Zoster Vaccine A New Vaccine for Preventing Herpes






















- Slides: 39

Zoster Vaccine: A New Vaccine for Preventing Herpes Zoster and Post-herpetic Neuralgia Jane Seward, MBBS, MPH Division Viral Diseases (proposed) National Center Immunization & Respiratory Diseases (proposed) Centers for Disease Control and Prevention 41 st National Immunization Conference Kansas City, March 5 th, 2007

Acknowledgements • Centers for Disease Control and Prevention • Food and Drug Administration • Advisory Committee on Immunization Practices (ACIP) – Herpes Zoster ACIP working group • Drs Michael Oxman and Ken Schmader

Outline • Herpes zoster – Clinical manifestations and complications – Epidemiology • Zoster vaccine – Efficacy and safety – ACIP vaccine policy recommendations • Vaccine program implementation and surveillance

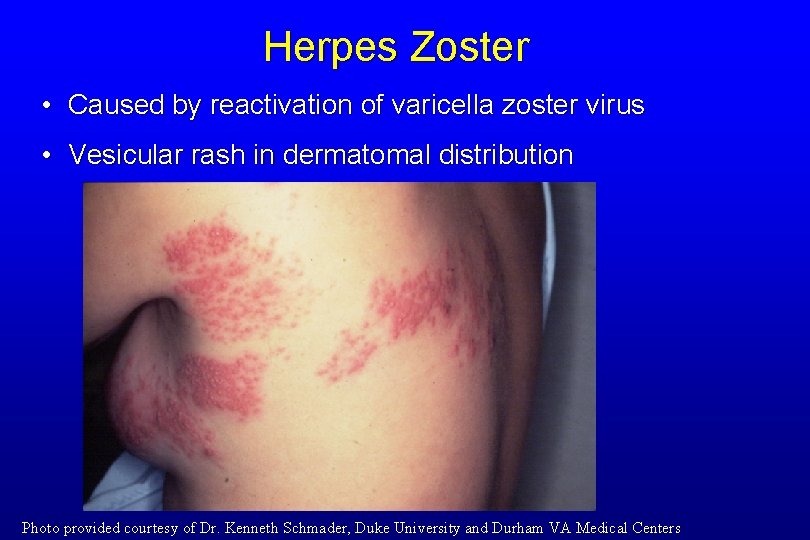
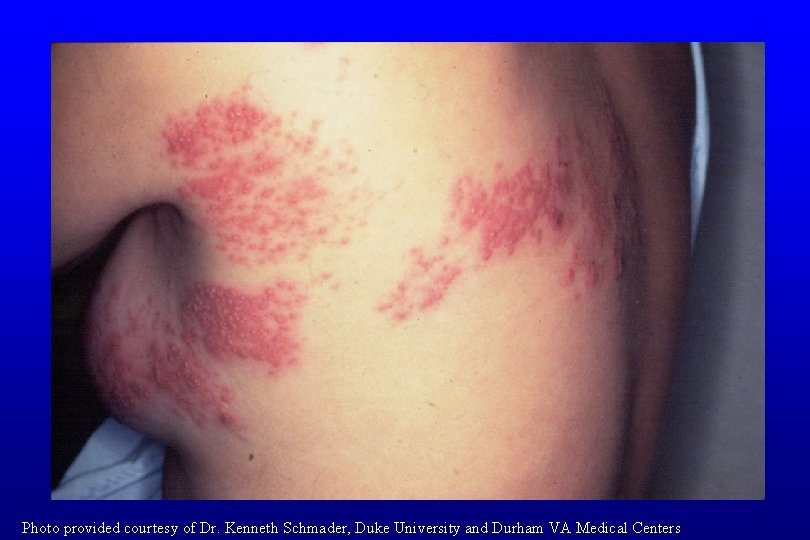
Herpes Zoster • Caused by reactivation of varicella zoster virus • Vesicular rash in dermatomal distribution Photo provided courtesy of Dr. Kenneth Schmader, Duke University and Durham VA Medical Centers


Herpes Zoster Epidemiology • Incidence – – ~ 3 -4 per 1, 000 person years May have been increasing before availability of varicella and zoster vaccines • 750, 000 to 1 million cases in U. S. annually • Lifetime risk – ~ 20% to 30% – 50% of individuals living until 85 years of age Gnann J et al. N Engl J Med. 2002; Katz J et al. Clin Infect Dis. 2004; Ragozzino M et al. Medicine 1982.

Herpes Zoster Epidemiology • Main risk factors – Age – Cellular immune deficiencies • Other risk factors – Race – Sex – Stress – Trauma

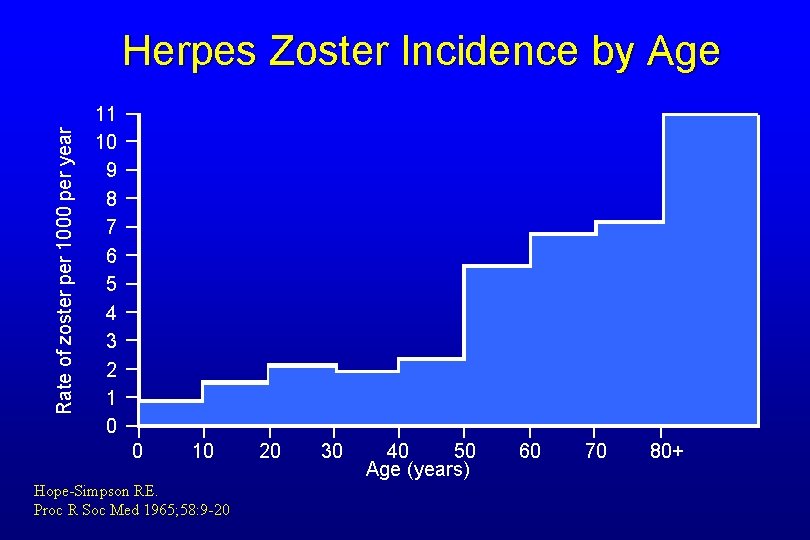
Rate of zoster per 1000 per year Herpes Zoster Incidence by Age 11 10 9 8 7 6 5 4 3 2 1 0 0 10 Hope-Simpson RE. Proc R Soc Med 1965; 58: 9 -20 20 30 40 50 Age (years) 60 70 80+

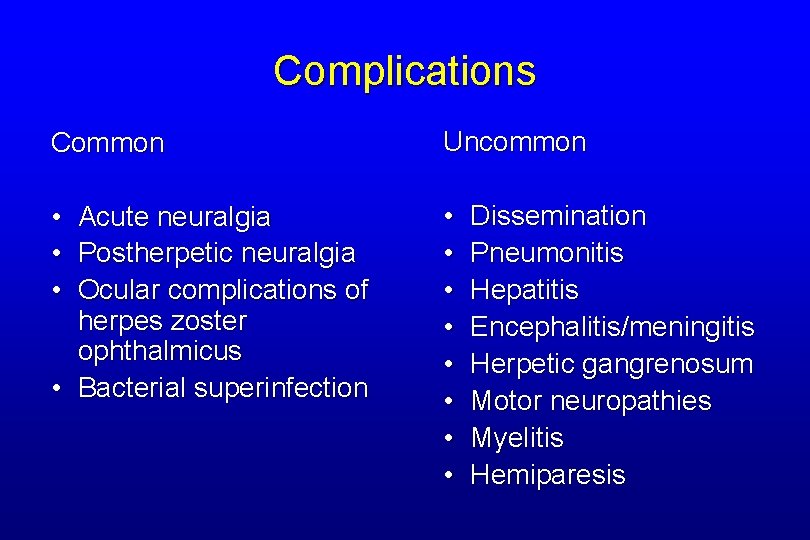
Complications Common Uncommon • • • Acute neuralgia Postherpetic neuralgia Ocular complications of herpes zoster ophthalmicus • Bacterial superinfection Dissemination Pneumonitis Hepatitis Encephalitis/meningitis Herpetic gangrenosum Motor neuropathies Myelitis Hemiparesis

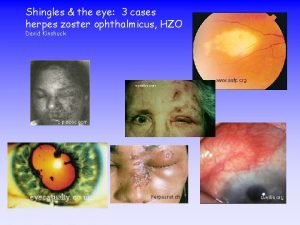
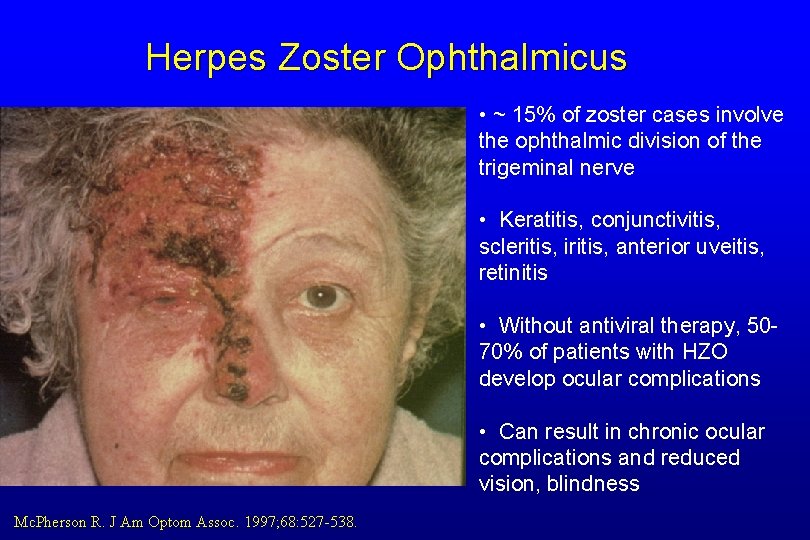
Herpes Zoster Ophthalmicus • ~ 15% of zoster cases involve the ophthalmic division of the trigeminal nerve • Keratitis, conjunctivitis, scleritis, iritis, anterior uveitis, retinitis • Without antiviral therapy, 5070% of patients with HZO develop ocular complications • Can result in chronic ocular complications and reduced vision, blindness Mc. Pherson R. J Am Optom Assoc. 1997; 68: 527 -538.

Herpes Zoster Pain • Can be excruciating (e. g. , like renal colic, childbirth) • Described as aching, burning, stabbing, shock-like • Continuous or paroxysmal • Often associated with: § Altered or painful sensitivity to touch (paresthesia, dysesthesia) § Provoked by trivial stimuli like bed sheets or breeze (allodynia) § Exaggerated, prolonged response to pain (hyperesthesia) § Unbearable itching

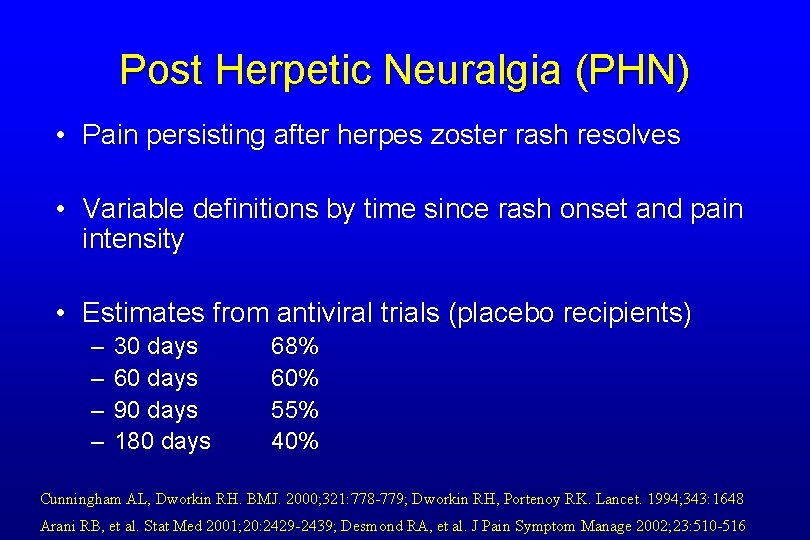
Post Herpetic Neuralgia (PHN) • Pain persisting after herpes zoster rash resolves • Variable definitions by time since rash onset and pain intensity • Estimates from antiviral trials (placebo recipients) – – 30 days 60 days 90 days 180 days 68% 60% 55% 40% Cunningham AL, Dworkin RH. BMJ. 2000; 321: 778 -779; Dworkin RH, Portenoy RK. Lancet. 1994; 343: 1648 Arani RB, et al. Stat Med 2001; 20: 2429 -2439; Desmond RA, et al. J Pain Symptom Manage 2002; 23: 510 -516

Risk Factors for Postherpetic Neuralgia • • • Age Severity of acute pain Severity of acute rash Painful prodrome Female sex Jung BF. Neurology. 2004; 62: 1545 -1551.

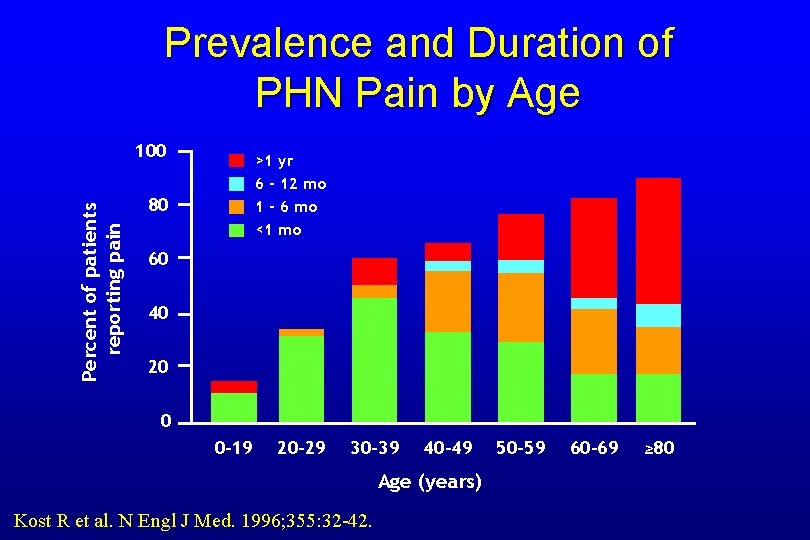
Prevalence and Duration of PHN Pain by Age Percent of patients reporting pain 100 >1 yr 6 - 12 mo 1 - 6 mo <1 mo 80 60 40 20 0 0 -19 20 -29 30 -39 40 -49 Age (years) Kost R et al. N Engl J Med. 1996; 355: 32 -42. 50 -59 60 -69 ≥ 80

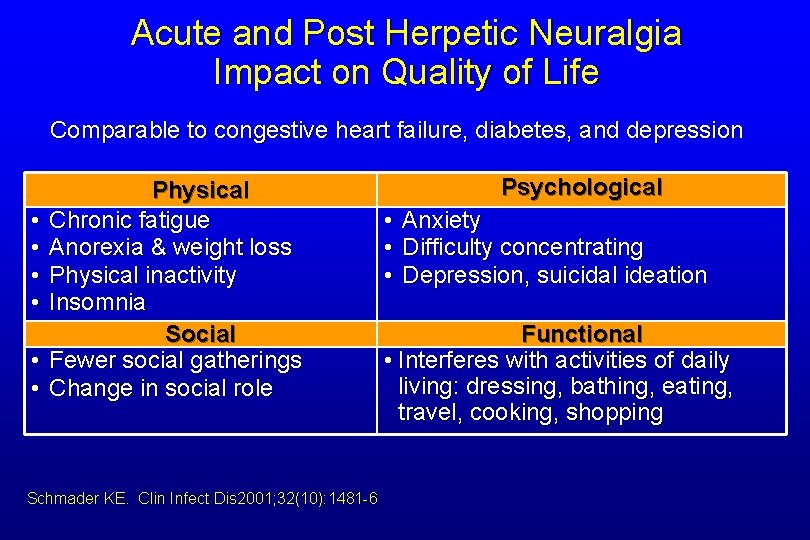
Acute and Post Herpetic Neuralgia Impact on Quality of Life Comparable to congestive heart failure, diabetes, and depression • • • Physical Chronic fatigue Anorexia & weight loss Physical inactivity Insomnia Social Fewer social gatherings Change in social role Schmader KE. Clin Infect Dis 2001; 32(10): 1481 -6 Psychological • • • Anxiety Difficulty concentrating Depression, suicidal ideation Functional • Interferes with activities of daily living: dressing, bathing, eating, travel, cooking, shopping

Zoster Vaccine • ZOSTAVAX®, Merck and Co. , Inc • Licensed by FDA in May 2006 • Live, attenuated Oka/Merck VZV vaccine • Minimum dose 19, 400 PFU at expiration [varicella vaccine 1, 350 PFU at expiration] [varicella vaccine

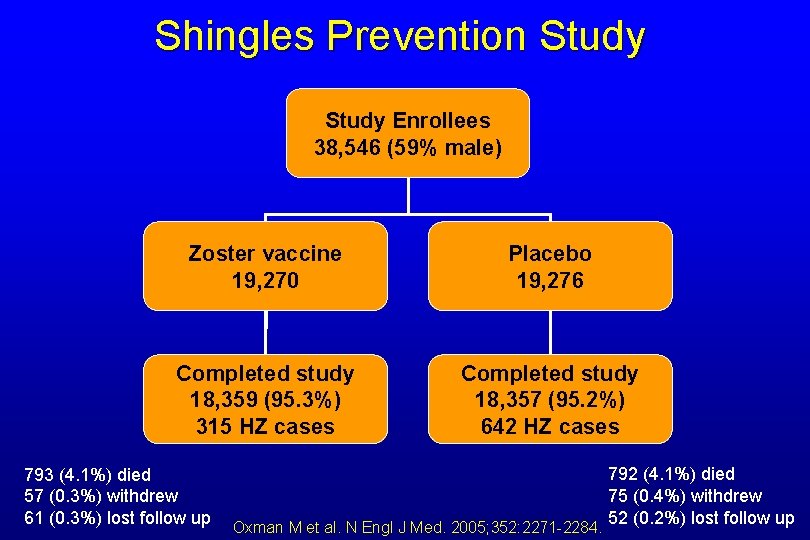
Shingles Prevention Study • • • Randomized, double blind placebo-controlled trial 38, 546 adults ≥ 60 years at 22 study sites Study end points – Primary: Burden of illness (Sum of HZ severity of illness scores ~ pain level and duration) – Secondary: Post herpetic neuralgia (PHN) and HZ Incidence • Prospective, active follow up median 3. 1 years • 94% cases laboratory confirmed Oxman M et al. N Engl J Med. 2005; 352: 2271 -2284.

Shingles Prevention Study Enrollees 38, 546 (59% male) Zoster vaccine 19, 270 Placebo 19, 276 Completed study 18, 359 (95. 3%) 315 HZ cases Completed study 18, 357 (95. 2%) 642 HZ cases 793 (4. 1%) died 57 (0. 3%) withdrew 61 (0. 3%) lost follow up Oxman M et al. N Engl J Med. 2005; 352: 2271 -2284. 792 (4. 1%) died 75 (0. 4%) withdrew 52 (0. 2%) lost follow up

Results

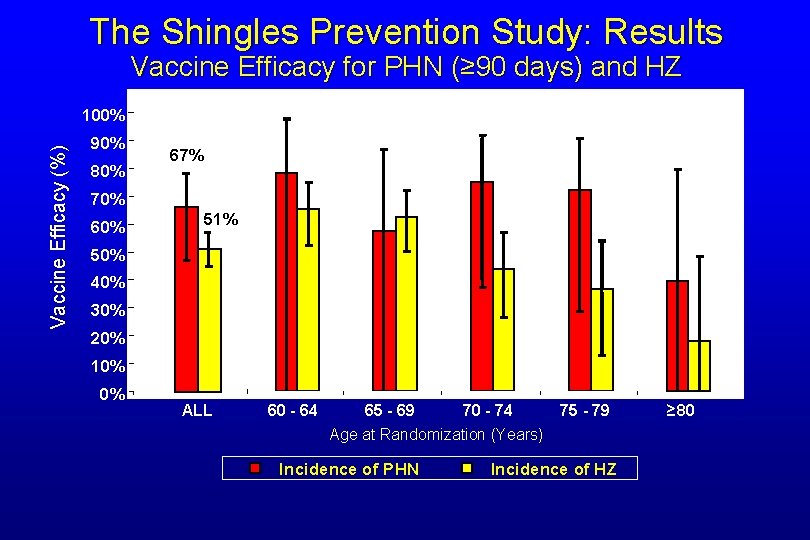
The Shingles Prevention Study: Results Vaccine Efficacy for PHN (≥ 90 days) and HZ Vaccine Efficacy (%) 100% 90% 80% 67% 70% 60% 51% 50% 40% 30% 20% 10% 0% ALL 60 - 64 65 - 69 70 - 74 75 - 79 Age at Randomization (Years) Incidence of PHN Incidence of HZ ≥ 80

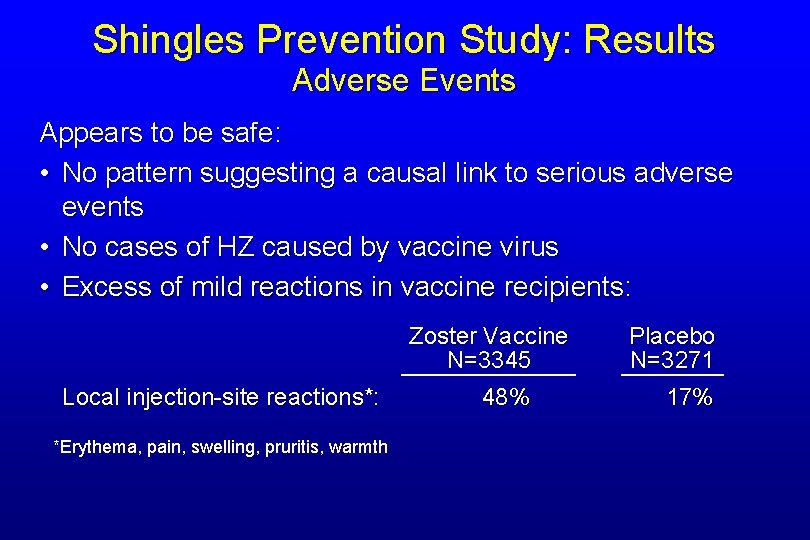
Shingles Prevention Study: Results Adverse Events Appears to be safe: • No pattern suggesting a causal link to serious adverse events • No cases of HZ caused by vaccine virus • Excess of mild reactions in vaccine recipients: Zoster Vaccine N=3345 Local injection-site reactions*: *Erythema, pain, swelling, pruritis, warmth 48% Placebo N=3271 17%

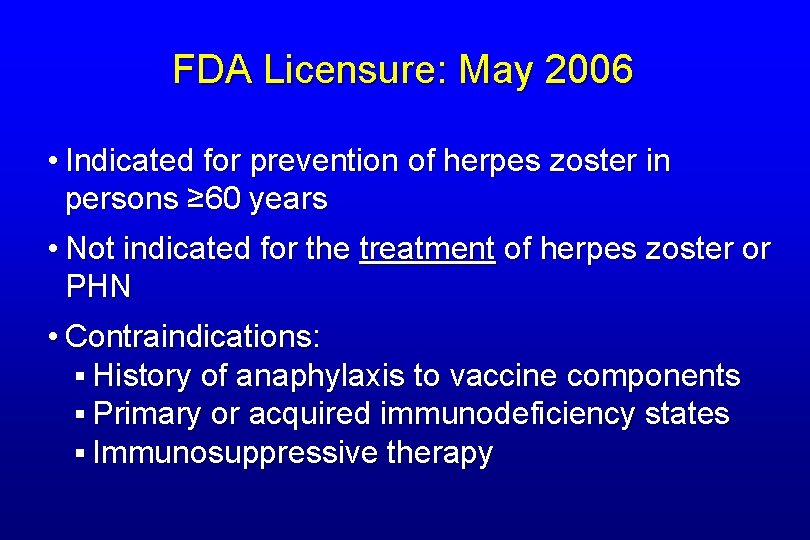
FDA Licensure: May 2006 • Indicated for prevention of herpes zoster in persons ≥ 60 years • Not indicated for the treatment of herpes zoster or PHN • Contraindications: § History of anaphylaxis to vaccine components § Primary or acquired immunodeficiency states § Immunosuppressive therapy

Zoster Vaccine Policy Recommendations Advisory Committee Immunization Practices • Recommended for adults 60 years of age and older whether or not they report a prior episode of herpes zoster • Persons with chronic medical conditions may be vaccinated unless a contraindication or precaution exists for their condition • Provisional recommendations available at http: //www. cdc. gov/nip/recs/provisional_recs/zoster-11 -20 -06. pdf

Vaccine Cost and Re-imbursement • • • Wholesale acquisition price = $152 Average wholesale price - $190. 60 CDC contract price = $107. 90 • Covered under Medicare part D • Will be covered under prevention services by private insurers • Merck financial assistance program

Vaccine Storage and Handling • Lyophilized preparation stored frozen at -15º C (+5ºF) or colder • Cannot be stored in refrigerator for any length of time • Reconstituted with diluent provided • Discard if not administered within 30 minutes

Monitoring Implementation and Impact of Vaccine Program

Post Licensure Surveillance Vaccine • Coverage - considering options for using – National data sources e. g. NHIS, Medicare – Modifying NIS [NAIS] • Safety – challenges with age group and co-existing medical conditions – VAERS – VSD rapid cycle analysis – Laboratory testing and VZV strain identification (national. VZVlab@cdc. gov)

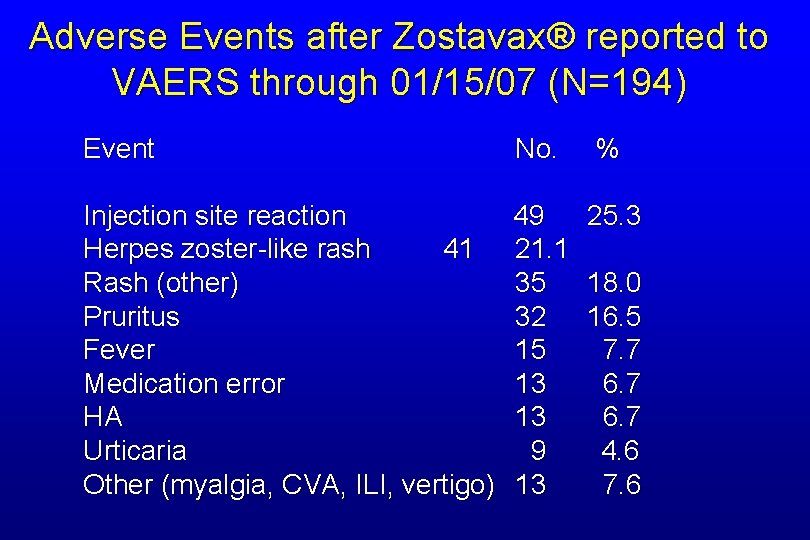
Post Licensure Safety Surveillance Reporting to VAERS through 1/15/07 • 194 reports, 2 serious • Median age = 65 years • Median interval from vaccination to symptom onset = 1 day

Adverse Events after Zostavax® reported to VAERS through 01/15/07 (N=194) Event No. % Injection site reaction 49 25. 3 Herpes zoster-like rash 41 21. 1 Rash (other) 35 18. 0 Pruritus 32 16. 5 Fever 15 7. 7 Medication error 13 6. 7 HA 13 6. 7 Urticaria 9 4. 6 Other (myalgia, CVA, ILI, vertigo) 13 7. 6

Post Licensure Surveillance Disease and Vaccine Effectiveness Herpes Zoster +/- PHN • National data sources e. g. NHIS, Medicare, Med. Stat • Varicella and HZ active surveillance sites Vaccine effectiveness in preventing HZ, PHN • National data sources – Medicare • Active surveillance sites: case control study

Summary • Herpes zoster causes considerable morbidity in elderly persons • Herpes zoster and its complications may now be prevented or modified by vaccination • Persons ≥ 60 years (and their health care providers) should be educated about HZ and offered vaccine

Thank You

Duration of Protection?


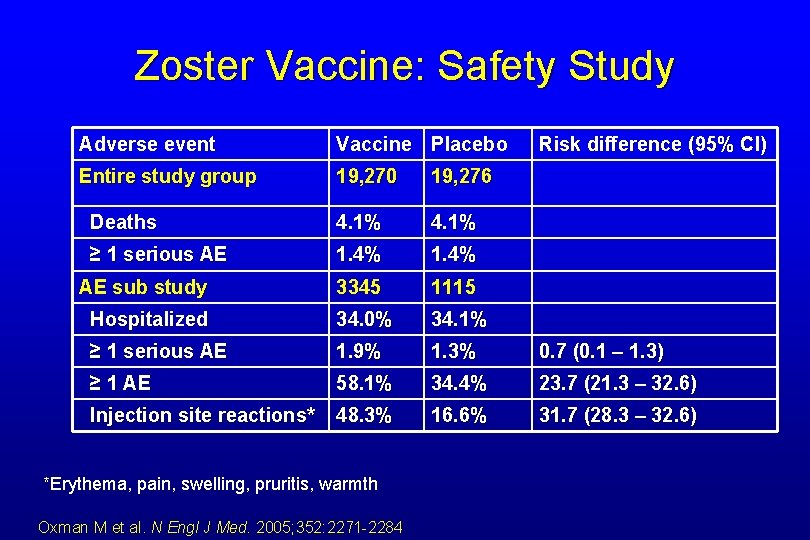
Zoster Vaccine: Safety Study • No cases of herpes zoster caused by vaccine virus among 919 PCR-confirmed cases tested • Vaccine did not induce herpes zoster – During the 30 days postvaccination • Placebo group → 18 cases • Vaccine group → 6 cases Oxman M et al. N Engl J Med. 2005; 352: 2271 -2284

Zoster Vaccine: Safety Study Adverse event Vaccine Placebo Entire study group 19, 270 19, 276 Deaths 4. 1% ≥ 1 serious AE 1. 4% 3345 1115 Hospitalized 34. 0% 34. 1% ≥ 1 serious AE 1. 9% 1. 3% 0. 7 (0. 1 – 1. 3) ≥ 1 AE 58. 1% 34. 4% 23. 7 (21. 3 – 32. 6) Injection site reactions* 48. 3% 16. 6% 31. 7 (28. 3 – 32. 6) AE sub study *Erythema, pain, swelling, pruritis, warmth Oxman M et al. N Engl J Med. 2005; 352: 2271 -2284 Risk difference (95% CI)

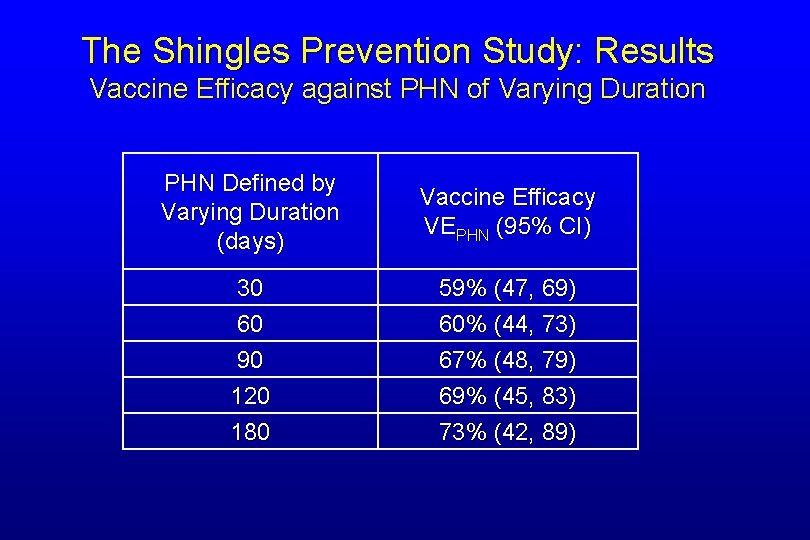
The Shingles Prevention Study: Results Vaccine Efficacy against PHN of Varying Duration PHN Defined by Varying Duration (days) Vaccine Efficacy VEPHN (95% CI) 30 60 90 120 180 59% (47, 69) 60% (44, 73) 67% (48, 79) 69% (45, 83) 73% (42, 89)

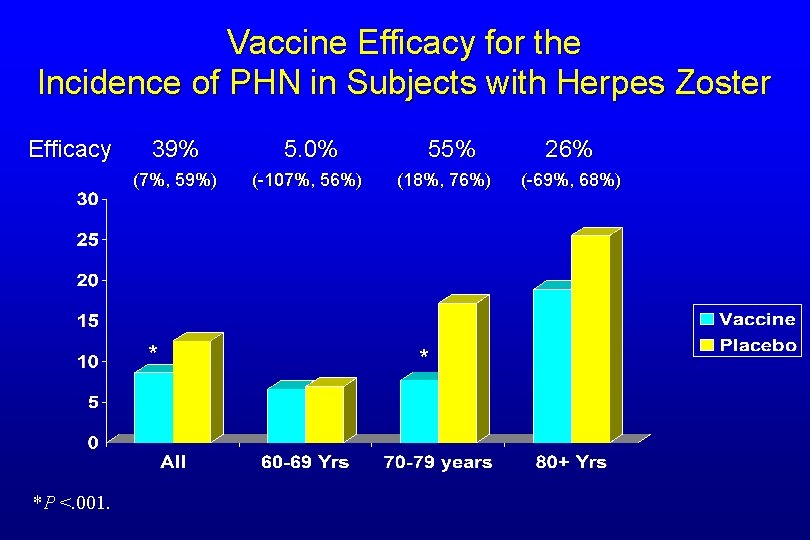
Vaccine Efficacy for the Incidence of PHN in Subjects with Herpes Zoster Efficacy 39% 5. 0% 55% 26% (7%, 59%) (-107%, 56%) (18%, 76%) (-69%, 68%) * *P <. 001. *

Photo provided courtesy of Dr. Kenneth Schmader, Duke University and Durham VA Medical Centers
 Herpes zoster infection
Herpes zoster infection How to mix shingrix vaccine
How to mix shingrix vaccine Herpes zoster
Herpes zoster Herpes zoster clasificacion
Herpes zoster clasificacion Herpes zoster
Herpes zoster Herpes zoster cicatriz
Herpes zoster cicatriz Tmp smx dosis pediatrica
Tmp smx dosis pediatrica Zoster eye disease study
Zoster eye disease study Zoster eye disease study
Zoster eye disease study Chapter 16 preventing infection
Chapter 16 preventing infection Chapter 13:2 preventing accidents and injuries
Chapter 13:2 preventing accidents and injuries Chapter 24 lesson 2 preventing and treating stds
Chapter 24 lesson 2 preventing and treating stds Preventing cuts
Preventing cuts Chapter 14:1 using body mechanics
Chapter 14:1 using body mechanics Chapter 4 preventing injuries through fitness
Chapter 4 preventing injuries through fitness Chapter 9 lesson 2 resolving conflicts
Chapter 9 lesson 2 resolving conflicts Preventing ageing unequally
Preventing ageing unequally How does robert vischer's theory prevent discrimination
How does robert vischer's theory prevent discrimination Preventing kitchen accidents worksheet
Preventing kitchen accidents worksheet Chapter 15 preventing infection
Chapter 15 preventing infection Chapter 20 preventing kitchen accidents
Chapter 20 preventing kitchen accidents Which is mainly responsible for preventing erosion
Which is mainly responsible for preventing erosion Chapter 9 resolving conflicts and preventing violence
Chapter 9 resolving conflicts and preventing violence Preventing hand injuries
Preventing hand injuries Infeccion de herpes
Infeccion de herpes Herpes genitalis gravid
Herpes genitalis gravid Herpes latency
Herpes latency Faringite
Faringite Tempat untuk memproduksi ovum
Tempat untuk memproduksi ovum Genial herpes
Genial herpes Cheilita angulara
Cheilita angulara Gonorrea
Gonorrea Klamydia syntom
Klamydia syntom Vezicula
Vezicula Historia natural de la enfermedad herpes genital
Historia natural de la enfermedad herpes genital Equine rhinopneumonitis
Equine rhinopneumonitis Human herpesvirus 2
Human herpesvirus 2 Herpes rugbiorum
Herpes rugbiorum Corrimento amarelo
Corrimento amarelo Herpes genital glande
Herpes genital glande