EVALUTE THE EFFICACY OF ADJUVANT REGIMEN 3 FEC











- Slides: 27

EVALUTE THE EFFICACY OF ADJUVANT REGIMEN 3 FEC- 3 T IN STAGE II BREAST CANCER LÊ THU HÀ, Ph. D. Nguyễn Khánh Hà, Dr. Hai Phong, 2017

Introduction v Breast cancer is the most commonly diagnosed cancer in women v Global (2012): 1, 6 million new cases. v Viet Nam (2010): 18. 424 new cases. v Rate of women diagnosed at the early stage (I, II) increases, mortality rate decreases. v Adjuvant chemotherapy, hormone therapy, target therapy are the common ways to treat breast cancer after mastectomy.

Introduction v Adjuvant chemotherapy can lower the risk cancer coming back and prolong survival v Regimens in the clinical : AC , CAF , CMF , 4 AC – 4 T TAC, 3 FEC – 3 T, 4 AC , TC… v Sequencial adjuvant 3 FEC – 3 T was demonstrated efficacy in breast cancer on the World. v Ha Noi Oncology Hospital started perform 3 FEC – 3 T in 2010.

OBJECTIVE v This study is aim to evaluate the efficacy and the toxicity of 3 FEC – 3 T regimen in breast cancer patients with stage II after mastectomy.

OVERVIEW v Diagnosis v Stage TNM v Multidemensional treatment v Adjuvant chemotherapy v Clinical trials

PATIENTS AND METHOD v Patients: Restrospective study of 65 stage II breast cancer patients underwent mastectomy given 3 FEC – 3 T regimen from 10/2010 to 31/12/2014 at Ha Noi Oncology Hospital.

PATIENTS AND METHOD v Method: • Clinical presentation • Staging TNM • Surgery • Mortality and morbidity • Toxicity of regimen

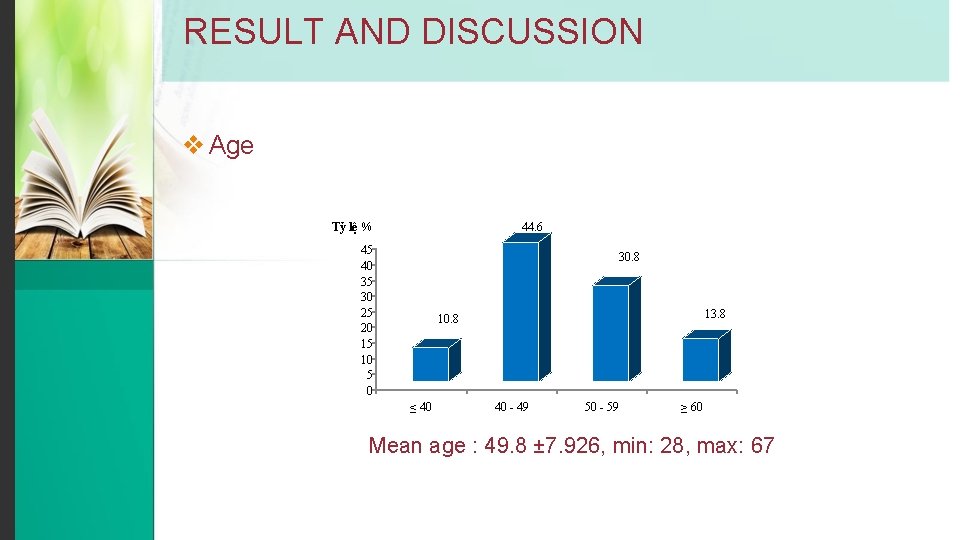
RESULT AND DISCUSSION v Age Tỷ lệ % 44. 6 45 40 35 30 25 20 15 10 5 0 30. 8 13. 8 10. 8 ≤ 40 40 - 49 50 - 59 ≥ 60 Mean age : 49. 8 ± 7. 926, min: 28, max: 67

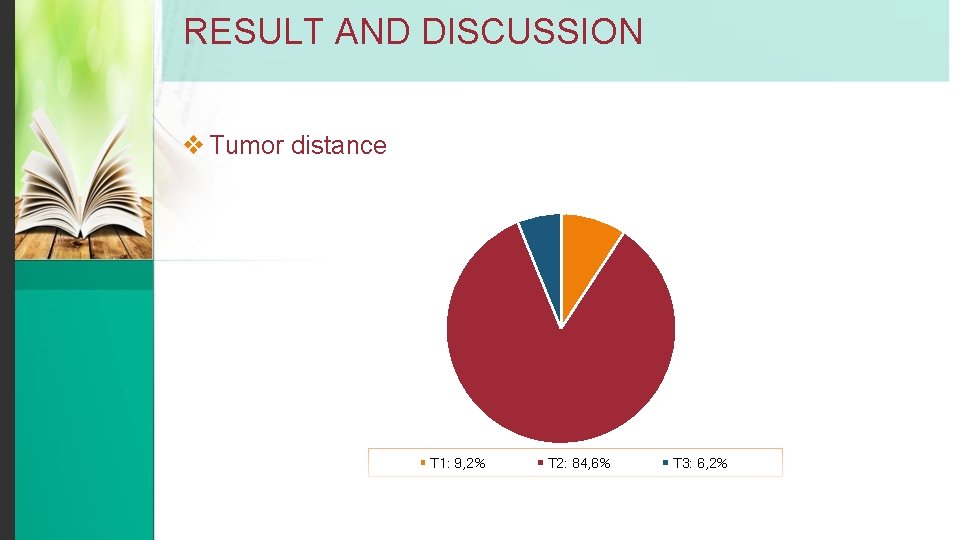
RESULT AND DISCUSSION v Tumor distance T 1: 9, 2% T 2: 84, 6% T 3: 6, 2%

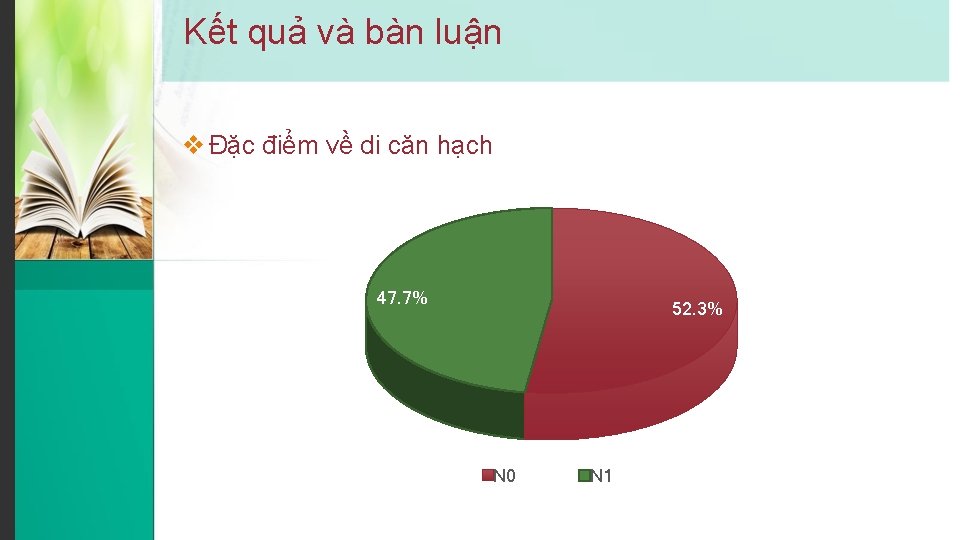
Kết quả và bàn luận v Đặc điểm về di căn hạch 47. 7% 52. 3% N 0 N 1

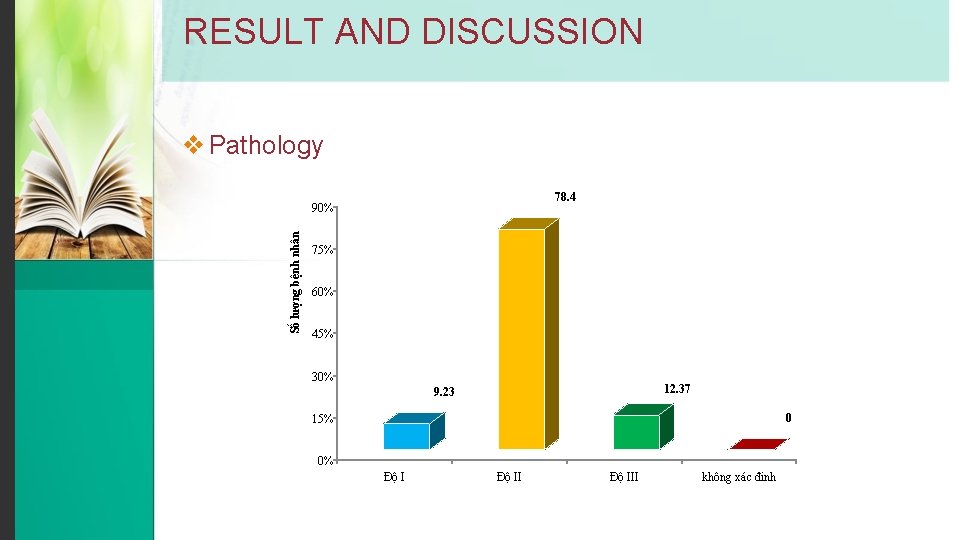
RESULT AND DISCUSSION v Pathology 78. 4 Số lượng bệnh nhân 90% 75% 60% 45% 30% 12. 37 9. 23 0 15% 0% Độ III không xác đinh

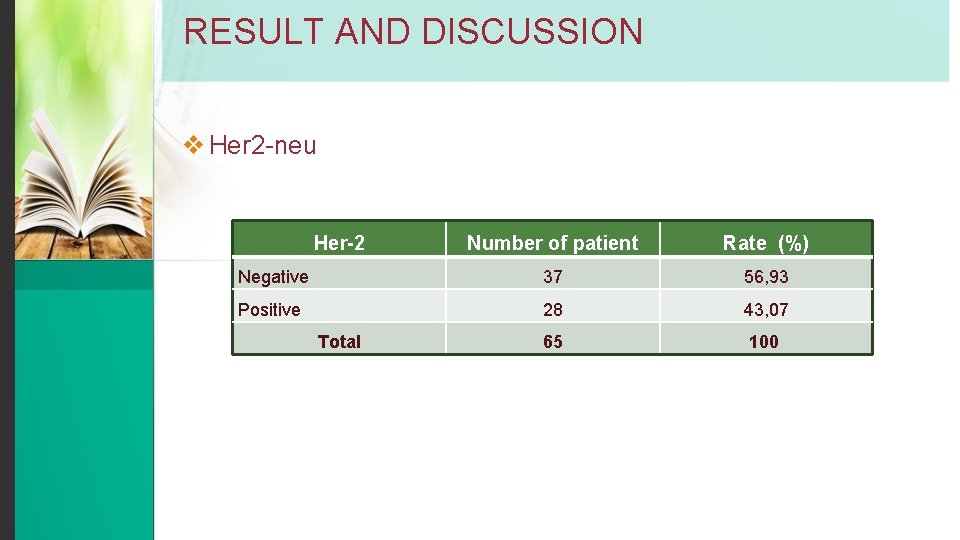
RESULT AND DISCUSSION v Her 2 -neu Her-2 Number of patient Rate (%) Negative 37 56, 93 Positive 28 43, 07 65 100 Total

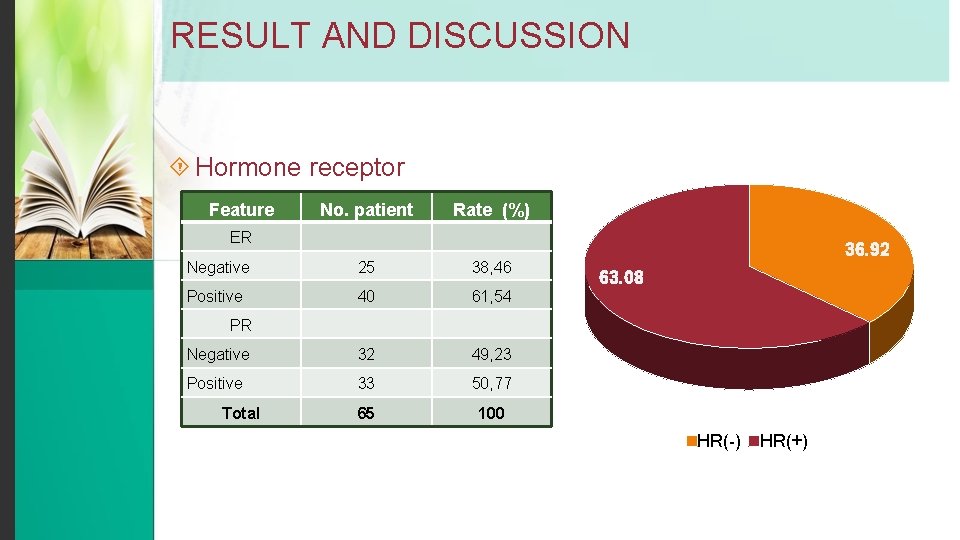
RESULT AND DISCUSSION Hormone receptor Feature No. patient Rate (%) ER Negative 25 38, 46 Positive 40 61, 54 Negative 32 49, 23 Positive 33 50, 77 65 100 PR Total 36. 92 63. 08 HR(-) HR(+)

Time to v Median follow – up time : 42, 75 ± 13, 95 months v Max : 78 months v Min : 25 months

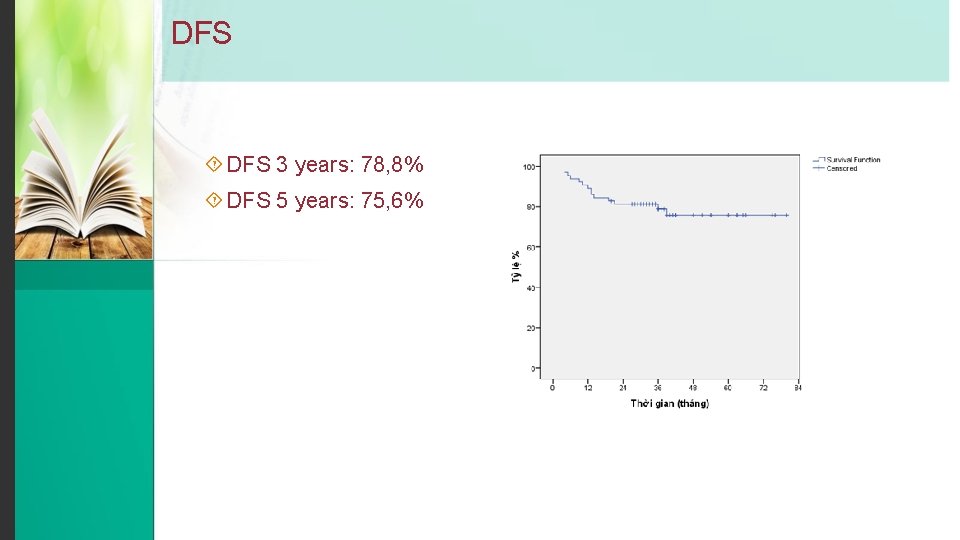
DFS 3 years: 78, 8% DFS 5 years: 75, 6%

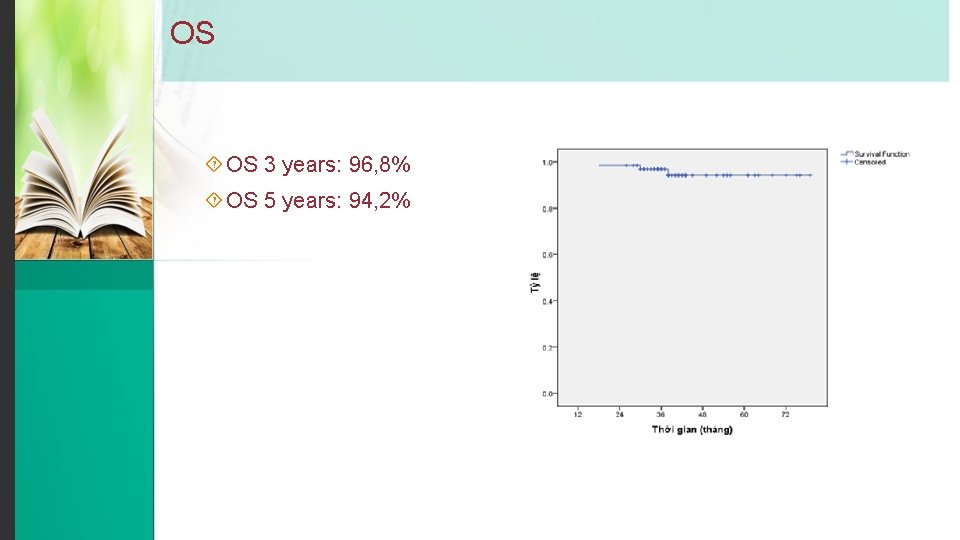
OS OS 3 years: 96, 8% OS 5 years: 94, 2%

RESULT AND DISCUSSION Clinical Trials PAC-01 GEICAM 9906 TACT We DFS (5 năm) 78. 4% 78. 5% 74, 3% 75. 6% OS (5 năm) 90. 7% - - 94. 2% Roche’ H et al (2006) Miguel Martín et al (2008) Ellis P et al (2009)

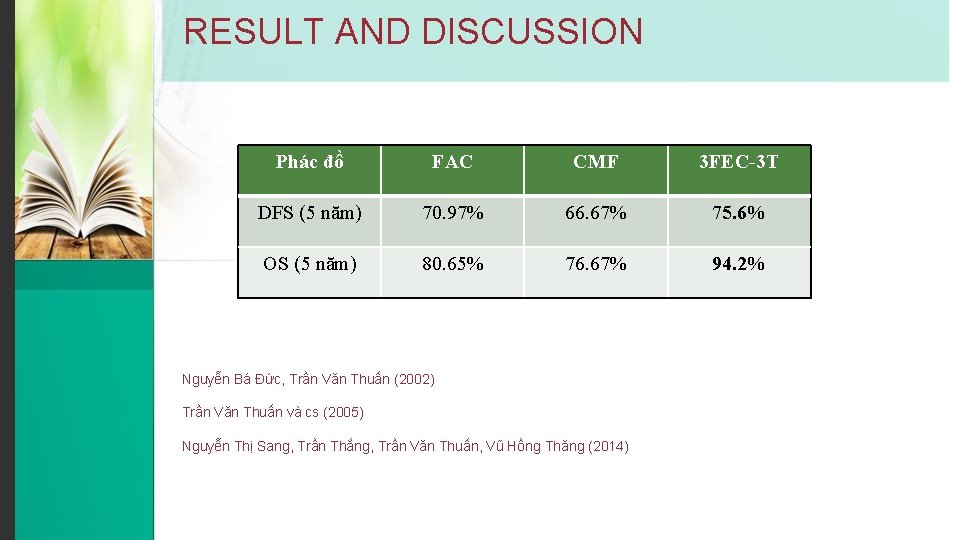
RESULT AND DISCUSSION Phác đồ FAC CMF 3 FEC-3 T DFS (5 năm) 70. 97% 66. 67% 75. 6% OS (5 năm) 80. 65% 76. 67% 94. 2% Nguyễn Bá Đức, Trần Văn Thuấn (2002) Trần Văn Thuấn và cs (2005) Nguyễn Thị Sang, Trần Thắng, Trần Văn Thuấn, Vũ Hồng Thăng (2014)

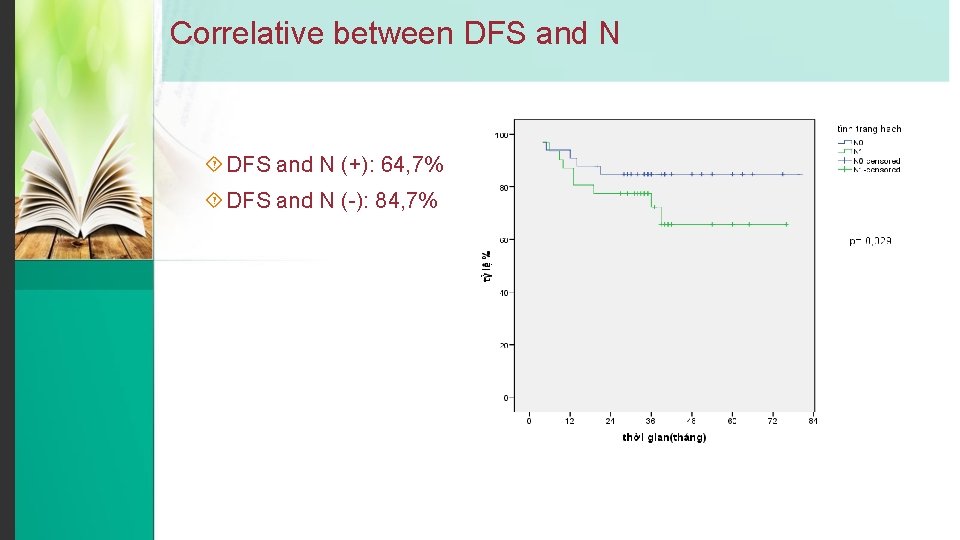
Correlative between DFS and N (+): 64, 7% DFS and N (-): 84, 7%

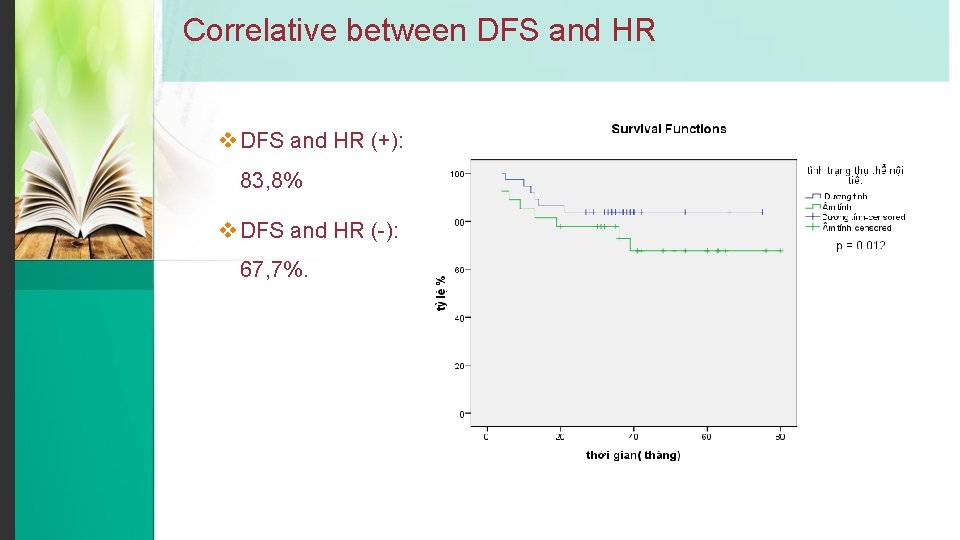
Correlative between DFS and HR v DFS and HR (+): 83, 8% v DFS and HR (-): 67, 7%.

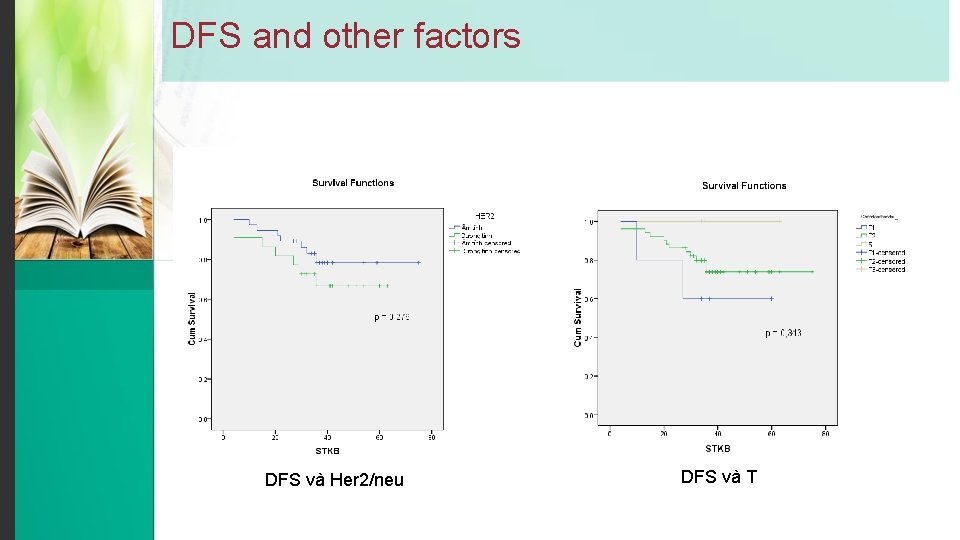
DFS and other factors DFS và Her 2/neu DFS và T

RESULT AND DISCUSSION v Hematology toxicity 120 100 7. 7 8. 9 9. 2 11. 8 80 19. 5 19. 2 51. 8 60 40 1. 2 4. 4 0 3. 1 96. 9 63. 1 59 46. 6 20 0 Hạ bạch cầu Độ 0 Hạ BC ĐNTT Độ 1 Hạ tiểu cầu Độ 2 Hạ huyết sắc tố Độ 3 Độ 4

Toxicity Nausea 70 60 58. 4 50 40 30 20 12. 5 10 1. 5 0 Độ 3 Độ 4 0 Độ 1 Độ 2

Toxicity Liver 18 16 15. 4 14 12 10 8 6 4 1. 5 2 0 0 Độ 1 Độ 2 Độ 3 Độ 4

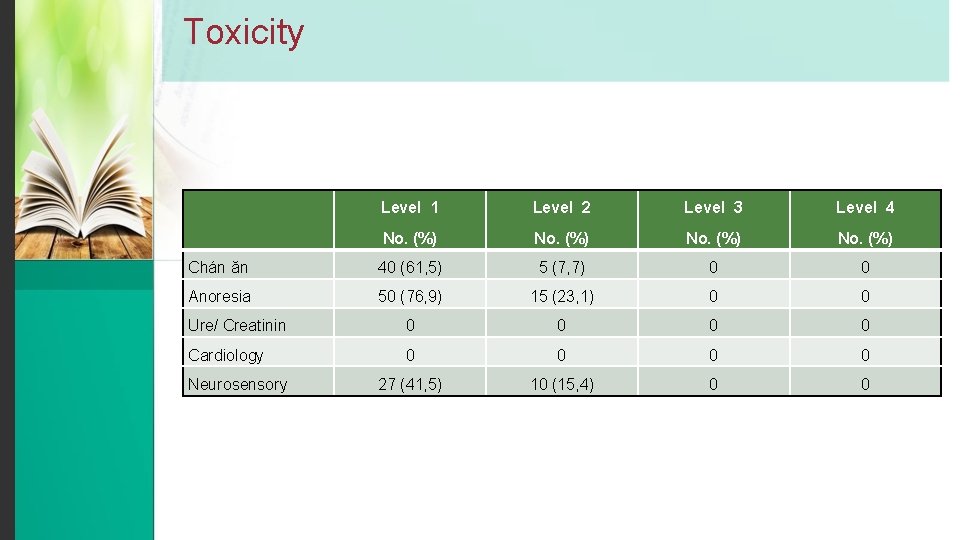
Toxicity Level 1 Level 2 Level 3 Level 4 No. (%) Chán ăn 40 (61, 5) 5 (7, 7) 0 0 Anoresia 50 (76, 9) 15 (23, 1) 0 0 Ure/ Creatinin 0 0 Cardiology 0 0 27 (41, 5) 10 (15, 4) 0 0 Neurosensory

Conclusion v DFS and OS improved. DFS after 3 year and 5 year are 78, 8%; 75, 6% respectivly and OS after 3 year and 5 year are 96, 8%; 94, 2%. v N (+) lead to decrease survival. DFS and N (+) is 84, 7%, DFS and N (-) is 64, 7% v DFS and HR (+) is higher than DFS and HR (-) (83, 8% và 67, 7%)

THANKS FOR YOUR ATTENTION
 Domenico galetta
Domenico galetta Adjuvant neoadjuvant palliative
Adjuvant neoadjuvant palliative Adjuvant nsclc
Adjuvant nsclc Gondor adjuvant
Gondor adjuvant Forward equivalence class
Forward equivalence class Fec software
Fec software øfan
øfan Fec dijagram
Fec dijagram Cme fec
Cme fec Freeswitch opus
Freeswitch opus Fec errors
Fec errors Metastabilní diagram
Metastabilní diagram I fec
I fec Efficacy therapy
Efficacy therapy Collective teacher efficacy
Collective teacher efficacy Potency vs efficacy
Potency vs efficacy Drug efficacy
Drug efficacy Self-efficacy theory
Self-efficacy theory Albert bandura self-efficacy
Albert bandura self-efficacy Efficacy potency
Efficacy potency Potency vs efficacy
Potency vs efficacy Luminous efficacy comparison chart
Luminous efficacy comparison chart Collective teacher efficacy
Collective teacher efficacy Self monitoring in organisational behaviour
Self monitoring in organisational behaviour Vaccine efficacy
Vaccine efficacy Nebido effectiveness
Nebido effectiveness Drug efficacy
Drug efficacy Collective teacher efficacy
Collective teacher efficacy