Nausea and Vomiting in Palliative Care Audit Presentation


























- Slides: 68

Nausea and Vomiting in Palliative Care Audit Presentation January 15 th 2015

Audit Group Members and Meetings Dr Richard Latten Dr Seamus Coyle Dr Laura Mc. Glynn Dr Paula Powell Dr Jamie Barfield Ann Griffiths Sian Rae Agnes Noble Tracey Hindley Tania Forrester Dave Hewison 27 th August 2014 10 th September 2014 1 st October 2014 27 th October 2014 12 th November 2014 3 rd December 2014 17 th December 2014 30 th December 2014 7 th January 2015 14 th January 2015

Session Overview • • • Patient, Carer and Public Involvement Literature Search and Review Existing Standards Audit Results Recommendations Questions

Patient, Carer and Public Involvement • Representative - Dave Hewison • Willowbrook Hospice Volunteer • Actively participated in several group audit meetings • No previous direct involvement with a specialist palliative care team

• Key areas to recognise from a patient, public and carer view 1. COMMUNICATION - Inform the patient of their management plan Discuss antiemetic choice and WHY Help the patient to understand WHY are they are nauseated/vomiting Assess how much information the patient would like If further information requested seek ways to direct the patient to information sources

2. EXPECTATIONS - Discuss various antiemetics may be required to achieve symptom control - Help the patient to remain confident that the healthcare professional is reviewing and managing appropriately 3. HONESTY AND PROFESSIONALISM - Open and honest approach

LITERATURE REVIEW

Question What is the evidence for the use of ANTIEMETIC ‘X’ in the management of nausea and vomiting in palliative care?

ANTIEMETICS SEARCHED • • Aprepitant Cyclizine Dexamethasone Domperidone Granisetron Haloperidol Hyoscine hydrobromide • • Hyoscine butylbromide Levomepromazine Lorazepam Metoclopramide Olanzapine Ondansetron Prochlorperazine

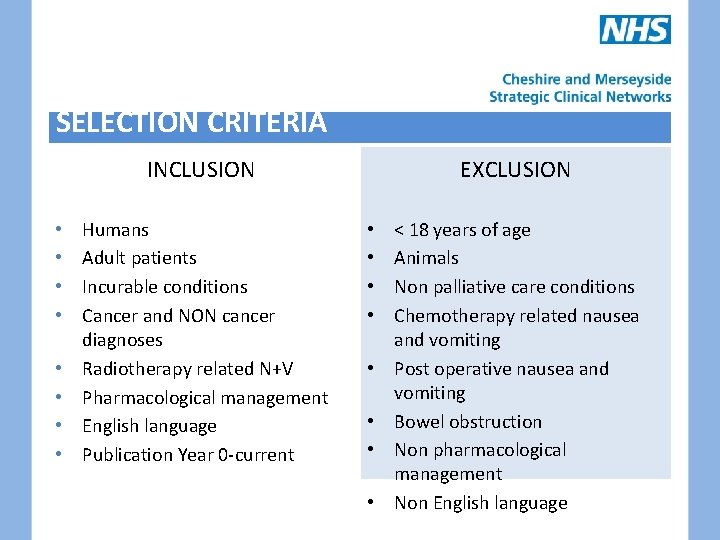
SELECTION CRITERIA EXCLUSION INCLUSION • • Humans Adult patients Incurable conditions Cancer and NON cancer diagnoses Radiotherapy related N+V Pharmacological management English language Publication Year 0 -current • • < 18 years of age Animals Non palliative care conditions Chemotherapy related nausea and vomiting Post operative nausea and vomiting Bowel obstruction Non pharmacological management Non English language

METHOD • Search via NHS Evidence search • MEDLINE/PUBMED/EMBASE/COCHRANE databases • Search terms: “Nausea” OR “Vomiting” AND “Antiemetic” NOT “chemotherapy” • 2 reviewers for each antiemetic searched

• Filters: “Clinical Trials” “Evidence base” “Cochrane” “Humans” “Evidence based medicine” • If zero articles from standard search some filters not selected and 2 nd search • Abstract selection…full text reviewed…. 2 reviewers

LITERATURE SEARCH RESULTS

Sian Rae; Clinical Pharmacist, Whiston Hospital & Willowbrook Hospice Aprepitant

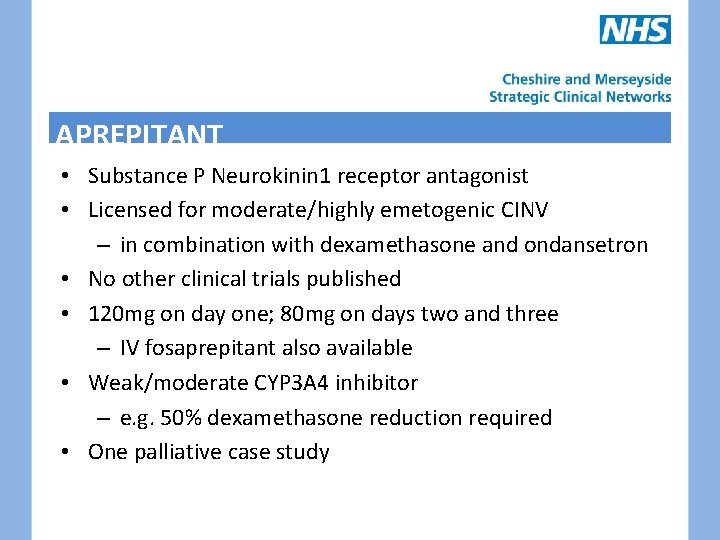
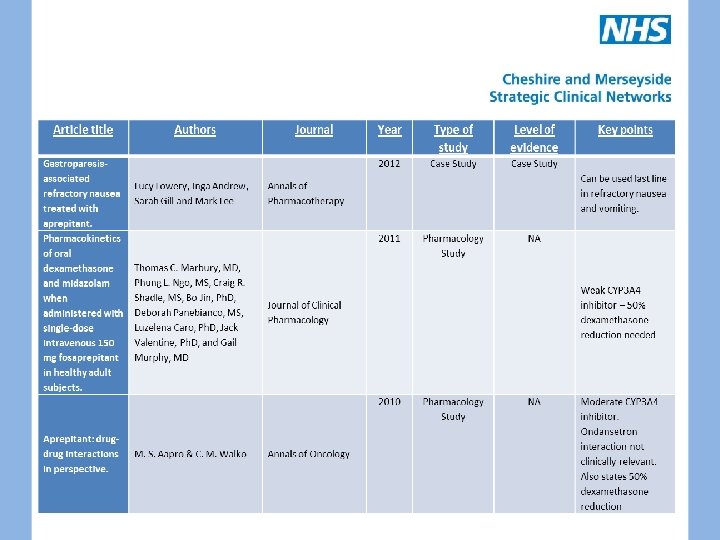
APREPITANT • Substance P Neurokinin 1 receptor antagonist • Licensed for moderate/highly emetogenic CINV – in combination with dexamethasone and ondansetron • No other clinical trials published • 120 mg on day one; 80 mg on days two and three – IV fosaprepitant also available • Weak/moderate CYP 3 A 4 inhibitor – e. g. 50% dexamethasone reduction required • One palliative case study


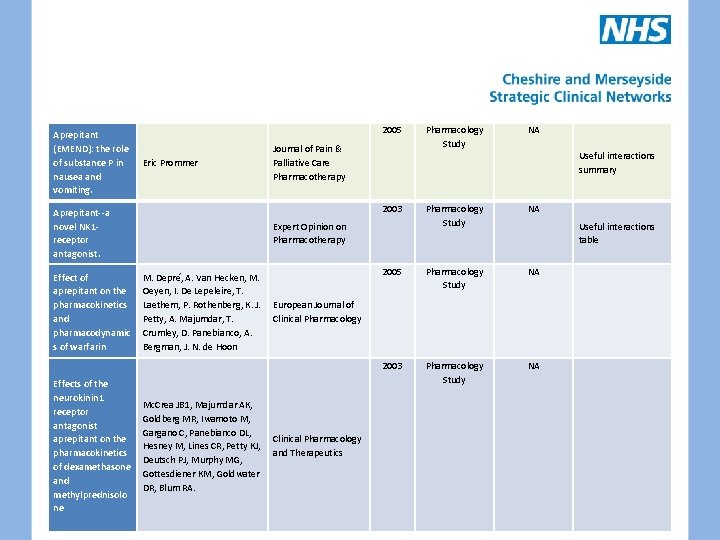
Aprepitant (EMEND): the role of substance P in nausea and vomiting. 2005 Eric Prommer Journal of Pain & Palliative Care Pharmacotherapy 2003 Aprepitant--a novel NK 1 receptor antagonist. Effect of aprepitant on the pharmacokinetics and pharmacodynamic s of warfarin M. Depré, A. Van Hecken, M. Oeyen, I. De Lepeleire, T. Laethem, P. Rothenberg, K. J. European Journal of Petty, A. Majumdar, T. Clinical Pharmacology Crumley, D. Panebianco, A. Bergman, J. N. de Hoon Expert Opinion on Pharmacotherapy 2005 Mc. Crea JB 1, Majumdar AK, Goldberg MR, Iwamoto M, Gargano C, Panebianco DL, Clinical Pharmacology Hesney M, Lines CR, Petty KJ, and Therapeutics Deutsch PJ, Murphy MG, Gottesdiener KM, Goldwater DR, Blum RA. NA Pharmacology Study NA Useful interactions summary Useful interactions table 2003 Effects of the neurokinin 1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolo ne Pharmacology Study NA

Tania Forrester; Community Palliative Care Nurse Specialist; WLS&F Cyclizine

Cyclizine • Well established antihistamine • Exact mechanism unknown – reduces lower oesophageal sphincter tone and labyrinthine apparatus sensitivity • 50 mg TDS oral, IV, SC • No relevant clinical trials • Can cause hallucinations and enhance euphoric effects of opioids • Limited evidence in relation to use in heart failure

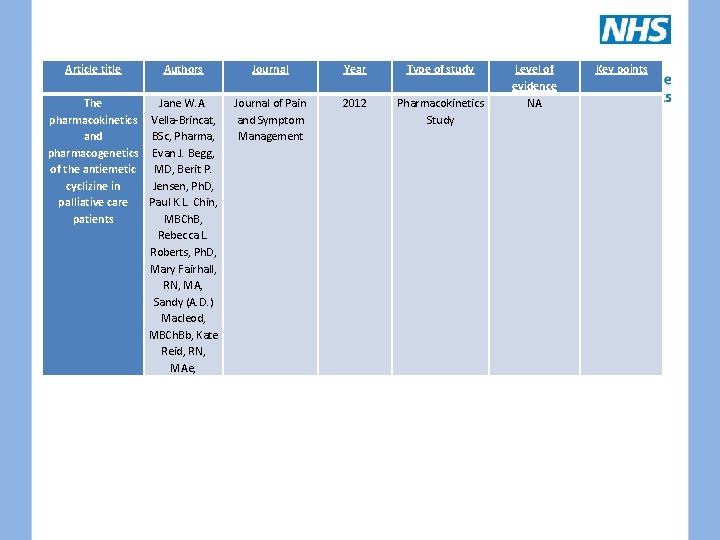
Article title Authors Journal Year Type of study The pharmacokinetics and pharmacogenetics of the antiemetic cyclizine in palliative care patients Jane W. A. Vella-Brincat, BSc, Pharma, Evan J. Begg, MD, Berit P. Jensen, Ph. D, Paul K. L. Chin, MBCh. B, Rebecca L. Roberts, Ph. D, Mary Fairhall, RN, MA, Sandy (A. D. ) Macleod, MBCh. Bb, Kate Reid, RN, MAe, Journal of Pain and Symptom Management 2012 Pharmacokinetics Study Level of evidence NA Key points

Dr Laura Mc. Glynn; Palliative Medicine ST 4; Aintree Hospital Metoclopramide

METOCLOPRAMIDE Prokinetic anti emetic D 2 antagonist and 5 HT 4 agonist Triggers a cholinergic system in the wall of the GI tract Block the dopamine break on gastric emptying induced by stress, anxiety and nausea • Useful in N+V caused by - GI disorders - Chemotherapy and Radiotherapy - Dysmotility dyspepsia, Heartburn, Migraine, Hiccups MHRA guidance • •

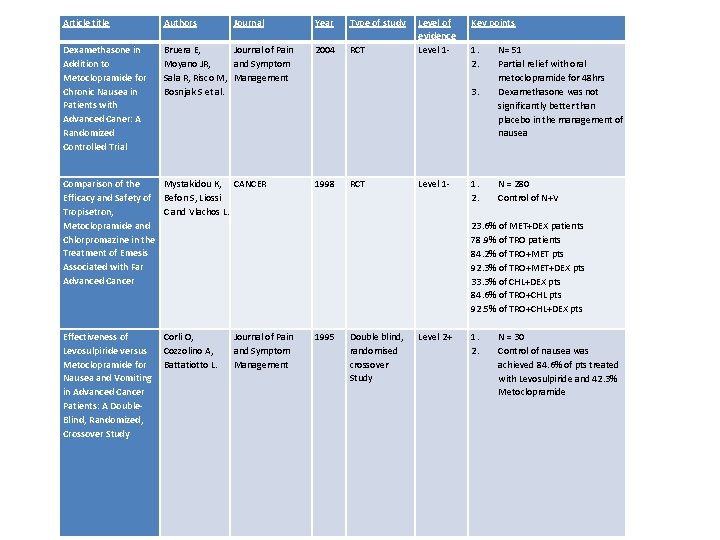
Article title Authors Journal Dexamethasone in Addition to Metoclopramide for Chronic Nausea in Patients with Advanced Caner: A Randomized Controlled Trial Bruera E, Journal of Pain Moyano JR, and Symptom Sala R, Risco M, Management Bosnjak S et al. Year Type of study 2004 RCT Level of evidence Level 1 - Key points 1. 2. 3. Comparison of the Mystakidou K, CANCER Efficacy and Safety of Befon S, Liossi Tropisetron, C and Vlachos L. Metoclopramide and Chlorpromazine in the Treatment of Emesis Associated with Far Advanced Cancer 1998 RCT Level 1 - Effectiveness of Levosulpiride versus Metoclopramide for Nausea and Vomiting in Advanced Cancer Patients: A Double. Blind, Randomized, Crossover Study Double blind, randomised crossover Study Level 2+ Corli O, Cozzolino A, Battatiotto L. Journal of Pain and Symptom Management 1995 N= 51 Partial relief with oral metoclopramide for 48 hrs Dexamethasone was not significantly better than placebo in the management of nausea 1. N = 280 2. Control of N+V 23. 6% of MET+DEX patients 78. 9% of TRO patients 84. 2% of TRO+MET pts 92. 3% of TRO+MET+DEX pts 33. 3% of CHL+DEX pts 84. 6% of TRO+CHL pts 92. 5% of TRO+CHL+DEX pts 1. N = 30 2. Control of nausea was achieved 84. 6% of pts treated with Levosulpiride and 42. 3% Metoclopramide

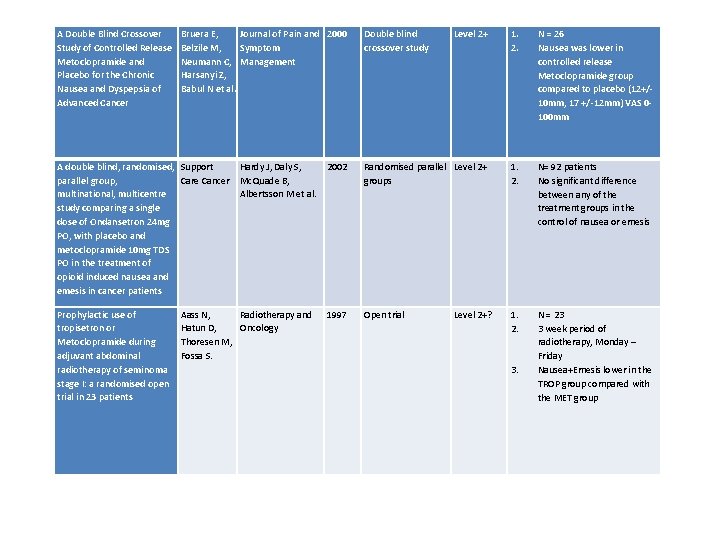
A Double Blind Crossover Study of Controlled Release Metoclopramide and Placebo for the Chronic Nausea and Dyspepsia of Advanced Cancer Bruera E, Journal of Pain and 2000 Belzile M, Symptom Neumann C, Management Harsanyi Z, Babul N et al. Double blind crossover study Level 2+ 1. 2. N = 26 Nausea was lower in controlled release Metoclopramide group compared to placebo (12+/- 10 mm, 17 +/-12 mm) VAS 0100 mm 1. 2. N= 92 patients No significant difference between any of the treatment groups in the control of nausea or emesis A double blind, randomised, Support parallel group, Care Cancer multinational, multicentre study comparing a single dose of Ondansetron 24 mg PO, with placebo and metoclopramide 10 mg TDS PO in the treatment of opioid induced nausea and emesis in cancer patients Prophylactic use of tropisetron or Metoclopramide during adjuvant abdominal radiotherapy of seminoma stage I: a randomised open trial in 23 patients Hardy J, Daly S, 2002 Mc. Quade B, Albertsson M et al. Aass N, Radiotherapy and Hatun D, Oncology Thoresen M, Fossa S. Randomised parallel Level 2+ groups 1997 Open trial Level 2+? 1. 2. 3. N = 23 3 week period of radiotherapy, Monday – Friday Nausea+Emesis lower in the TROP group compared with the MET group

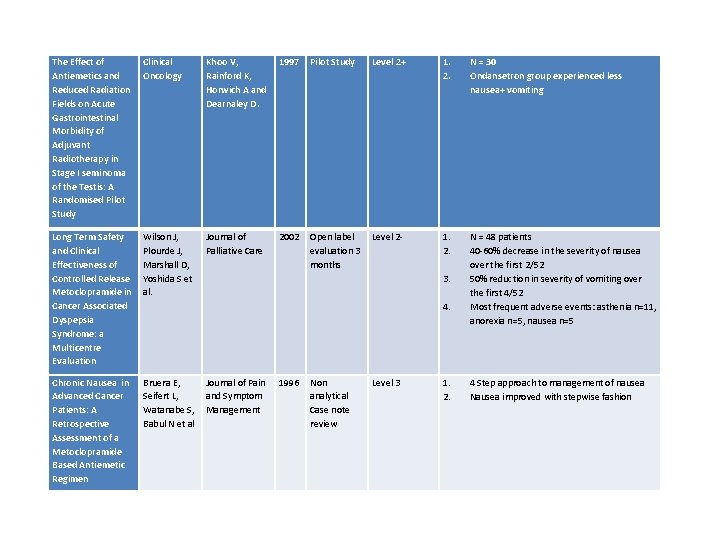
The Effect of Antiemetics and Reduced Radiation Fields on Acute Gastrointestinal Morbidity of Adjuvant Radiotherapy in Stage I seminoma of the Testis: A Randomised Pilot Study Clinical Oncology Khoo V, Rainford K, Horwich A and Dearnaley D. 1997 Long Term Safety and Clinical Effectiveness of Controlled Release Metoclopramide in Cancer Associated Dyspepsia Syndrome: a Multicentre Evaluation Wilson J, Plourde J, Marshall D, Yoshida S et al. Journal of Palliative Care 2002 Chronic Nausea in Advanced Cancer Patients: A Retrospective Assessment of a Metoclopramide Based Antiemetic Regimen Bruera E, Journal of Pain Seifert L, and Symptom Watanabe S, Management Babul N et al Pilot Study Level 2+ 1. 2. N = 30 Ondansetron group experienced less nausea+ vomiting Open label Level 2 evaluation 3 months 1. 2. N = 48 patients 40 -60% decrease in the severity of nausea over the first 2/52 50% reduction in severity of vomiting over the first 4/52 Most frequent adverse events: asthenia n=11, anorexia n=5, nausea n=5 3. 4. 1996 Non analytical Case note review Level 3 1. 2. 4 Step approach to management of nausea Nausea improved with stepwise fashion

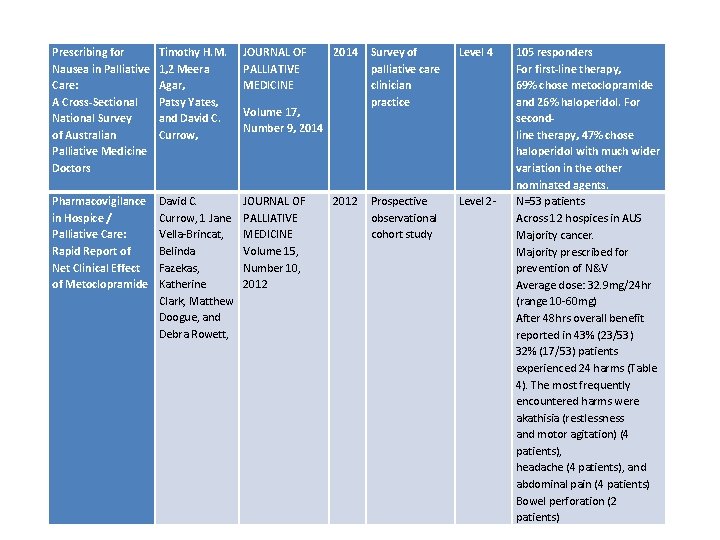
Prescribing for Nausea in Palliative Care: A Cross-Sectional National Survey of Australian Palliative Medicine Doctors Timothy H. M. 1, 2 Meera Agar, Patsy Yates, and David C. Currow, JOURNAL OF PALLIATIVE MEDICINE Pharmacovigilance in Hospice / Palliative Care: Rapid Report of Net Clinical Effect of Metoclopramide David C. Currow, 1 Jane Vella-Brincat, Belinda Fazekas, Katherine Clark, Matthew Doogue, and Debra Rowett, JOURNAL OF PALLIATIVE MEDICINE Volume 15, Number 10, 2012 2014 Survey of palliative care clinician practice Level 4 2012 Prospective observational cohort study Level 2 - Volume 17, Number 9, 2014 105 responders For first-line therapy, 69% chose metoclopramide and 26% haloperidol. For secondline therapy, 47% chose haloperidol with much wider variation in the other nominated agents. N=53 patients Across 12 hospices in AUS Majority cancer. Majority prescribed for prevention of N&V Average dose: 32. 9 mg/24 hr (range 10 -60 mg) After 48 hrs overall benefit reported in 43% (23/53) 32% (17/53) patients experienced 24 harms (Table 4). The most frequently encountered harms were akathisia (restlessness and motor agitation) (4 patients), headache (4 patients), and abdominal pain (4 patients) Bowel perforation (2 patients)

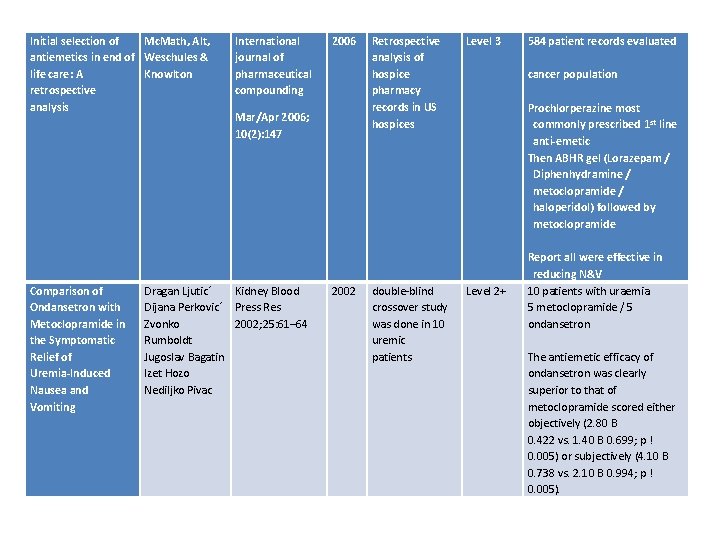
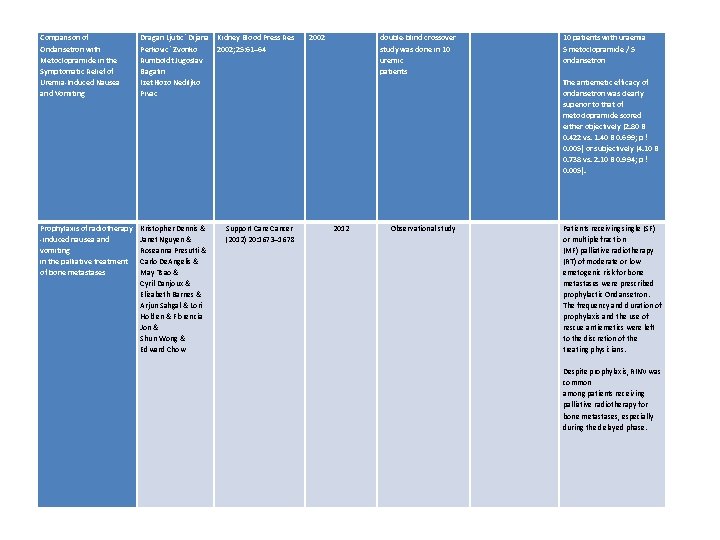
Initial selection of Mc. Math, Alt, antiemetics in end of Weschules & life care: A Knowlton retrospective analysis Comparison of Ondansetron with Metoclopramide in the Symptomatic Relief of Uremia-Induced Nausea and Vomiting International journal of pharmaceutical compounding 2006 Retrospective analysis of hospice pharmacy records in US hospices Level 3 2002 double-blind crossover study was done in 10 uremic patients Level 2+ Mar/Apr 2006; 10(2): 147 Dragan Ljutic´ Kidney Blood Dijana Perkovic´ Press Res Zvonko 2002; 25: 61– 64 Rumboldt Jugoslav Bagatin Izet Hozo Nediljko Pivac 584 patient records evaluated cancer population Prochlorperazine most commonly prescribed 1 st line anti-emetic Then ABHR gel (Lorazepam / Diphenhydramine / metoclopramide / haloperidol) followed by metoclopramide Report all were effective in reducing N&V 10 patients with uraemia 5 metoclopramide / 5 ondansetron The antiemetic efficacy of ondansetron was clearly superior to that of metoclopramide scored either objectively (2. 80 B 0. 422 vs. 1. 40 B 0. 699; p ! 0. 005) or subjectively (4. 10 B 0. 738 vs. 2. 10 B 0. 994; p ! 0. 005).

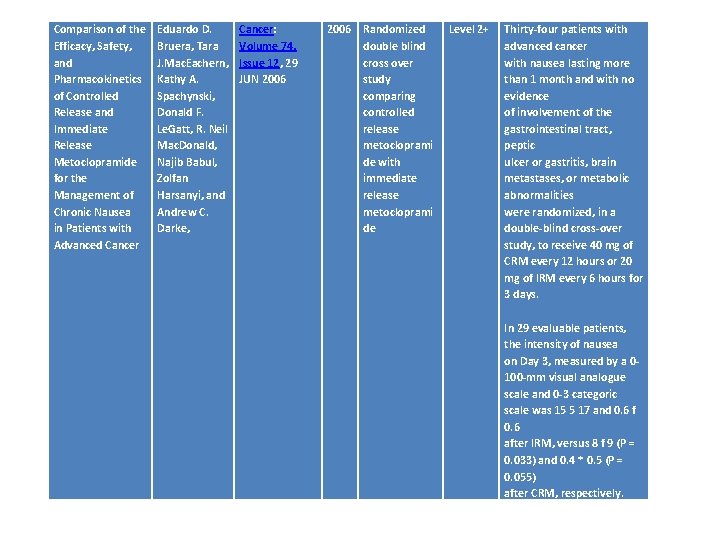
Comparison of the Efficacy, Safety, and Pharmacokinetics of Controlled Release and Immediate Release Metoclopramide for the Management of Chronic Nausea in Patients with Advanced Cancer Eduardo D. Bruera, Tara J. Mac. Eachern, Kathy A. Spachynski, Donald F. Le. Gatt, R. Neil Mac. Donald, Najib Babul, Zolfan Harsanyi, and Andrew C. Darke, Cancer: Volume 74, Issue 12, 29 JUN 2006 Randomized double blind cross over study comparing controlled release metocloprami de with immediate release metocloprami de Level 2+ Thirty-four patients with advanced cancer with nausea lasting more than 1 month and with no evidence of involvement of the gastrointestinal tract, peptic ulcer or gastritis, brain metastases, or metabolic abnormalities were randomized, in a double-blind cross-over study, to receive 40 mg of CRM every 12 hours or 20 mg of IRM every 6 hours for 3 days. In 29 evaluable patients, the intensity of nausea on Day 3, measured by a 0100 -mm visual analogue scale and 0 -3 categoric scale was 15 5 17 and 0. 6 f 0. 6 after IRM, versus 8 f 9 (P = 0. 033) and 0. 4 * 0. 5 (P = 0. 055) after CRM, respectively.

Dr Richard Latten Hyoscine Hydrobromide

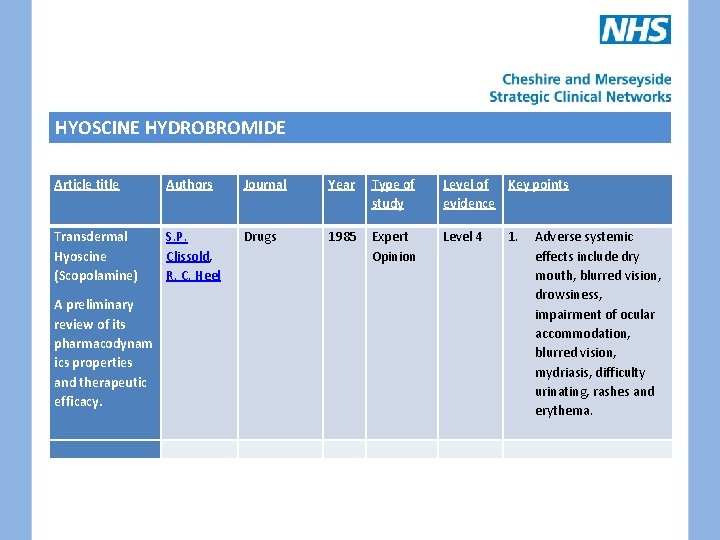
HYOSCINE HYDROBROMIDE • Antimuscarinic drug • Smooth muscle relaxant and anti secretory properties • Indicated for prevention of motion sickness, drying secretions, smooth muscle spasm and paraneoplastic pyrexia and sweating • Drug available as oral, subcut and transdermal preparations • No evidence from literature search regarding use in nausea and vomiting

HYOSCINE HYDROBROMIDE Article title Authors Journal Year Type of study Level of Key points evidence Transdermal Hyoscine (Scopolamine) S. P. Clissold, R. C. Heel Drugs 1985 Expert Opinion Level 4 1. A preliminary review of its pharmacodynam ics properties and therapeutic efficacy. Adverse systemic effects include dry mouth, blurred vision, drowsiness, impairment of ocular accommodation, blurred vision, mydriasis, difficulty urinating, rashes and erythema.

Dr Richard Latten; Consultant in Palliative Medicine; Marie Curie Ondansetron

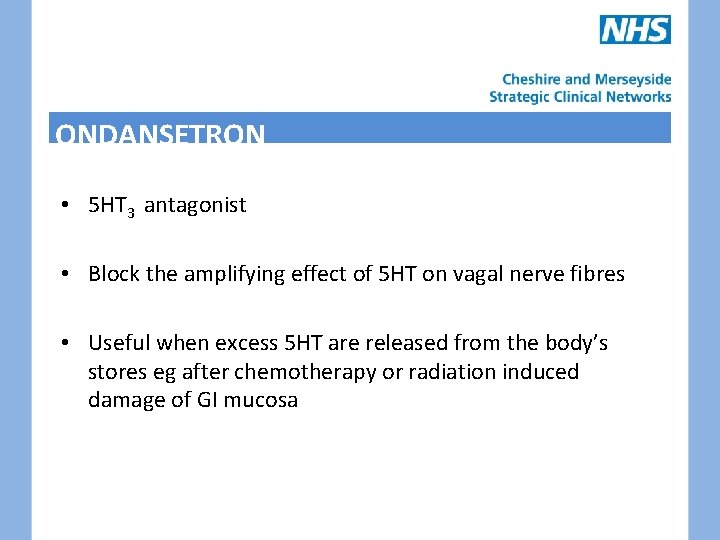
ONDANSETRON • 5 HT 3 antagonist • Block the amplifying effect of 5 HT on vagal nerve fibres • Useful when excess 5 HT are released from the body’s stores eg after chemotherapy or radiation induced damage of GI mucosa

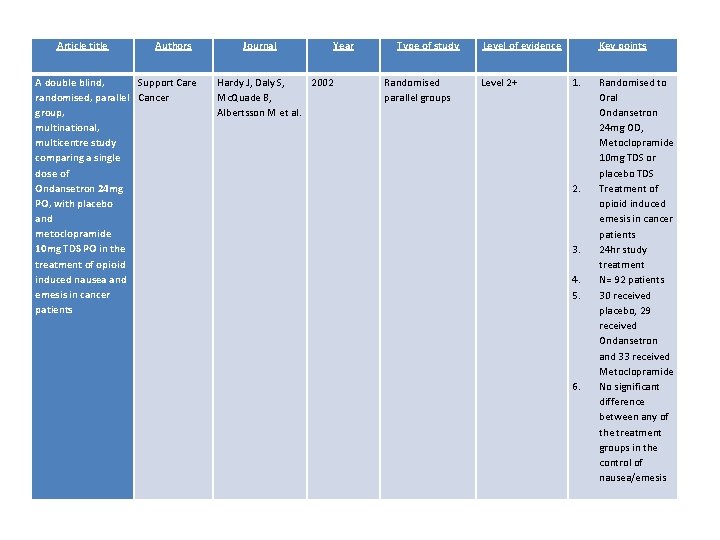
Article title Authors A double blind, Support Care randomised, parallel Cancer group, multinational, multicentre study comparing a single dose of Ondansetron 24 mg PO, with placebo and metoclopramide 10 mg TDS PO in the treatment of opioid induced nausea and emesis in cancer patients Journal Year Hardy J, Daly S, 2002 Mc. Quade B, Albertsson M et al. Type of study Randomised parallel groups Level of evidence Level 2+ Key points 1. Randomised to Oral Ondansetron 24 mg OD, Metoclopramide 10 mg TDS or placebo TDS Treatment of opioid induced emesis in cancer patients 24 hr study treatment N= 92 patients 30 received placebo, 29 received Ondansetron and 33 received Metoclopramide No significant difference between any of the treatment groups in the control of nausea/emesis 2. 3. 4. 5. 6.

Comparison of Ondansetron with Metoclopramide in the Symptomatic Relief of Uremia-Induced Nausea and Vomiting Dragan Ljutic´ Dijana Kidney Blood Press Res Perkovic´ Zvonko 2002; 25: 61– 64 Rumboldt Jugoslav Bagatin Izet Hozo Nediljko Pivac Prophylaxis of radiotherapy -induced nausea and vomiting in the palliative treatment of bone metastases Kristopher Dennis & Janet Nguyen & Roseanna Presutti & Carlo De. Angelis & May Tsao & Cyril Danjoux & Elizabeth Barnes & Arjun Sahgal & Lori Holden & Florencia Jon & Shun Wong & Edward Chow Support Care Cancer (2012) 20: 1673– 1678 2002 double-blind crossover study was done in 10 uremic patients 2012 Observational study 10 patients with uraemia 5 metoclopramide / 5 ondansetron The antiemetic efficacy of ondansetron was clearly superior to that of metoclopramide scored either objectively (2. 80 B 0. 422 vs. 1. 40 B 0. 699; p ! 0. 005) or subjectively (4. 10 B 0. 738 vs. 2. 10 B 0. 994; p ! 0. 005). Patients receiving single (SF) or multiple fraction (MF) palliative radiotherapy (RT) of moderate or low emetogenic risk for bone metastases were prescribed prophylactic Ondansetron. The frequency and duration of prophylaxis and the use of rescue antiemetics were left to the discretion of the treating physicians. Despite prophylaxis, RINV was common among patients receiving palliative radiotherapy for bone metastases, especially during the delayed phase.

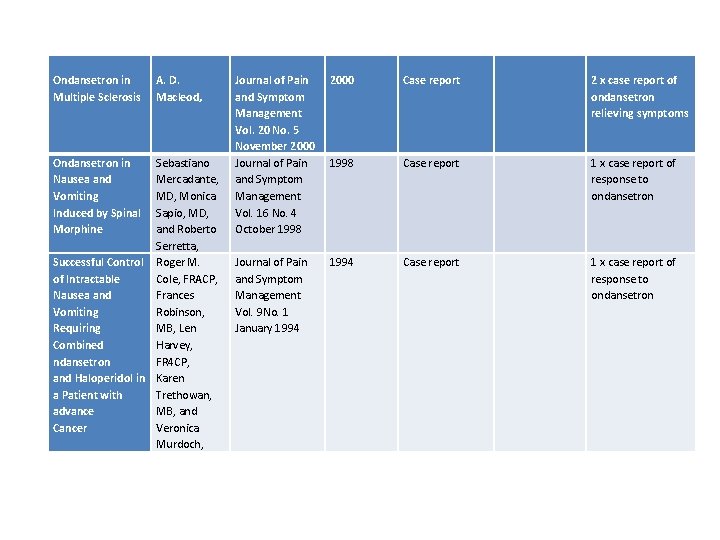
Ondansetron in Multiple Sclerosis Ondansetron in Nausea and Vomiting Induced by Spinal Morphine A. D. Macleod, Sebastiano Mercadante, MD, Monica Sapio, MD, and Roberto Serretta, Successful Control Roger M. of Intractable Cole, FRACP, Nausea and Frances Vomiting Robinson, Requiring MB, Len Combined Harvey, ndansetron FR 4 CP, and Haloperidol in Karen a Patient with Trethowan, advance MB, and Cancer Veronica Murdoch, Journal of Pain and Symptom Management Vol. 20 No. 5 November 2000 Journal of Pain and Symptom Management Vol. 16 No. 4 October 1998 2000 Case report 2 x case report of ondansetron relieving symptoms 1998 Case report 1 x case report of response to ondansetron Journal of Pain and Symptom Management Vol. 9 No. 1 January 1994 Case report 1 x case report of response to ondansetron

Agnes Noble; Palliative Care CNS; Clatterbridge Cancer Centre Dexamethasone

Dexamethasone Corticosteroid Mechanism of action unclear Dose 8 mg-16 mg Extensive side-effect profile ‘Add-on’ anti-emetic when all else fails 2 Randomised controlled trials

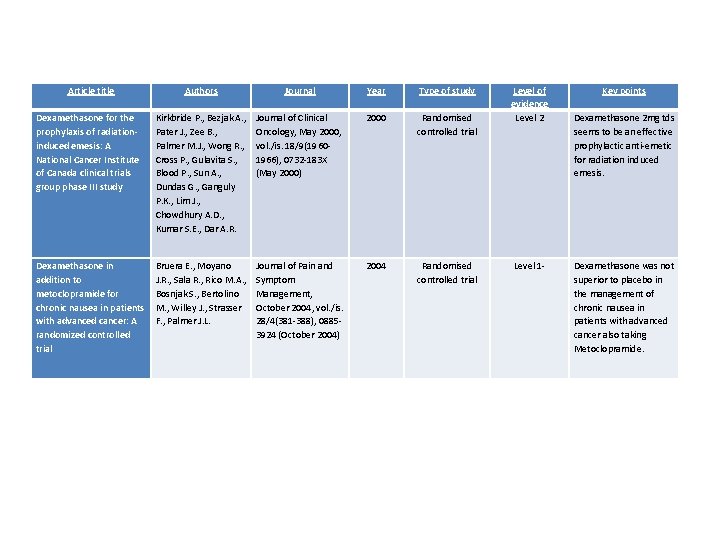
Article title Authors Journal Year Type of study Dexamethasone for the prophylaxis of radiationinduced emesis: A National Cancer Institute of Canada clinical trials group phase III study Kirkbride P. , Bezjak A. , Pater J. , Zee B. , Palmer M. J. , Wong R. , Cross P. , Gulavita S. , Blood P. , Sun A. , Dundas G. , Ganguly P. K. , Lim J. , Chowdhury A. D. , Kumar S. E. , Dar A. R. Journal of Clinical Oncology, May 2000, vol. /is. 18/9(19601966), 0732 -183 X (May 2000) 2000 Randomised controlled trial 2004 Randomised controlled trial Dexamethasone in Bruera E. , Moyano addition to J. R. , Sala R. , Rico M. A. , metoclopramide for Bosnjak S. , Bertolino chronic nausea in patients M. , Willey J. , Strasser with advanced cancer: A F. , Palmer J. L. randomized controlled trial Level of evidence Level 2 Key points Dexamethasone 2 mg tds seems to be an effective prophylactic anti-emetic for radiation induced emesis. Journal of Pain and Symptom Management, October 2004, vol. /is. 28/4(381 -388), 08853924 (October 2004) Level 1 - Dexamethasone was not superior to placebo in the management of chronic nausea in patients with advanced cancer also taking Metoclopramide.

Agnes Noble; Palliative Care CNS; Clatterbridge Cancer Centre Haloperidol

Haloperidol Antipsychotic D 2 antagonist Precise mechanism of action unknown Extrapyramidal side-effects Dose: 1. 5 mg-5 mg orally; 1. 5 mg-3 mg SC (PCF/BNF dose) 4 studies

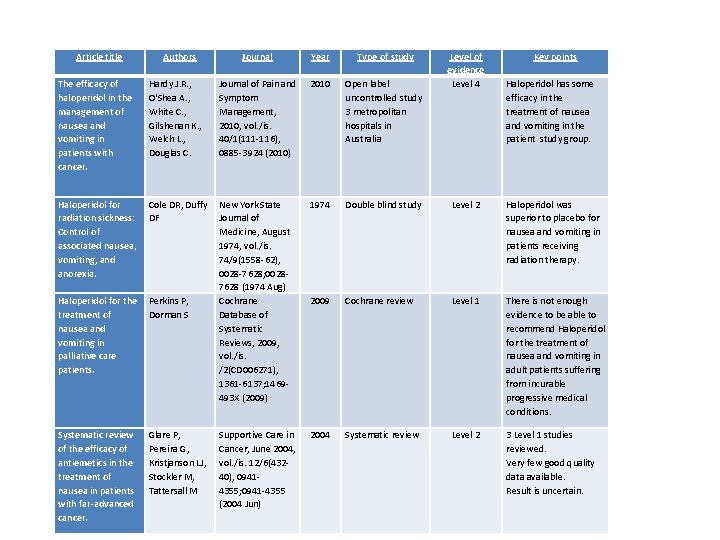
Article title The efficacy of haloperidol in the management of nausea and vomiting in patients with cancer. Authors Hardy J. R. , O'Shea A. , White C. , Gilshenan K. , Welch L. , Douglas C. Journal Year Type of study Journal of Pain and Symptom Management, 2010, vol. /is. 40/1(111 -116), 0885 -3924 (2010) 2010 Open label uncontrolled study 3 metropolitan hospitals in Australia 1974 Double blind study Level 2 Haloperidol was superior to placebo for nausea and vomiting in patients receiving radiation therapy. 2009 Cochrane review Level 1 There is not enough evidence to be able to recommend Haloperidol for the treatment of nausea and vomiting in adult patients suffering from incurable progressive medical conditions. 2004 Systematic review Level 2 3 Level 1 studies reviewed. Very few good quality data available. Result is uncertain. Cole DR, Duffy New York State DF Journal of Medicine, August 1974, vol. /is. 74/9(1558 -62), 0028 -7628; 00287628 (1974 Aug) Haloperidol for the Perkins P, Cochrane treatment of Dorman S Database of nausea and Systematic vomiting in Reviews, 2009, palliative care vol. /is. patients. /2(CD 006271), 1361 -6137; 1469 493 X (2009) Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M Key points Haloperidol has some efficacy in the treatment of nausea and vomiting in the patient study group. Haloperidol for radiation sickness: Control of associated nausea, vomiting, and anorexia. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Level of evidence Level 4 Supportive Care in Cancer, June 2004, vol. /is. 12/6(43240), 09414355; 0941 -4355 (2004 Jun)

Agnes Noble; Palliative Care CNS; Clatterbridge Cancer Centre Domperidone

Domperidone D 2 receptor antagonist Acts on gastro-oesophageal and gastro-duodenal junctions – Pro-kinetic Acts on dopamine receptors in CTZ Less likely to cause central effects Increased risk of serious cardiac side-effects: MHRA guidance No relevant evidence within audit criteria

Agnes Noble; Palliative Care CNS; Clatterbridge Cancer Centre Hyoscine Butylbromide

Hyoscine Butlybromide Antimuscarinic Doesn’t cross blood-brain barrier – No central anti-emetic effects No drowsiness No relevant evidence within audit criteria

Dr Jamie Barfield; Palliative Medicine ST 3; Aintree Hospital Granisetron

Granisetron • A serotonin 5 -HT 3 receptor antagonist • Main effect is to reduce the activity of the vagus nerve, which activates the vomiting centre in the medulla oblongata • metabolized slowly by the liver, giving it a longer than average half-life. One doses usually lasts 4 to 9 hours and is usually administered once or twice daily • Excreted by the liver and kidneys

Granisetron • 3 open label trials, 2 RCT, 1 review • Good control of radiation induced nausea with between 3 -6 mg IV over 24 hours or 2 mg PO, • Also used in bowel obstruction at 3 mg IV daily with dexamethasone

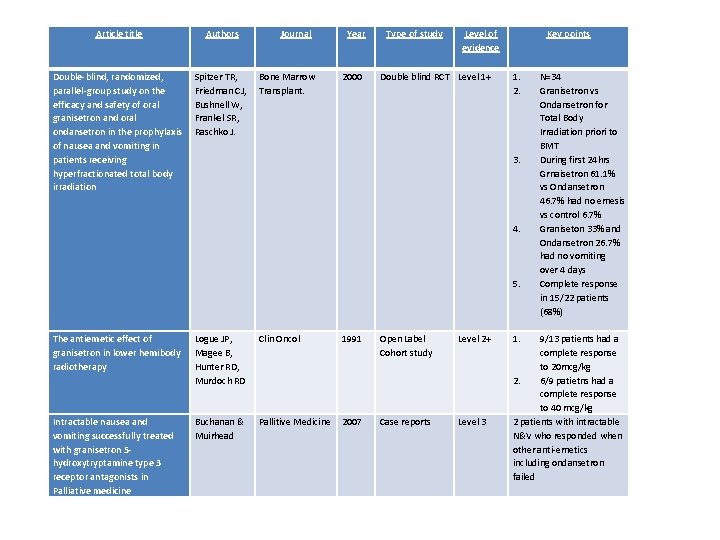
Article title Authors Double-blind, randomized, parallel-group study on the efficacy and safety of oral granisetron and oral ondansetron in the prophylaxis of nausea and vomiting in patients receiving hyperfractionated total body irradiation Spitzer TR, Friedman CJ, Bushnell W, Frankel SR, Raschko J. Journal Bone Marrow Transplant. Year 2000 Type of study Level of evidence Double blind RCT Level 1+ Key points 1. 2. N=34 Granisetron vs Ondansetron for Total Body Irradiation priori to BMT During first 24 hrs Grnaisetron 61. 1% vs Ondansetron 46. 7% had no emesis vs control 6. 7% Graniseton 33% and Ondansetron 26. 7% had no vomiting over 4 days Complete response in 15/22 patients (68%) 3. 4. 5. The antiemetic effect of granisetron in lower hemibody radiotherapy Logue JP, Magee B, Hunter RD, Murdoch RD Clin Oncol 1991 Open Label Cohort study Level 2+ Intractable nausea and vomiting successfully treated with granisetron 5 hydroxytryptamine type 3 receptor antagonists in Palliative medicine Buchanan & Muirhead Pallitive Medicine 2007 Case reports Level 3 1. 9/13 patients had a complete response to 20 mcg/kg 2. 6/9 patietns had a complete response to 40 mcg/kg 2 patients with intractable N&V who responded when other anti-emetics including ondansetron failed

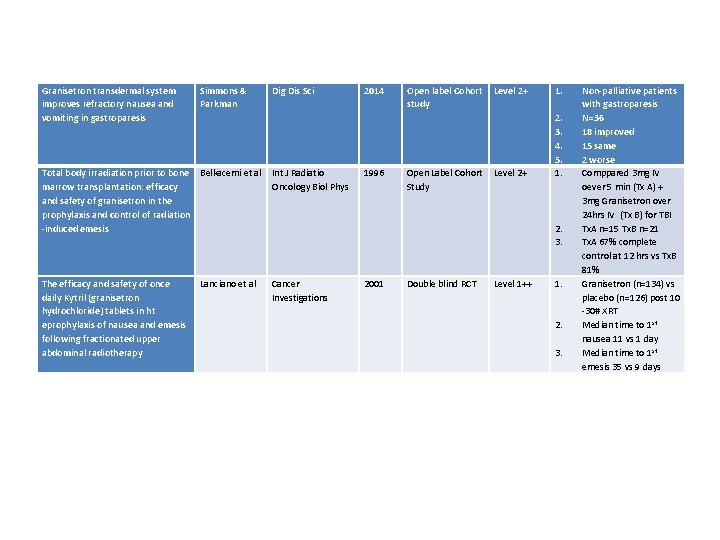
Granisetron transdermal system improves refractory nausea and vomiting in gastroparesis Simmons & Parkman Dig Dis Sci Total body irradiation prior to bone Belkacemi et al marrow transplantation: efficacy and safety of granisetron in the prophylaxis and control of radiation -induced emesis Int J Radiatio Oncology Biol Phys The efficacy and safety of once daily Kytril (granisetron hydrochloride) tablets in ht eprophylaxis of nausea and emesis following fractionated upper abdominal radiotherapy Cancer Investigations Lanciano et al 2014 1996 Open label Cohort Level 2+ study Open Label Cohort Level 2+ Study 1. 2. 3. 4. 5. 1. 2. 3. 2001 Double blind RCT Level 1++ 1. 2. 3. Non-palliative patients with gastroparesis N=36 18 improved 15 same 2 worse Comppared 3 mg IV oever 5 min (Tx A) + 3 mg Granisetron over 24 hrs IV (Tx B) for TBI Tx. A n=15 Tx. B n=21 Tx. A 67% complete control at 12 hrs vs Tx. B 81% Granisetron (n=134) vs placebo (n=126) post 10 -30# XRT Median time to 1 st nausea 11 vs 1 day Median time to 1 st emesis 35 vs 9 days

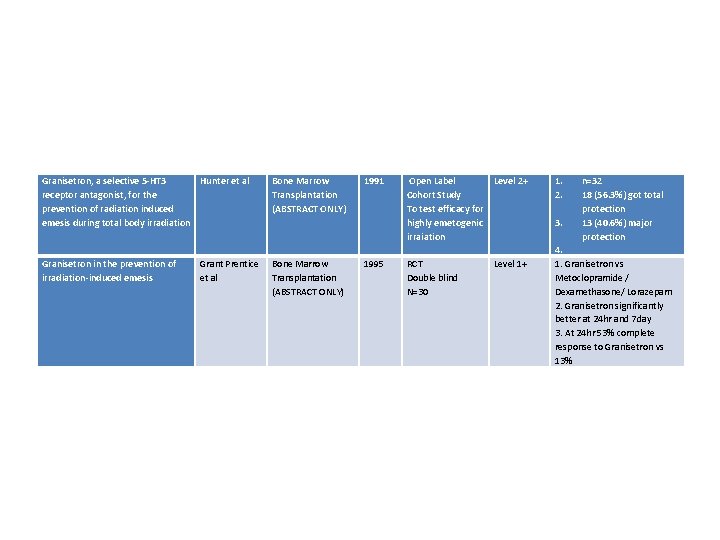
Granisetron, a selective 5 -HT 3 Hunter et al receptor antagonist, for the prevention of radiation induced emesis during total body irradiation Bone Marrow Transplantation (ABSTRACT ONLY) 1991 Open Label Level 2+ Cohort Study To test efficacy for highly emetogenic irraiation Granisetron in the prevention of irradiation-induced emesis Bone Marrow Transplantation (ABSTRACT ONLY) 1995 RCT Double blind N=30 Grant Prentice et al Level 1+ 1. 2. n=32 18 (56. 3%) got total protection 3. 13 (40. 6%) major protection 4. 1. Granisetron vs Metoclopramide / Dexamethasone/ Lorazepam 2. Granisetron significantly better at 24 hr and 7 day 3. At 24 hr 53% complete response to Granisetron vs 13%

Dr Jamie Barfield; Palliative Medicine ST 3; Aintree Hospital Levomepromazine

Levomepromazine • Phenothiazine • Low potency antipsychotic – (approximately half as potent as chlorpromazine) • Exerts its effects by blocking a variety of receptors – Adrenergic, dopamine, histamine, muscarinic, acetylcholine and serotonin • Serious side effects – tardive dyskinesia, akathisia, arrhythmias, hypotension and neuroleptic malignant syndrome

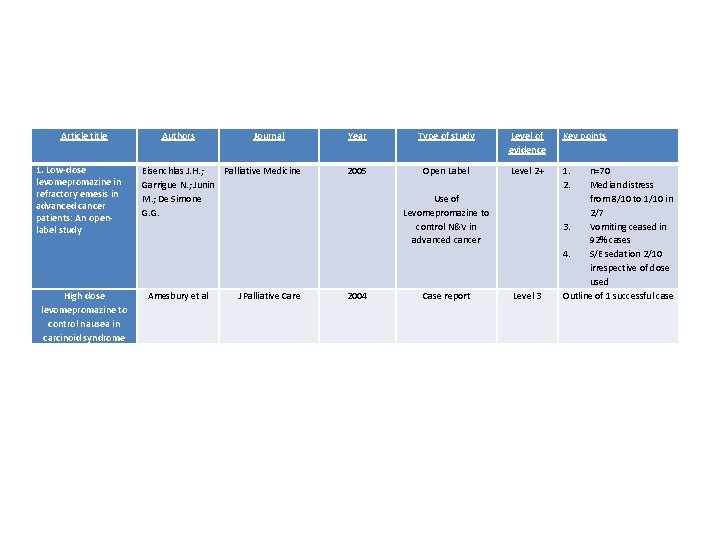
Levomepromazine • 1 case report of a man with carcinoid treated with high dose levomepromazine that found 25 mg TDS to be effective • 1 open label study of 70 patients given levomepromazine at doses from 3. 12 mg to 25 mg subcut daily. No association between dose and efficacy or sedation

Article title 1. Low-dose levomepromazine in refractory emesis in advanced cancer patients: An openlabel study High dose levomepromazine to control nausea in carcinoid syndrome Authors Journal Eisenchlas J. H. ; Palliative Medicine Garrigue N. ; Junin M. ; De Simone G. G. Amesbury et al J Palliative Care Year Type of study Level of evidence Key points 2005 Open Label Use of Levomepromazine to control N&V in advanced cancer Level 2+ 1. 2. 2004 Case report Level 3 n=70 Median distress from 8/10 to 1/10 in 2/7 3. Vomiting ceased in 92% cases 4. S/E sedation 2/10 irrespective of dose used Outline of 1 successful case

Dr Paula Powell; Community Consultant in Palliative Medicine Olanzapine

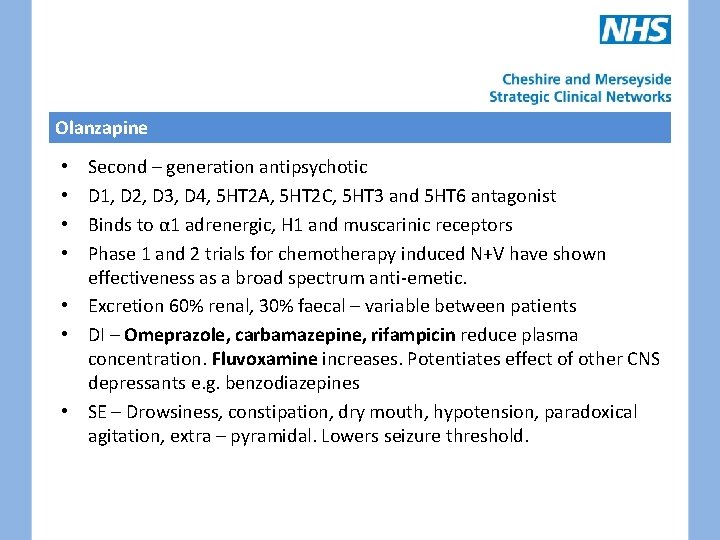
Olanzapine Second – generation antipsychotic D 1, D 2, D 3, D 4, 5 HT 2 A, 5 HT 2 C, 5 HT 3 and 5 HT 6 antagonist Binds to α 1 adrenergic, H 1 and muscarinic receptors Phase 1 and 2 trials for chemotherapy induced N+V have shown effectiveness as a broad spectrum anti-emetic. • Excretion 60% renal, 30% faecal – variable between patients • DI – Omeprazole, carbamazepine, rifampicin reduce plasma concentration. Fluvoxamine increases. Potentiates effect of other CNS depressants e. g. benzodiazepines • SE – Drowsiness, constipation, dry mouth, hypotension, paradoxical agitation, extra – pyramidal. Lowers seizure threshold. • •

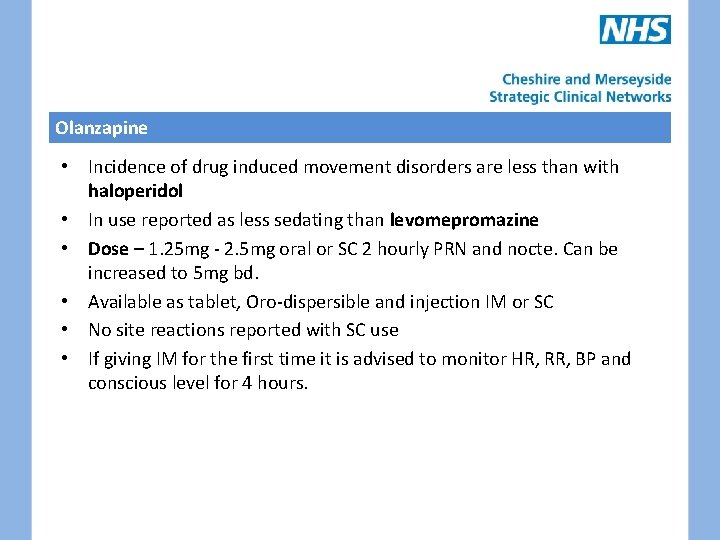
Olanzapine • Incidence of drug induced movement disorders are less than with haloperidol • In use reported as less sedating than levomepromazine • Dose – 1. 25 mg - 2. 5 mg oral or SC 2 hourly PRN and nocte. Can be increased to 5 mg bd. • Available as tablet, Oro-dispersible and injection IM or SC • No site reactions reported with SC use • If giving IM for the first time it is advised to monitor HR, RR, BP and conscious level for 4 hours.

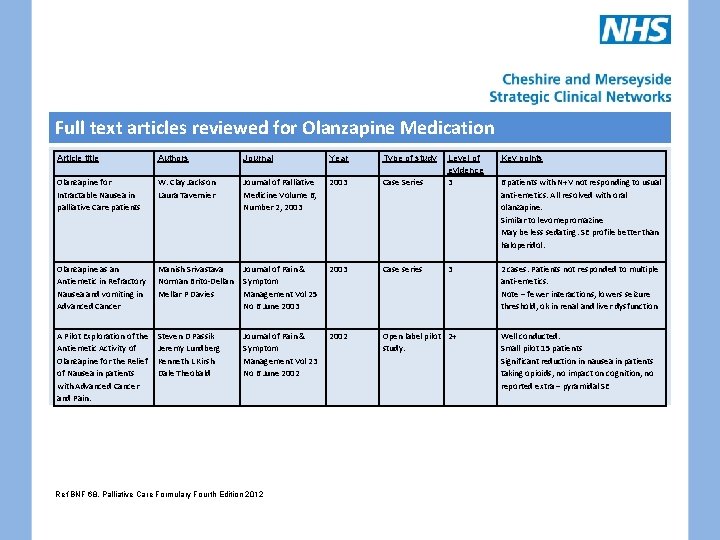
Full text articles reviewed for Olanzapine Medication Article title Olanzapine for Intractable Nausea in palliative Care patients Authors Journal Year Type of study Key points Case Series Level of evidence 3 W. Clay Jackson Laura Tavernier Journal of Palliative Medicine Volume 6, Number 2, 2003 Olanzapine as an Antiemetic in Refractory Nausea and vomiting in Advanced Cancer Manish Srivastava Norman Brito-Dellan Mellar P Davies Journal of Pain & Symptom Management Vol 25 No 6 June 2003 Case series 3 2 cases. Patients not responded to multiple anti-emetics. Note – fewer interactions, lowers seizure threshold, ok in renal and liver dysfunction A Pilot Exploration of the Antiemetic Activity of Olanzapine for the Relief of Nausea in patients with Advanced Cancer and Pain. Steven D Passik Jeremy Lundberg Kenneth L Kirsh Dale Theobald Journal of Pain & Symptom Management Vol 23 No 6 June 2002 Open label pilot 2+ study. Ref BNF 68, Palliative Care Formulary Fourth Edition 2012 6 patients with N+V not responding to usual anti-emetics. All resolved with oral olanzapine. Similar to levomepromazine May be less sedating. SE profile better than haloperidol. Well conducted. Small pilot 15 patients Significant reduction in nausea in patients taking opioids, no impact on cognition, no reported extra – pyramidal SE

Dr Paula Powell; Community Consultant in Palliative Medicine Prochlorperazine

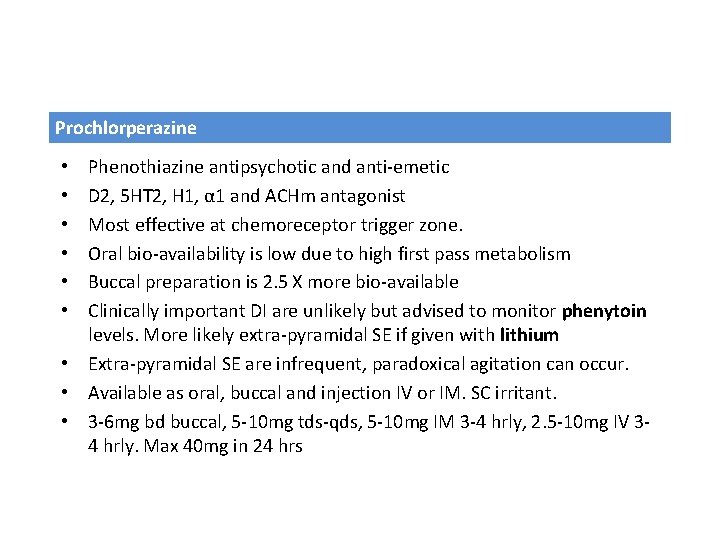
Prochlorperazine Phenothiazine antipsychotic and anti-emetic D 2, 5 HT 2, H 1, α 1 and ACHm antagonist Most effective at chemoreceptor trigger zone. Oral bio-availability is low due to high first pass metabolism Buccal preparation is 2. 5 X more bio-available Clinically important DI are unlikely but advised to monitor phenytoin levels. More likely extra-pyramidal SE if given with lithium • Extra-pyramidal SE are infrequent, paradoxical agitation can occur. • Available as oral, buccal and injection IV or IM. SC irritant. • 3 -6 mg bd buccal, 5 -10 mg tds-qds, 5 -10 mg IM 3 -4 hrly, 2. 5 -10 mg IV 34 hrly. Max 40 mg in 24 hrs • • •

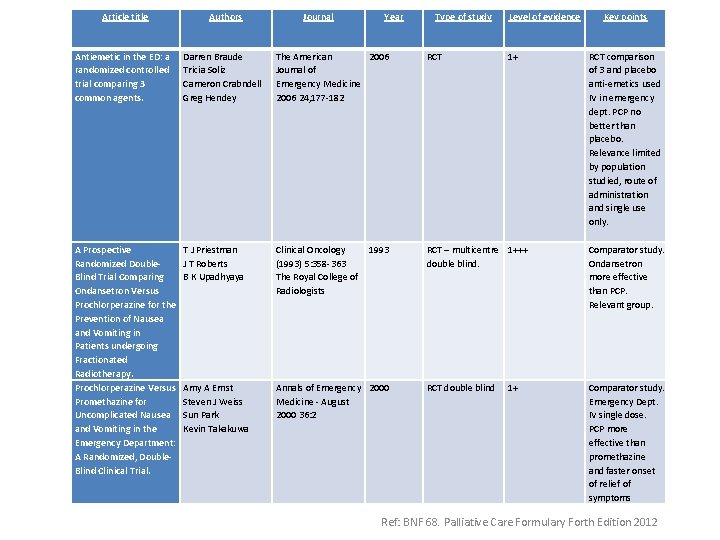
Article title Authors Journal Year Type of study Level of evidence Antiemetic in the ED: a randomized controlled trial comparing 3 common agents. Darren Braude Tricia Soliz Cameron Crabndell Greg Hendey The American 2006 Journal of Emergency Medicine 2006 24, 177 -182 RCT 1+ A Prospective Randomized Double. Blind Trial Comparing Ondansetron Versus Prochlorperazine for the Prevention of Nausea and Vomiting in Patients undergoing Fractionated Radiotherapy. Prochlorperazine Versus Promethazine for Uncomplicated Nausea and Vomiting in the Emergency Department: A Randomized, Double. Blind Clinical Trial. T J Priestman J T Roberts B K Upadhyaya Clinical Oncology 1993 (1993) 5: 358 -363 The Royal College of Radiologists RCT – multicentre 1+++ double blind. Amy A Ernst Steven J Weiss Sun Park Kevin Takakuwa Annals of Emergency 2000 Medicine - August 2000 36: 2 RCT double blind 1+ Key points RCT comparison of 3 and placebo anti-emetics used IV in emergency dept. PCP no better than placebo. Relevance limited by population studied, route of administration and single use only. Comparator study. Ondansetron more effective than PCP. Relevant group. Comparator study. Emergency Dept. IV single dose. PCP more effective than promethazine and faster onset of relief of symptoms Ref: BNF 68. Palliative Care Formulary Forth Edition 2012

Systematic Reviews – Dr Richard Latten, Consultant Palliative Medicine • A Systemic Review of the Treatment of Nausea and/or Vomiting in Cancer Unrelated to Chemotherapy or Radiation (2010) • Systemic review of the efficacy of antiemetics in the treatment of nausea in patients with far advanced cancer (2004)

Dr Richard Latten CURRENT STANDARDS

Current Standards 1. Any patient with a history of nausea and vomiting should have a full assessment including a history, clinical examination and appropriate investigations to try and identify a cause for the symptoms. (Grade D) 2. Appropriate anti emetics should be prescribed on a regular and as required basis. (Grade D) 3. If a patient is vomiting or nauseated for more than 24 hours, anti emetics should be given via the parenteral route. (Grade D)

4. If combinations of anti emetics are required, drugs with different but complementary actions should be used. (Grade D) 5. Metoclopramide should not be prescribed if the vomiting is caused by complete intestinal obstruction. (Grade D) 6. Symptoms of nausea and vomiting should be controlled within 72 hours. (Grade D)

Questions to consider • Cyclizine & heart failure • Max dose cyclizine • Rationale for antiemetic choice incl combinations – E. g. Why not Prochlorperazine / Olanzapine / Granisetron – Especially given available evidence level • Process of deciding choice – patient centred approach