Advances in immunotherapy for lymphomas and myelomas Larry














- Slides: 25

Advances in immunotherapy for lymphomas and myelomas Larry W. Kwak, M. D. , Ph. D. Chairman, Department of Lymphoma/Myeloma Justin Distinguished Chair in Leukemia Research Co-Director, Center for Cancer Immunology Research MD Anderson Cancer Center

CME Disclosures • • • Biovest International (consultant) Xeme Biopharma, Inc. (equity) Celgene (research support) Celltrion (consultant) Onco. Pep (consultant)

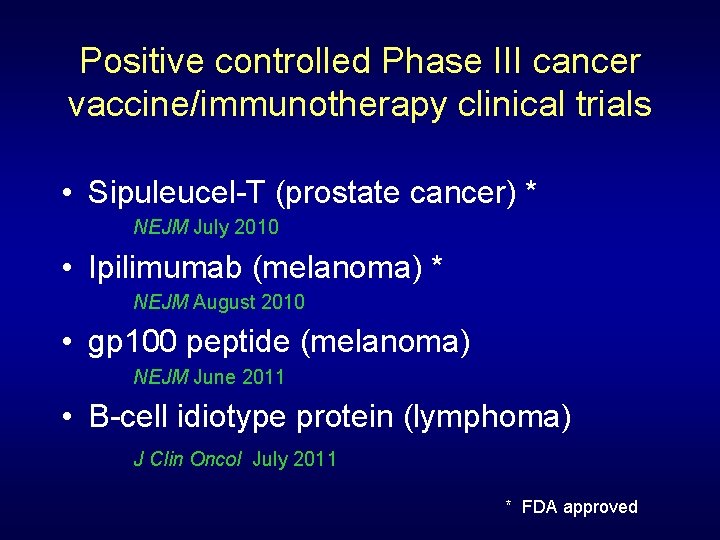
Positive controlled Phase III cancer vaccine/immunotherapy clinical trials • Sipuleucel-T (prostate cancer) * NEJM July 2010 • Ipilimumab (melanoma) * NEJM August 2010 • gp 100 peptide (melanoma) NEJM June 2011 • B-cell idiotype protein (lymphoma) J Clin Oncol July 2011 * FDA approved

Bench-to-bedside development of a homegrown therapeutic agent from an academic laboratory Y Isolate antigen Phase I/II Clinical Trial (Nature Med 1999): Lymphoma Tumor • Vaccine induces molecular remissions Preclinical • Addition of GM-CSF Adjuvant improves vaccine potency • (Kwak et al. PNAS 1996) Package in Delivery system Tumor protein Phase III Controlled Clinical Trial (J Clin Oncol 2011) • Vaccine prolongs DFS in patients in a chemotherapyinduced remission (n=117, p=0. 045) CD 4+ CD 8+ cytokines

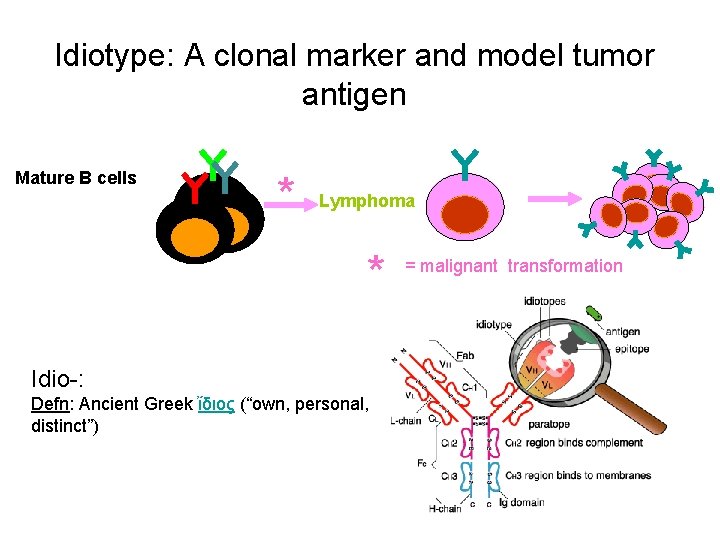
Idiotype: A clonal marker and model tumor antigen Mature B cells * Lymphoma * Idio-: Defn: Ancient Greek ἴδιος (“own, personal, distinct”) = malignant transformation

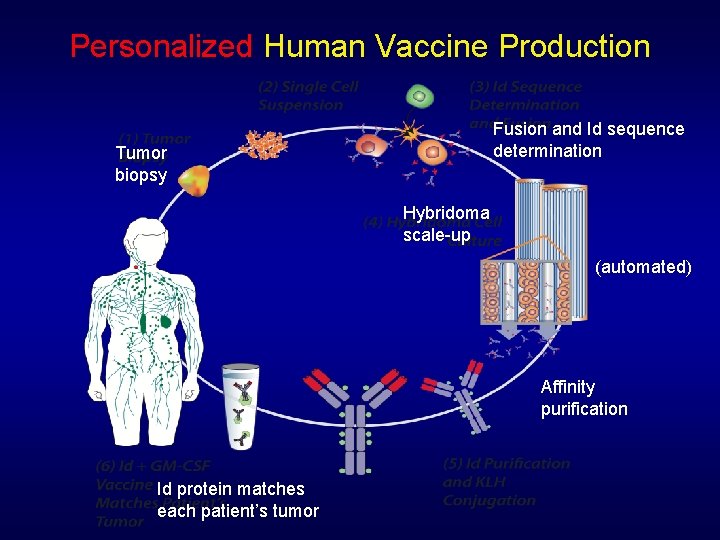
Personalized Human Vaccine Production Fusion and Id sequence determination Tumor biopsy Hybridoma scale-up (automated) Affinity purification Id protein matches each patient’s tumor

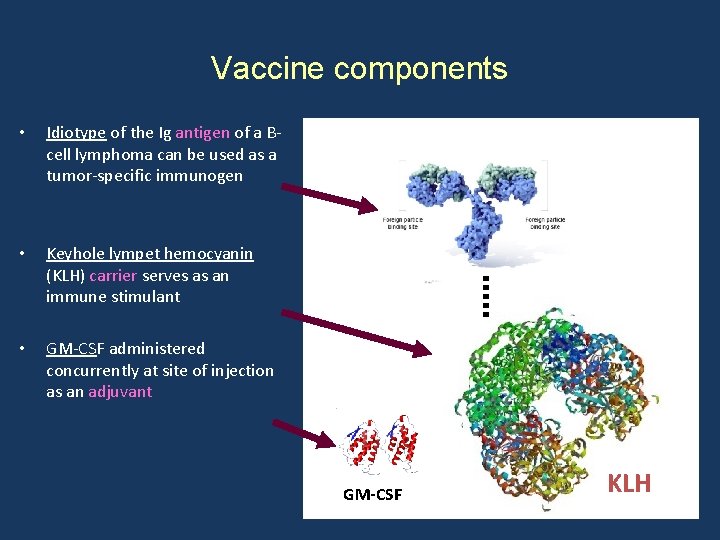
Vaccine components • Idiotype of the Ig antigen of a Bcell lymphoma can be used as a tumor-specific immunogen • Keyhole lympet hemocyanin (KLH) carrier serves as an immune stimulant • GM-CSF administered concurrently at site of injection as an adjuvant GM-CSF KLH

Types of vaccines • Therapeutic • Secondary prevention • Prevention (e. g. infectious diseases)

NCI/Biovest Phase III Trial: 2 -Stage Study Design (Id Vaccine) Chemo LN Bx Stratify for IPI 1, cycles of PACE 2 2: 1 Randomization Assign CR (Control) 6 - 12 months 6 - 8 months ITT • 2 prospective efficacy analyses • Primary endpoint: disease-free survival • 14 sites enrolled patients from 2000 -2007 6 months m. ITT 1 low, low-intermediate or high-intermedia high groups 2 < 8 or > 8 cycles

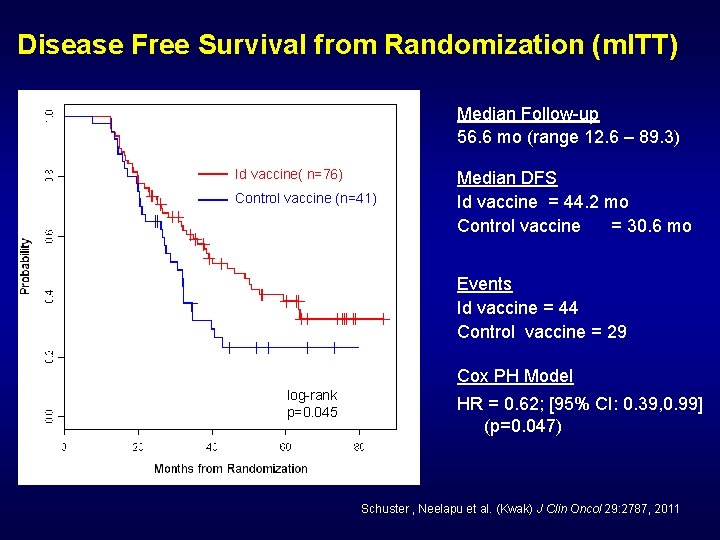
Disease Free Survival from Randomization (m. ITT) Median Follow-up 56. 6 mo (range 12. 6 – 89. 3) Id vaccine( n=76) Control vaccine (n=41) Median DFS Id vaccine = 44. 2 mo Control vaccine = 30. 6 mo Events Id vaccine = 44 Control vaccine = 29 Cox PH Model log-rank p=0. 045 HR = 0. 62; [95% CI: 0. 39, 0. 99] (p=0. 047) Schuster , Neelapu et al. (Kwak) J Clin Oncol 29: 2787, 2011

Disease Free Survival for Patients with Ig. M-isotype lymphomas: a potential predictive biomarker Median Follow-up 56. 6 mo (range 12. 6 – 89. 3) N = 60 Ig. M-Id vaccine N = 35 Control N = 25 Median DFS Ig. M-Id vaccine = 52. 9 mo [95% CI: 40. 2, NA] Control = 28. 7 mo [95% CI: 21. 0, 39. 8] Events Ig. M-Id vaccine = 17 Control = 20 Schuster , Neelapu nd et al. (Kwak) J Clin Oncol 29: 2787, 2011 52 ASH Annual Meeting, Orlando, FL

Positive Phase III trial: Potential challenges to “Delivery” • Patient accrual stopped early/treatment effect apparent only in modified ITT • requirement for biopsy and personalized manufacture (“high commercial risk”) • optimal treatment requires sustained complete remission

Future directions • Excisional biopsies serve as a rich source of residual tumor on all patients for genomic profiling/biomarkers • Additional clinical trials combining this vaccine with anti. CD 20 m. Ab (rituximab)-containing chemotherapy regimens • Making further improvements in the vaccine product (e. g. 2 nd generation DNA fusion vaccines)

2 nd generation DNA Vaccine Strategy • Maintain or improve efficacy • Reduce Manufacturing Time – For Protein Vaccines: 3 -6 months – For DNA Vaccines: 4 -5 weeks


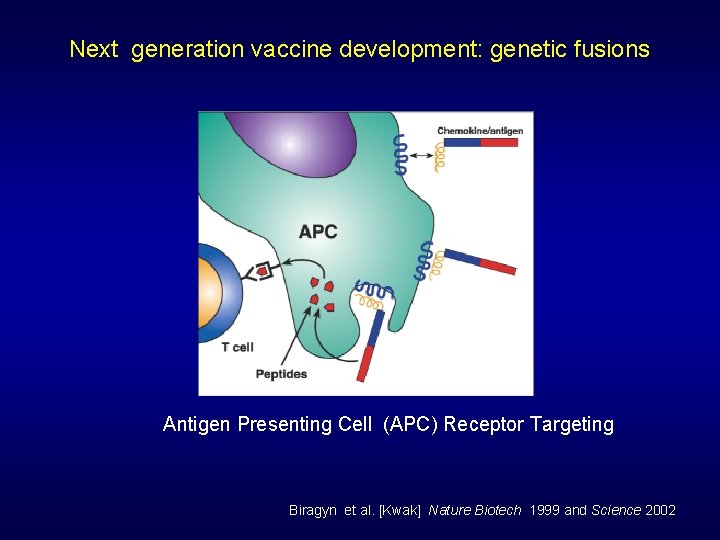
Next generation vaccine development: genetic fusions Antigen Presenting Cell (APC) Receptor Targeting Biragyn et al. [Kwak] Nature Biotech 1999 and Science 2002

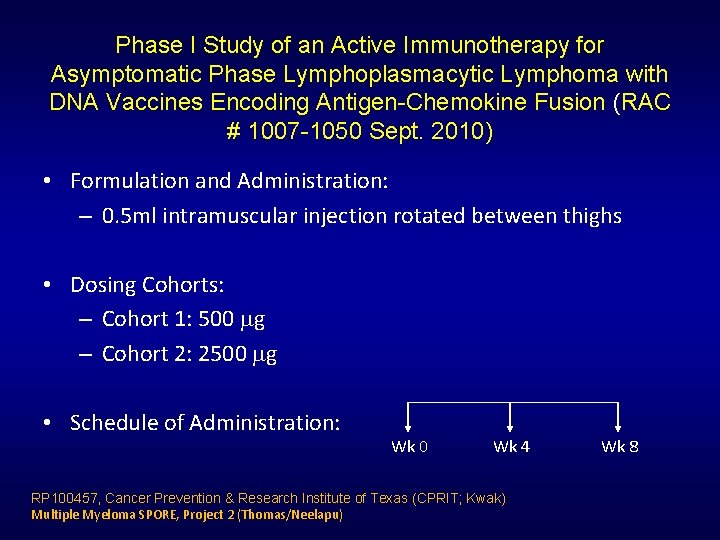
Phase I Study of an Active Immunotherapy for Asymptomatic Phase Lymphoplasmacytic Lymphoma with DNA Vaccines Encoding Antigen-Chemokine Fusion (RAC # 1007 -1050 Sept. 2010) • Formulation and Administration: – 0. 5 ml intramuscular injection rotated between thighs • Dosing Cohorts: – Cohort 1: 500 g – Cohort 2: 2500 g • Schedule of Administration: Wk 0 Wk 4 RP 100457, Cancer Prevention & Research Institute of Texas (CPRIT; Kwak) Multiple Myeloma SPORE, Project 2 (Thomas/Neelapu) Wk 8

Activated T Cell Production with Artificial APCs Cellular and Vaccine Production Facility CVPF Artificial APC: Bead Anti-CD 3 Anti-CD 28 Tc. R/CD 4 Signal 1 CD 28 CTLA 4 Signal 2 Growth J Immunol 1997; 159: 5921 Science 1997; 276: 273 Immunol. Rev. 1997; 160: 43 Mol. Ther. 2004; 9; 902 Exp. Opin. Biol. Ther. 2008; 8: 475

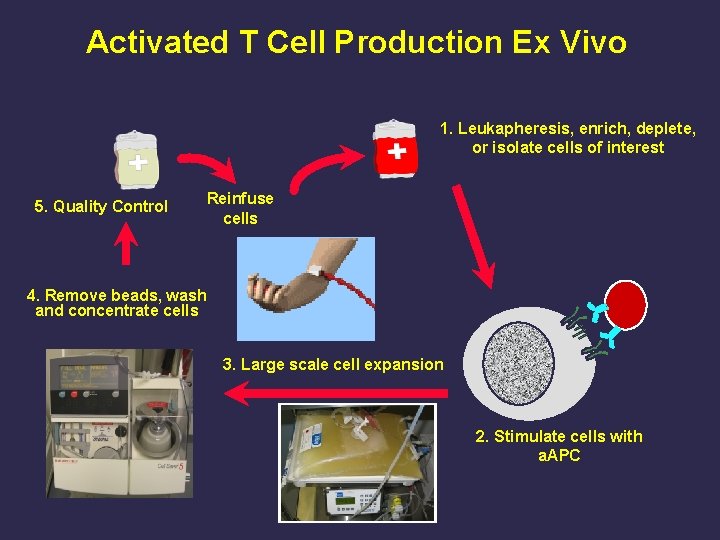
Activated T Cell Production Ex Vivo 1. Leukapheresis, enrich, deplete, or isolate cells of interest 5. Quality Control Reinfuse cells 4. Remove beads, wash and concentrate cells 3. Large scale cell expansion 2. Stimulate cells with a. APC

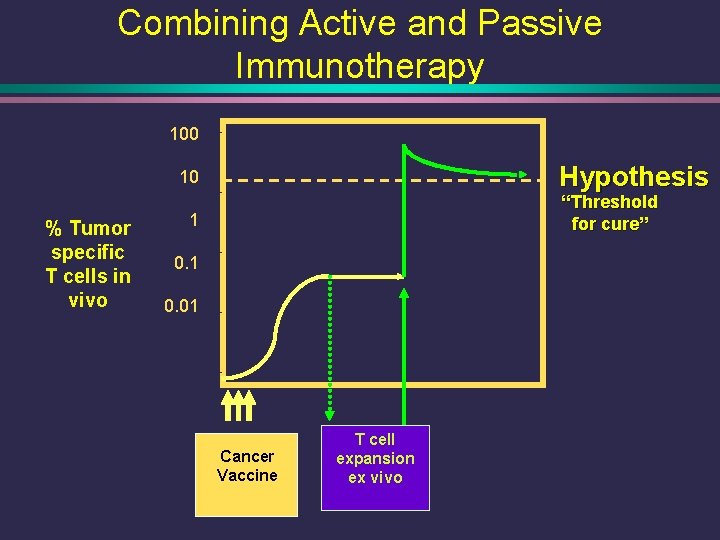
Combining Active and Passive Immunotherapy 100 Hypothesis 10 % Tumor specific T cells in vivo “Threshold for cure” 1 0. 01 Cancer Vaccine T cell expansion ex vivo

Central Hypothesis • Idiotype (Id-KLH) vaccine + the vaccine-primed adoptive T cell transfer will result in a robust Id-specific humoral and cellular response, compared to a control vaccine (KLH only)

Objectives • Primary – Whether infusions of Id-KLH primed CD 3/CD 28 activated autologous lymphocytes mediate a more intense Id-specific immunity than KLH-primed CD 3/CD 28 activated autologous lymphocytes • Secondary – To demonstrate that Id-KLH primed CD 3/CD 28 autologous lymphocytes can be infused safely and effectively in more than 80% of eligible patients – To determine whether Id-KLH primed CD 3/CD 28 activated autologous lymphocytes are as safe and as well tolerated as the KLH-primed CD 3/CD 28 activated autologous lymphocytes – To determine if the presence of Id-specific immunity correlates with disease response

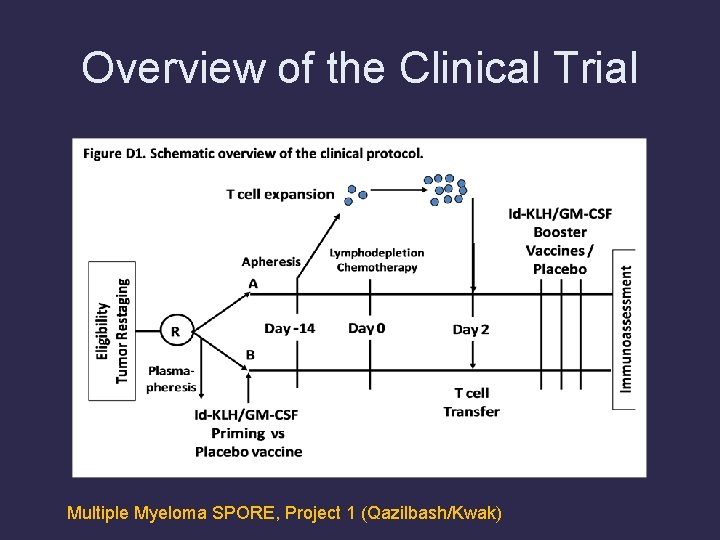
Overview of the Clinical Trial Multiple Myeloma SPORE, Project 1 (Qazilbash/Kwak)

Conclusions • Lymphoma vaccination improves disease-free survival following chemotherapy in patients already in complete remission at time of vaccination (secondary prevention) • The clinical effect of the vaccine is validated by the subgroup analysis of patients expressing the Ig. M isotype • Long-term clinical experience with the vaccine demonstrates low toxicity, making it ideal for consolidation or maintenance therapy (standard of care) • Future cancer vaccine strategies should feature combinations with adoptive T-cell therapy or immunologic checkpoint blockade

Acknowledgements Kwak Laboratory • Soung-chul Cha • Hong Qin • Sheetal Rao • Daniel Paick • Ippei Sakamaki • Flavio Baio • James Weng • Beata Lerman • Guowei Wei • Kunhwa Kim • Sung Doo Kim Center for Cancer Immunology Research Sapna Parshottam Sattva Neelapu Sheeba Thomas Dept. of SCTCT Richard Champlin Muzaffar Qazilbash EJ Shpall/Ian Mc. Niece Grant support • Leukemia & Lymphoma Society SCOR (7262 -08) • Myeloma SPORE (NCI P 50 CA 142509) • Do. D CDMRP (W 81 XWH-07 -1 -0345) • CPRIT (RP 100457)
 Rds mcas
Rds mcas How to draw a motor bike
How to draw a motor bike Delmovate
Delmovate Payroll in tally erp 9
Payroll in tally erp 9 Short term loans and advances
Short term loans and advances Global oncology trends 2017 advances complexity and cost
Global oncology trends 2017 advances complexity and cost Chapter 17 section 2 the axis advances
Chapter 17 section 2 the axis advances Advances in technology during wwii
Advances in technology during wwii Chapter 9 intellectual development of infants
Chapter 9 intellectual development of infants Advances in real time rendering
Advances in real time rendering Photodesintegration
Photodesintegration Opto-electronic advances
Opto-electronic advances Recent advances in ceramics
Recent advances in ceramics Irac guidelines
Irac guidelines Advances in memory technology
Advances in memory technology Formuö
Formuö Typiska drag för en novell
Typiska drag för en novell Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Returpilarna
Returpilarna Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Personalliggare bygg undantag
Personalliggare bygg undantag Tidbok
Tidbok A gastrica
A gastrica Densitet vatten
Densitet vatten Datorkunskap för nybörjare
Datorkunskap för nybörjare

















































