Pneumococcal vaccine Polyvalent Untoward reaction in older children






















- Slides: 27

Pneumococcal vaccine • Polyvalent • Untoward reaction in older children and adult • Responsiveness is unpredictable in children younger than 2 • 23 -valent pneumococcal vaccine contains purified polysaccharide from 23 pneumococcal serotype responsible for more than 95% of cases of bacteremia and meningitis and 85% of cases of otitis media.

Pneumococcal vaccine • Clinical efficacy is controversial • Pneumococcal serotype 6 A is one of the strains most likely to produce disease in children. • Antigens 6 A, 14, 19 F, 23 F, are poorly immunogenic in children younger than 6. • New protein-conjucate pneumococcal polysaccharide vaccine are being evaluated.

Indication for immunization • • Children older than 2 years Sickle cell anemia Functional or anatomic asplenia Nephrotic syndrome Splenectomy after staging laparatomy Cerebrospinal fluid leaks HIV infection Chronic cardiovascular, pulmonary, or liver disease

Not recommended for: • Prevention of recurrent otitis media or sinusitis

Revaccination • Need for revaccination is uncertain • Children 10 years of age or younger at the time of revaccination • A single revaccination 3 years after the first dose • Children 10 years of age or older at the time of revaccination • A single revaccination 5 years after the first dose

Pneumococcal vaccine • Immunization dose not prevent pneumococcal disease related to serotypes not found in the vaccine • Many report of serious and even fatal infections in vaccinated children have been described

Meningococcal vaccine • A quadrivalent vaccine composed of capsular polysaccharide of meningococcal groups A, C, Y, W 135 is licensed. • Immunogenic in adult but not reliable in children younger than 2 • No vaccine is available for serogroup B • Routine use is not recommend

indication • • Military recruits Control of outbreaks Travelers to high endemic area Close contact of individual with A, C, Y, W 135, . • Anatomic and functional asplenia. • Complement component deficiency

Haemophylus influenza • The most important element of host defense is antibody directed against the type b capsular polysaccharide , PRP. • it is related to it’s opsonic activity • Antibody against outer membrane protein and lipopolysaccharide may also have role in opsonization.

• Lack of antibody in young infant may reflect maturational delay in immunologic response to thymus independent antigen like unconjugated PRP • The conjugated vaccine has thymus independent properties and elicit antibody response in young infant.

• Currently available combination are PRPOMP combined with hepatitis B vaccine • Attempts to combine DTa. P with H influenza vaccine have resulted in decreased anti-PRP antibody.

Varicella zoster immune globulin • Post exposure prophylaxis is recommended in • • immunocompromised children Pregnant women Newborn exposed to maternal varicella Close contact of a susceptible with herpes zoster 1 vial (125 unit) for each 10 kg (max 5 vial) Should be given IM As soon as possible but within 96 hours It is not indicated after onset of symptoms

• New born whose mother develop varicella 5 days before or 2 days after delivery should receive 1 vial. • Adult should be tested before VZIG administration. • VZIG may ameliorate disease but dose not eliminate the possibility of progressive disease

• Patient who have received high dose IVIG (100 -400 mg/kg )within 2 -3 weeks ago have serum antibody to VZV. Vaccine given to normal children within 3 days exposure may be effective

Rubella post exposure prophylaxis • Nonpregnant susceptible contacts of person with rubella should be vaccinated (dose not prevent infection but ensure protection) All pregnant women regardless of immunization history should make effort to avoid exposure to rubella

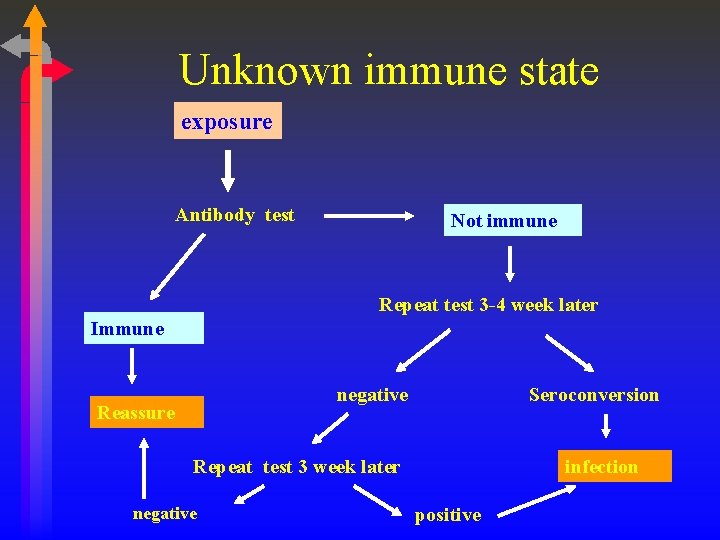
Unknown immune state exposure Antibody test Not immune Repeat test 3 -4 week later Immune negative Reassure Seroconversion Repeat test 3 week later negative infection positive

• If termination of pregnancy is not an option immunoglobulin administration is indicated. • The dose is 0. 55 mg/kg

Measles post exposure prophylaxis • Immunoglobulin is effective for prevention and attenuation of measles within 6 days of exposure. -indication Susceptible pregnant (0. 25 ml/kg ) Children below 12 mon(0. 25 ml/kg ) Immunocompromised (0. 5 ml/kg ) Susceptible children 6 -12 should also be vaccinated

Rabies posexposure prophylaxis • It is indicated for all persons with a history of a rabid animal bite. • Prevention depend on three complementary means of reducing the risk. • Local wound care • Passive immunization with human rabies Ig • Immediate blockage of attachment of virus to the nerve ending

Passive immunization for Rabies • Is available as HRIG • Dose is 20 IU /kg with as much as possible of the full dose of HRIG infiltrated in the area around the wound • Any remaining should be administered IM at a site distant from vaccine inoculation. • If vaccine was started previously, passive immunization should not be given once 8 days have elapsed • Corticosteroid should be avoided in the treatment of reaction because it activate rabies virus

Diphteria antitoxin • Specific antitoxin is the mainstay of therapy and should be administered on the basis of clinical diagnosis • It neutralize only free toxin • Antitoxin is administered once at empiric dosage based on the degree of toxicity site and size of the membrane and duration of illness. • Probably of no value for local manifestation

Diphtheria Antitoxin • First used in 1891 • Produced in horses • Used only for treatment of diphtheria • Neutralizes only unbound toxin

Basis of dose • • • antitoxin dosage Cutaneous lesion only 20000 -40000 Pharyngeal laryngeal disease 20000 -40000 Nasopharyngeal lesions 40000 -60000 Extensive disease 80000 -100000 Diffuse swelling of the neck 80000 -100000

Tetanus Wound Management Clean, minor wounds All other wounds Vaccination History Td TIG Unknown or <3 doses Yes No Yes 3+ doses No* No No** No * Yes, if >10 years since last dose ** Yes, if >5 years since last dose

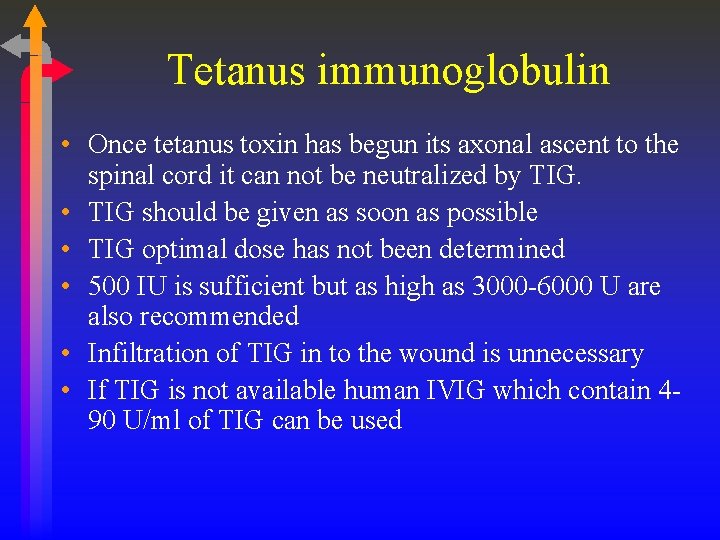
Tetanus immunoglobulin • Once tetanus toxin has begun its axonal ascent to the spinal cord it can not be neutralized by TIG. • TIG should be given as soon as possible • TIG optimal dose has not been determined • 500 IU is sufficient but as high as 3000 -6000 U are also recommended • Infiltration of TIG in to the wound is unnecessary • If TIG is not available human IVIG which contain 490 U/ml of TIG can be used

Tetanus Toxoid • Formalin-inactivated tetanus toxin • Schedule Three or four doses + booster Booster every 10 years • Efficacy Approximately 100% • Duration Approximately 10 years • Should be administered with diphtheria toxoid as DTa. P, DT, or Td

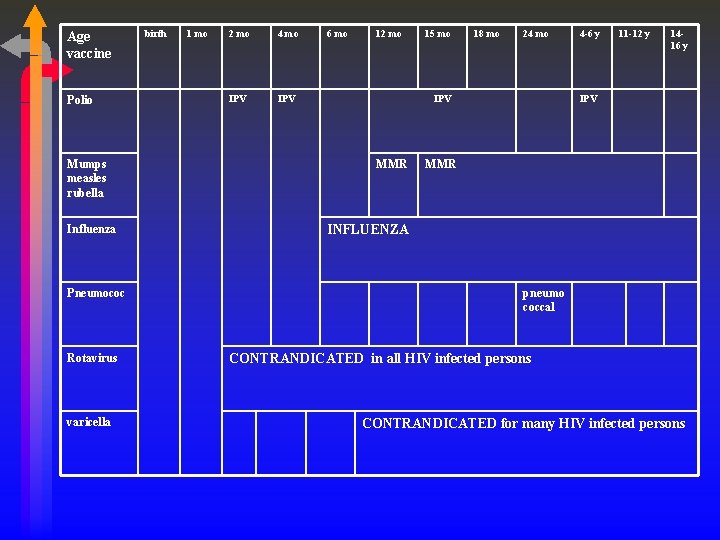
Age vaccine Polio Mumps measles rubella Influenza Pneumococ Rotavirus varicella birth 1 mo 2 mo 4 mo IPV 6 mo 12 mo 15 mo 18 mo 24 mo IPV MMR 4 -6 y 11 -12 y 1416 y IPV MMR INFLUENZA pneumo coccal CONTRANDICATED in all HIV infected persons CONTRANDICATED for many HIV infected persons
 Life is older than the trees
Life is older than the trees Lpo la jetée
Lpo la jetée Pneumococcal
Pneumococcal Pekka nuorti
Pekka nuorti Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Unit of rate of reaction for first order reaction
Unit of rate of reaction for first order reaction Addition reaction and substitution reaction
Addition reaction and substitution reaction Growing older poem
Growing older poem Renal
Renal Taller stronger sister
Taller stronger sister Covids older adults
Covids older adults Bartosz older owl
Bartosz older owl Thank you mr falker summary
Thank you mr falker summary Dq98 assessment form
Dq98 assessment form The virtues of growing older
The virtues of growing older My rotten redheaded older brother
My rotten redheaded older brother As people grow older
As people grow older Younger cells cuboidal older cells flattened
Younger cells cuboidal older cells flattened Future medical
Future medical Syncope in the older patient is
Syncope in the older patient is Moses older sister
Moses older sister Physical examination conclusion
Physical examination conclusion Older individuals who are blind program
Older individuals who are blind program Older women's cohousing
Older women's cohousing Late adulthood intellectual development
Late adulthood intellectual development Sos spelling for older students
Sos spelling for older students Ajayan is ten years older than vijayan
Ajayan is ten years older than vijayan