 23: 1289 -1294

ASCO 2006/NCCN 2009 Recommendations by Risk Category High (>90% emetic risk) Including AC containing regimens Three-drug combination of a HT 3 serotonin receptor antagonist, (palonosetron preferred-NCCN) dexamethasone, and aprepitant Moderate (>30% to 90% emetic risk) Two-drug combination of a HT 3 serotonin receptor antagonist and dexamethasone (+/-aprepitant for selected patients) Low (10% to 30% emetic risk) Dexamethasone 8 -12 mg Minimal (<10% emetic risk No antiemetic routinely

How Can We Improve the Value of Care in CINV? Value = Quality Cost • Direct • Indirect CR Nausea or Emesis Functioning Side Effects Compliance or Patient Inconvenience Access to Care

Summary ► 1 st generation 5 HT 3 RA’s therapeutically equivalent & major advance in supportive care for control of acute emesis ► No major progress in CINV for ~ 10+ yrs until aprepitant & palonosetron ► Treatment guidelines have changed ● ● ► Degree of nausea incurred has been refined for many agents Delayed CINV recommendations are updated Prevention of CINV has improved, but challenges remain ● ● Improving detection of CINV, especially after 24 hours Educating patients and oncology healthcare givers The development and evaluation of clinically useful assessment tools Further development of regimens to treat delayed CINV
 Nausea elderly woman
Nausea elderly woman Seamus hesney
Seamus hesney Anorexia, nausea and vomiting
Anorexia, nausea and vomiting Vapor pressure intermolecular forces
Vapor pressure intermolecular forces Bsa calculation formula for chemotherapy
Bsa calculation formula for chemotherapy Icd 9 code for oral thrush
Icd 9 code for oral thrush Principles of chemotherapy
Principles of chemotherapy General principles of chemotherapy
General principles of chemotherapy Chemotherapy
Chemotherapy Debra forman
Debra forman What replaced the common assessment framework in 2014
What replaced the common assessment framework in 2014 4ac 4t chemotherapy
4ac 4t chemotherapy Nausea vomitting
Nausea vomitting Secchezza vulvare
Secchezza vulvare Menopausa precoce
Menopausa precoce Hiatal hernia weird symptoms
Hiatal hernia weird symptoms Existetialism
Existetialism Nice guidelines diarrhoea and vomiting in adults
Nice guidelines diarrhoea and vomiting in adults Besigheidsplan
Besigheidsplan Disruptive and radical innovation
Disruptive and radical innovation Hyperkalemia symptoms and signs
Hyperkalemia symptoms and signs Nursing intervention of vomiting
Nursing intervention of vomiting Vomiting centre in brain
Vomiting centre in brain Small intestine peristalsis
Small intestine peristalsis Substance p vomiting
Substance p vomiting Vomiting case presentation
Vomiting case presentation Vomiting case presentation
Vomiting case presentation Vomiting
Vomiting Vomiting types
Vomiting types Vomiting blood
Vomiting blood Xyphoidynia
Xyphoidynia Cyclic vomiting syndrome
Cyclic vomiting syndrome Abdominal pain history taking sample
Abdominal pain history taking sample Does pregnancy feel like period cramps
Does pregnancy feel like period cramps Explain the lock and key hypothesis
Explain the lock and key hypothesis Induced fit vs lock and key
Induced fit vs lock and key Scrapyard crane diagram
Scrapyard crane diagram How long to boil eggs
How long to boil eggs Ch2cl intermolecular forces
Ch2cl intermolecular forces