Induce New vaccine Pneumococcal disease Is one of

























- Slides: 38

Induce New vaccine

Pneumococcal disease: Is one of leading causes of death in the world. • As WHO estimation , more than 1. 6 million people including more than 800, 000 children under 5 years die every year from pneumococcal infection. • Up to 60% of children carry pneumococcal bacteria. • There are more than 90 subtypes of SP.

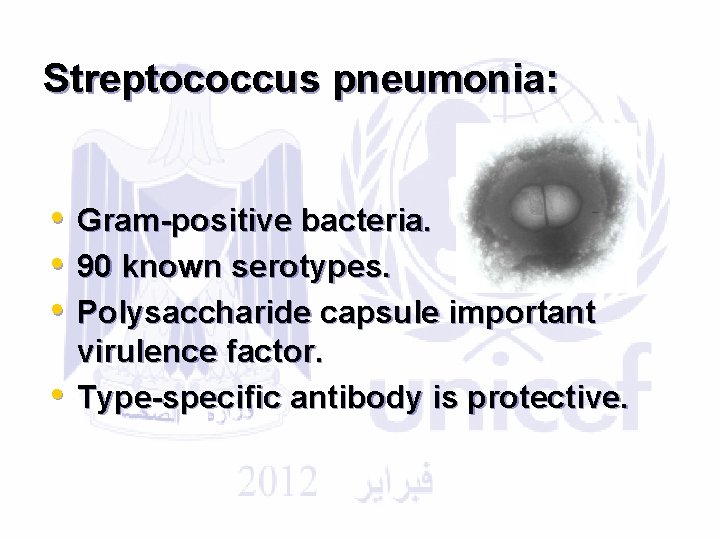
Streptococcus pneumonia: • • Gram-positive bacteria. 90 known serotypes. Polysaccharide capsule important virulence factor. Type-specific antibody is protective.

Pneumococcal Disease Burden: 1. Pneumococcal infections are estimated to cause >700, 000 deaths annually among children under 5 worldwide; most deaths occur in developing countries. 2. Important clinical syndromes include pneumonia, bloodstream infections, meningitis, and acute otitis media. 3. Emerging antibiotic resistance increases the cost of treatment of pneumococcal infections and can result in treatment failures

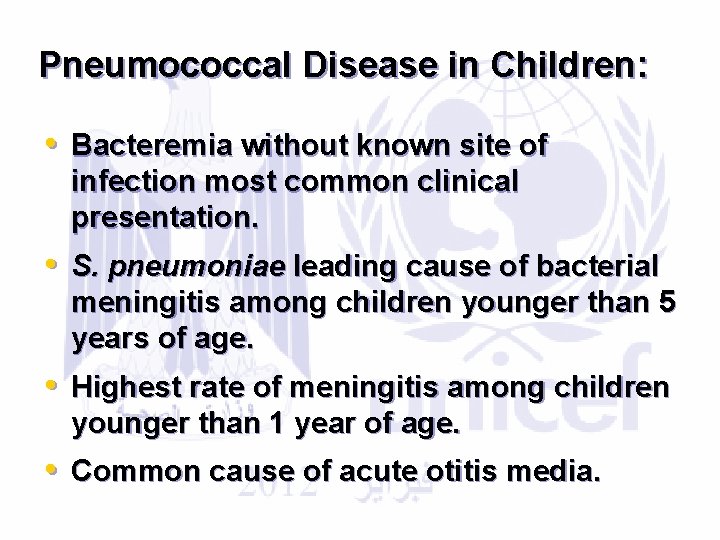
Pneumococcal Disease in Children: • Bacteremia without known site of infection most common clinical presentation. • S. pneumoniae leading cause of bacterial meningitis among children younger than 5 years of age. • Highest rate of meningitis among children younger than 1 year of age. • Common cause of acute otitis media.

Diagnosing Pneumococcal Disease: • The diagnosis of pneumococcal disease is difficult and microbiological proof of infection may not be possible. • There are various ways of finding out whether a patient has a pneumococcal infection.

• Which tests are used will depend on the patient’s signs and symptoms. • As other types of bacteria can also cause infections with similar symptoms, testing specifically for the presence of S. pneumoniae is important.

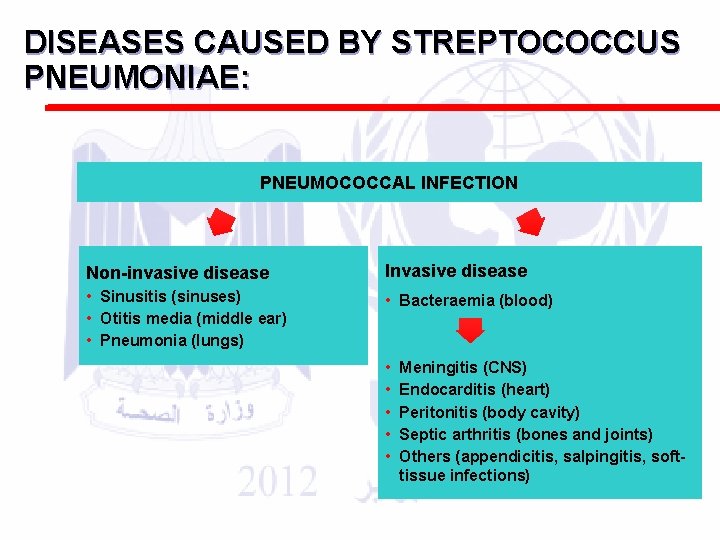
Types of pneumococcal diseases: • 1) Non-invasive pneumococcal diseases. • 2) Invasive pneumococcal diseases (IPD).

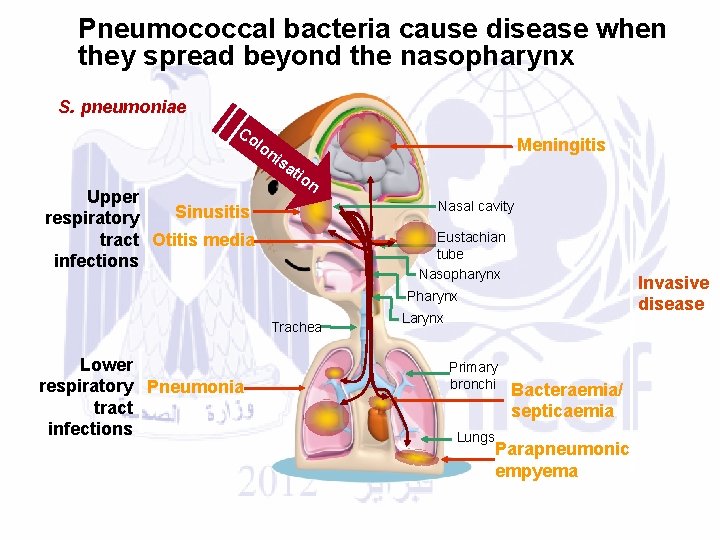
Types: • 1) Non-invasive pneumococcal diseases. • Infection occures through the nasopharynx (nose and throat) to the upper and lower respiratory tract and can cause:

Pneumococcal Pneumonia: Clinical Features • • • Abrupt onset. Fever. Shaking chills. Pleuritic chest pain. Productive cough. Dyspnea, tachypnea, hypoxia.

Pneumococcal acute otitis media signs and symptoms: • • • Earache. An elevated body temperature (fever). Vomiting. Diarrhea. Temporary hearing loss. Ear discharge.

2 - Invasive pneumococcal diseases (IPD): These tend to be more serious and occur inside a major organ, or in the blood. Examples of IPDs include: • Bacteremia (sepsis) - bacterial infection of the blood. • Bacteremia refers to the presence of live bacteria in the blood, while sepsis means a blood infection which is associated with capillary leak, shock and an increased risk of mortality.

Pneumococcal bacteremia - signs and symptoms may include: • • • An elevated body temperature (fever). Headache. Muscular aches and pains. Rapid heart rate. Rapid breathing.

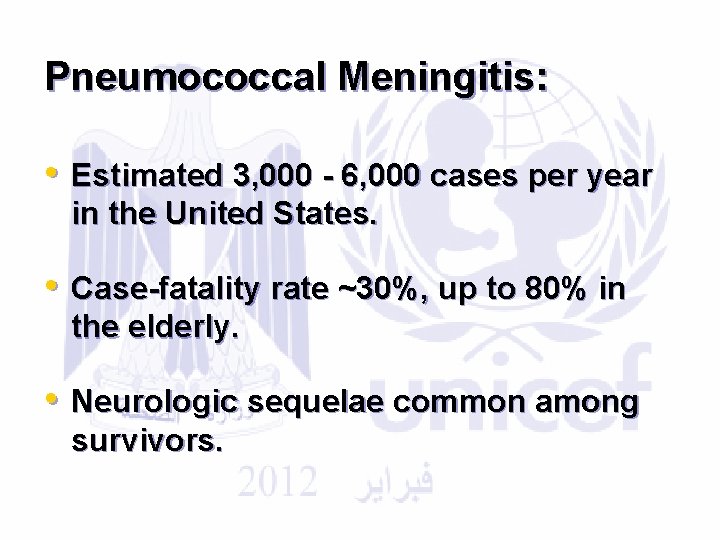
Pneumococcal Meningitis: • Estimated 3, 000 - 6, 000 cases per year in the United States. • Case-fatality rate ~30%, up to 80% in the elderly. • Neurologic sequelae common among survivors.

Pneumococcal meningitis - signs and symptoms may include: • • • An elevated body temperature (fever). Headache. Nausea. Vomiting. Sleepiness. Irritability. Stiff neck. Seizures. Sometimes coma.

Treatment: • in case the S. pneumoniae has developed resistance. • The emergence of resistant pneumococcal strains over the last few years is making treatment more difficult, extending the period of many hospitalizations, as well as increasing the likelihood of more expensive alternative therapy.

Treatment Options for Pneumococcal Disease: • Despite early and adequate treatment, • • there can still be serious and potentially life threatening complications arising from pneumococcal infection. Otitis media. May need to be treated with antibiotics. Bacterial pneumonia. Needs to be treated with antibiotics.

Invasive pneumococcal infections treatment: • antibiotics for invasive pneumococcal infections. If the infection is mild the patient will take oral antibiotics. • Serious infections will require intravenous administration a solution containing antibiotics will be administered directly into the venous circulation via a syringe or intravenous catheter.

Pneumococcal Disease Transmition: • S. pneumoniae, the bacterium, is most commonly found in the throat and nose (nasopharynx) of infants and young children. • They may also exist in the nasopharynx of adults, but this is less likely.

• The bacterium spreads from person to person via respiratory droplets if the infected person coughs or sneezes in close proximity to other people, the other people may become infected.

DISEASES CAUSED BY STREPTOCOCCUS PNEUMONIAE: PNEUMOCOCCAL INFECTION Non-invasive disease Invasive disease • Sinusitis (sinuses) • Otitis media (middle ear) • Pneumonia (lungs) • Bacteraemia (blood) • • • Meningitis (CNS) Endocarditis (heart) Peritonitis (body cavity) Septic arthritis (bones and joints) Others (appendicitis, salpingitis, softtissue infections)

Pneumococcal bacteria cause disease when they spread beyond the nasopharynx S. pneumoniae Co lo Upper Sinusitis respiratory tract Otitis media infections Meningitis ni sa tio n Nasal cavity Eustachian tube Nasopharynx Invasive disease Pharynx Trachea Lower respiratory Pneumonia tract infections Larynx Primary bronchi Lungs Bacteraemia/ septicaemia Parapneumonic empyema

Pneumococcal Vaccine: • There are two kinds of pneumococcal vaccine: 1 - Pneumococcal polysaccharide vaccine (PPV). 2 - Pneumococcal conjugate vaccine (PCV)

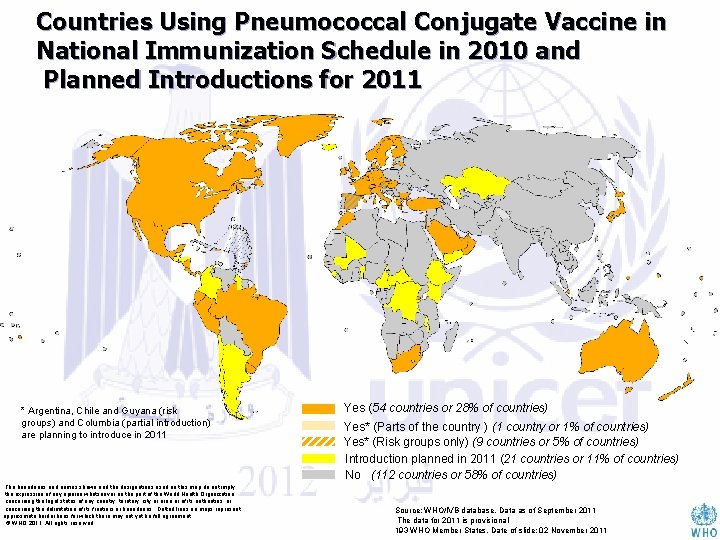
Countries Using Pneumococcal Conjugate Vaccine in National Immunization Schedule in 2010 and Planned Introductions for 2011 * Argentina, Chile and Guyana (risk groups) and Columbia (partial introduction) are planning to introduce in 2011 The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. © WHO 2011. All rights reserved Yes (54 countries or 28% of countries) Yes* (Parts of the country ) (1 country or 1% of countries) Yes* (Risk groups only) (9 countries or 5% of countries) Introduction planned in 2011 (21 countries or 11% of countries) No (112 countries or 58% of countries) Source: WHO/IVB database, Data as of September 2011 The data for 2011 is provisional 193 WHO Member States. Date of slide: 02 November 2011

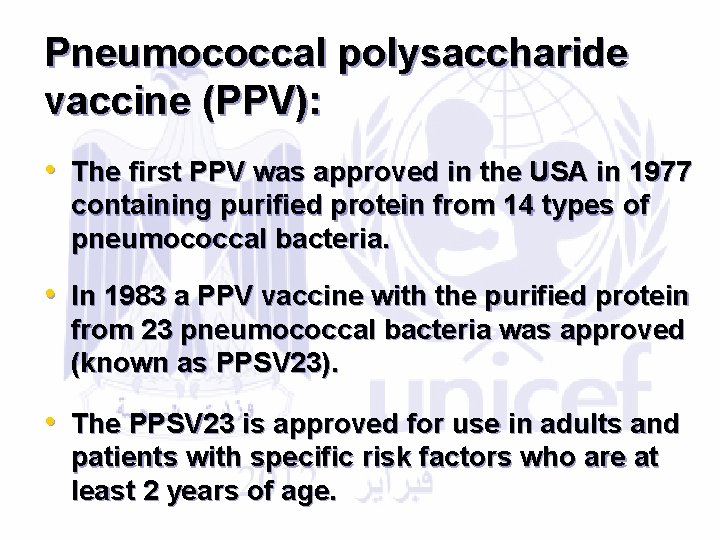
Pneumococcal polysaccharide vaccine (PPV): • The first PPV was approved in the USA in 1977 containing purified protein from 14 types of pneumococcal bacteria. • In 1983 a PPV vaccine with the purified protein from 23 pneumococcal bacteria was approved (known as PPSV 23). • The PPSV 23 is approved for use in adults and patients with specific risk factors who are at least 2 years of age.

Pneumococcal conjugate vaccine (PCV): • The first PCV was approved in the USA in 2000 for use in infants and young children aged six weeks to 5 years for the prevention of pneumococcal disease. • This vaccine is commonly known as PCV 7.

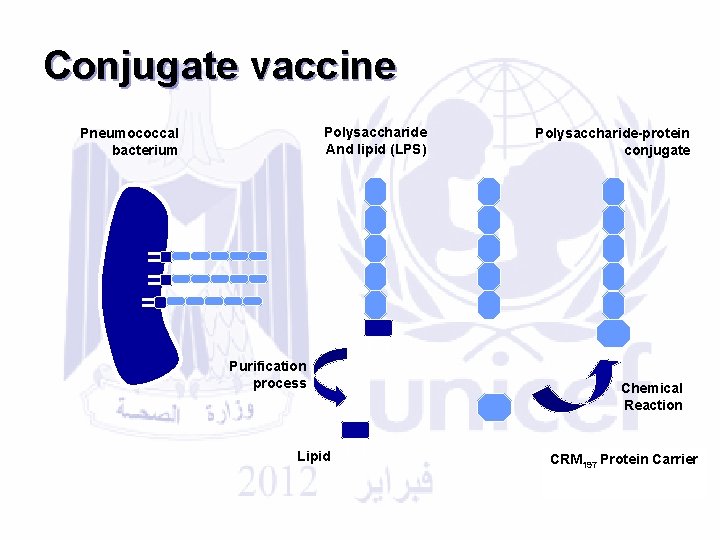
Pneumococcal Conjugate Vaccine: A conjugate vaccine is created by covalently attaching a poor (polysaccharide organism) antigen to a carrier protein, thereby conferring the immunological attributes of the carrier on the attached antigen. This technique for the creation of an effective immunogen is most often applied to bacterial polysaccharides for the prevention of invasive bacterial disease.

Polysaccharide vaccines: Made from polysaccharide from the capsule surrounding the bacteria. Works in adults: • Two major problems: – Not immunogenic in babies. – No immune memory.

Pnemoumoccal Conjugate Vaccine pcv for infant and children: The first PCV was approved in the USA in 2000 known as PCV 7. (PCV 7): vaccine protects against 7 serotypes, (PCV 10): vaccine protects against 10 serotypes 2009. (PCV 13): vaccine protects against 13 serotypes. This type will be used in Palestin.

Conjugate vaccine Polysaccharide And lipid (LPS) Pneumococcal bacterium Purification process Lipid Polysaccharide-protein conjugate Chemical Reaction CRM 197 Protein Carrier

DOSES OF VACCINE: • First dose: 2 month. • Second dose: 4 month. • Third dose: 6 month.

Symptoms of an allergic reaction may include: • Shortness of breath. • Wheezing or difficulty breathing. • Swelling of the face, lips, tongue or other parts of the body. • Rash, itching or hives on the skin.

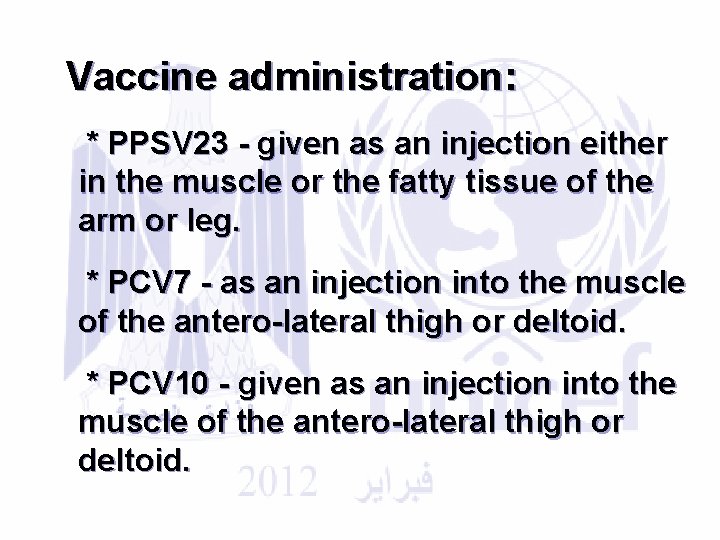
Vaccine administration: * PPSV 23 - given as an injection either in the muscle or the fatty tissue of the arm or leg. * PCV 7 - as an injection into the muscle of the antero-lateral thigh or deltoid. * PCV 10 - given as an injection into the muscle of the antero-lateral thigh or deltoid.

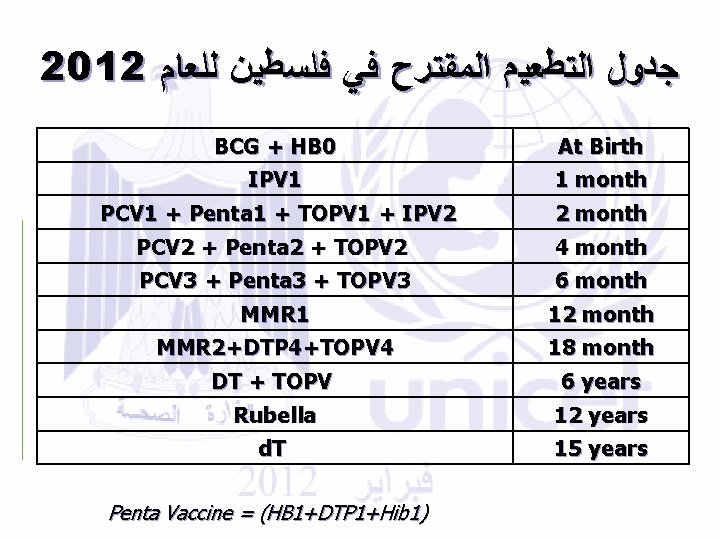
2012 ﺟﺪﻭﻝ ﺍﻟﺘﻄﻌﻴﻢ ﺍﻟﻤﻘﺘﺮﺡ ﻓﻲ ﻓﻠﺴﻄﻴﻦ ﻟﻠﻌﺎﻡ BCG + HB 0 IPV 1 At Birth 1 month PCV 1 + Penta 1 + TOPV 1 + IPV 2 PCV 2 + Penta 2 + TOPV 2 2 month 4 month PCV 3 + Penta 3 + TOPV 3 MMR 1 6 month 12 month MMR 2+DTP 4+TOPV 4 DT + TOPV 18 month 6 years Rubella d. T 12 years 15 years Penta Vaccine = (HB 1+DTP 1+Hib 1)

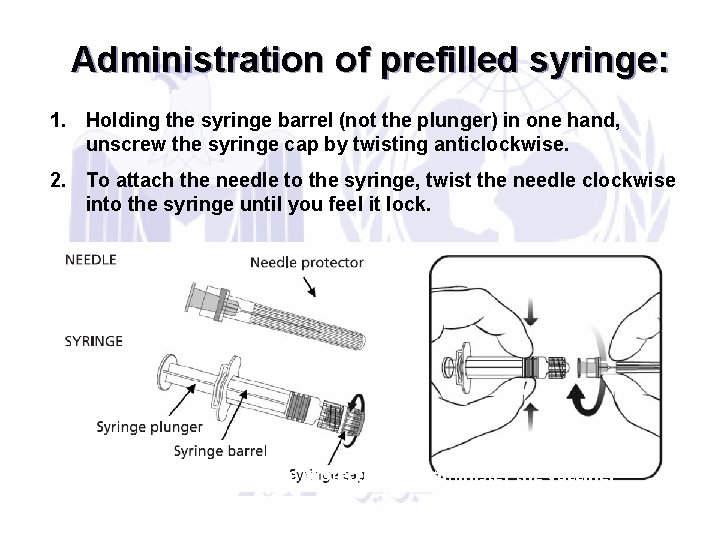
Administration of prefilled syringe: 1. Holding the syringe barrel (not the plunger) in one hand, unscrew the syringe cap by twisting anticlockwise. 2. To attach the needle to the syringe, twist the needle clockwise into the syringe until you feel it lock. 3. Remove the needle protector and administer the vaccine.

Side effects of PCV 7: • Vaccine is safe. • Between 10% and 20% of children develop redness. • Tenderness. • Swelling at the injection site. • Approximately 11% get a mild fever.

How effective is PCV 7? • A large clinical study showed that PCV 7 is 97% effective in preventing invasive disease caused by the pneumococci contained in the vaccine. • Children with chronic diseases, such as HIV infection and sickle cell disease appear to respond well to PCV 7.

Contraindications of Vaccine: • Anaphylactic shock due to previous dose of vaccine. • Moderate or sever illness (temporary). • Pregnancy.
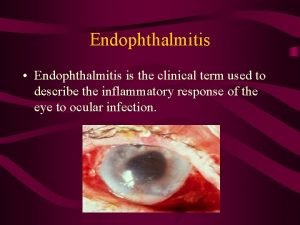
 Pneumococcal
Pneumococcal Pekka nuorti
Pekka nuorti Deduce and induce
Deduce and induce Positively by temporary induction
Positively by temporary induction Vaccine preventable disease
Vaccine preventable disease Bharathi viswanathan
Bharathi viswanathan One empire one god one emperor
One empire one god one emperor Little dog run
Little dog run One king one law one faith
One king one law one faith One empire one god one emperor
One empire one god one emperor One ford
One ford See one do one teach one
See one do one teach one See one, do one, teach one
See one, do one, teach one Structure of twelfth night
Structure of twelfth night See one do one teach one
See one do one teach one Asean tourism strategic plan
Asean tourism strategic plan Graphic organizer with the aims of la liga filipina
Graphic organizer with the aims of la liga filipina Edible vaccine definition
Edible vaccine definition Edible vaccines pros and cons
Edible vaccines pros and cons Global vaccine safety blueprint
Global vaccine safety blueprint Rabies incubation period
Rabies incubation period Imocolibov vaccine price
Imocolibov vaccine price Hepatitis b vaccine series adults
Hepatitis b vaccine series adults Herpes zoster
Herpes zoster Georgia registry of immunizations and transactions login
Georgia registry of immunizations and transactions login Hpv vaccine schedule adults
Hpv vaccine schedule adults Dr sears vaccine schedule
Dr sears vaccine schedule Next generation vaccine
Next generation vaccine Mcv4 vaccine schedule
Mcv4 vaccine schedule Vcxin
Vcxin Rabies vaccine dose
Rabies vaccine dose Shingles vaccine side effects
Shingles vaccine side effects Ennicav
Ennicav Vaccine temperature monitor kenya
Vaccine temperature monitor kenya Smallpox vaccine inventor industrial revolution
Smallpox vaccine inventor industrial revolution Mcb vaccine
Mcb vaccine Vaccine administration technique
Vaccine administration technique Activity 1 where do i belong vaccine
Activity 1 where do i belong vaccine Tetadif td
Tetadif td