HPV Infection and Cervical Cancer Introduction Human papillomavirus

































- Slides: 43

HPV Infection and Cervical Cancer


Introduction Human papillomavirus (HPV) is the most common sexually transmitted infection in the United States; an estimated 14 million persons are newly infected every year Although most infections cause no symptoms and are selflimited, persistent HPV infection cause cervical cancer in women as well as other anogenital cancers, oropharyngeal cancer, and genital warts in men and women. Persistent oncogenic HPV infection is the strongest risk factor for development of HPV-associated precancers and cancers.

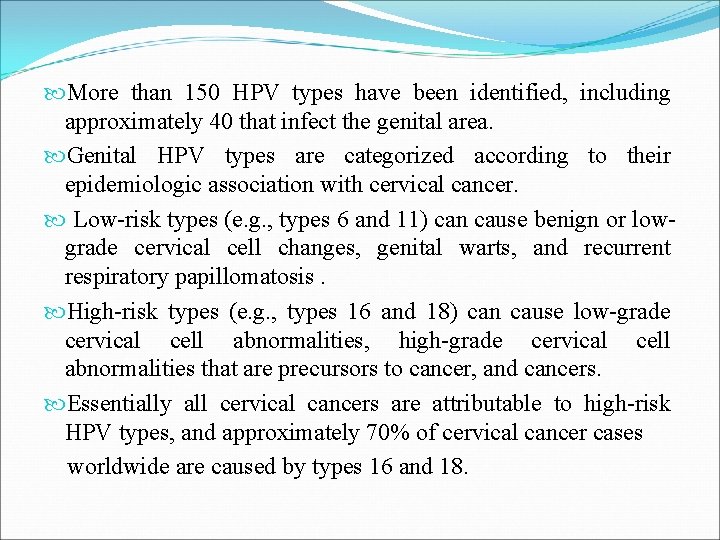
More than 150 HPV types have been identified, including approximately 40 that infect the genital area. Genital HPV types are categorized according to their epidemiologic association with cervical cancer. Low-risk types (e. g. , types 6 and 11) can cause benign or lowgrade cervical cell changes, genital warts, and recurrent respiratory papillomatosis. High-risk types (e. g. , types 16 and 18) can cause low-grade cervical cell abnormalities, high-grade cervical cell abnormalities that are precursors to cancer, and cancers. Essentially all cervical cancers are attributable to high-risk HPV types, and approximately 70% of cervical cancer cases worldwide are caused by types 16 and 18.

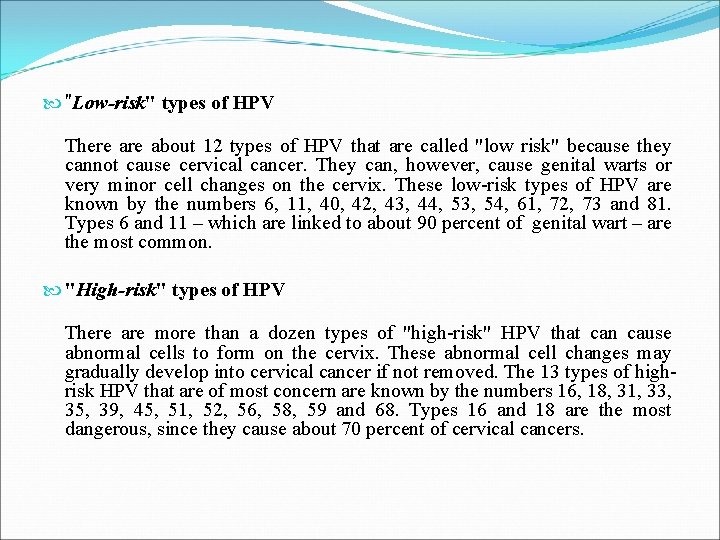
"Low-risk" types of HPV There about 12 types of HPV that are called "low risk" because they cannot cause cervical cancer. They can, however, cause genital warts or very minor cell changes on the cervix. These low-risk types of HPV are known by the numbers 6, 11, 40, 42, 43, 44, 53, 54, 61, 72, 73 and 81. Types 6 and 11 – which are linked to about 90 percent of genital wart – are the most common. "High-risk" types of HPV There are more than a dozen types of "high-risk" HPV that can cause abnormal cells to form on the cervix. These abnormal cell changes may gradually develop into cervical cancer if not removed. The 13 types of highrisk HPV that are of most concern are known by the numbers 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68. Types 16 and 18 are the most dangerous, since they cause about 70 percent of cervical cancers.

In addition to cervical cancer, HPV infection also is the cause of some other anogenital cancers such as cancer of the vulva, vagina, penis, and anus, as well as cancer of the oropharynx, esophagus and larynx.

HPV-Associated Oropharyngeal Cancers Prevalence increased from 16. 3% (1984 -89) to 71. 7% (200004) Population-level incidence of HPV-positive cancers increased by 225% while HPV-negative cancers declined by 50% If trends continue, the annual number of HPV-positive oropharyngeal cancers is expected to surpass the annual number of cervical cancers by the year 2020 Chaturvedi, 2011, J Clin Oncol- data from SEER

HPV and Cervical Cancer The only HPV-associated cancer for which screening is recommended is cervical cancer. Cervical cancer screening is based on exfoliated cytology (Pap test).

Transmission Genital HPV infection is transmitted primarily by genital contact, usually through sexual intercourse but also through other intimate contact (e. g. , oral-genital or genital). Intercourse is not necessary for infection Nonsexual routes of genital HPV transmission are less common and can include intrapartum transmission from mother to infant

In virtually all studies of HPV prevalence and incidence, the most consistent predictors of infection have been measures of sexual activity, most importantly the number of sex partners (lifetime and recent). Transmission is very common between sex partners, and likely more frequent from females to males than from males to females.

Most HPV infections are transient and asymptomatic and cause no clinical problems; 70% of persons with new cervical HPV infection will clear the infection within 1 year, and approximately 90% within 2 years. The median duration of new infections is about 8 months for genital infection among both females and males. The risk for persistence and progression to cancer precursor lesions varies by HPV type as well as host factors (including those with HIV infection). HPV 16 is more likely to persist and progress to cancer than other high-risk HPV types. The usual time between initial HPV infection and development of cervical cancer is decades but more rapid progression has occurred. Women persistently infected with high risk HPV strains are at risk of the development of high grade cervical intraepithelial neoplasia (CIN) and invasive cervical cancer (CC).

Studies have shown that the virus does not "ping-pong" back and forth. This means that a couple cannot re-infect each other within their own relationship. After exposure to HPV and subsequent clearance of the virus, a person probably has immunity to that type. He or she cannot be re-infected, but could be exposed to a new type of HPV. If a person enters a new relationship, he or she could put a new partner at risk for HPV. A person can be exposed to a new viral type of HPV with a new partner.

Can a person be re-infected with HPV? There appears to be humoral and probably cellular immunity that develops to a specific type of HPV after a person has been infected with it and “has cleared” it. However, a person can be infected with more than one type of HPV. The risk for re-infection with that specific type of HPV appears is rare.

Re-detection of the same HPV type is relatively common, occurring in at least 10– 20% of women observed to have “cleared” the virus. Furthermore, convincing data from multiple studies of immune compromised, sexually abstinent, older, less sexually active populations and adolescents with longterm intensive follow-up support the phenomena of immunologically controlled re-detection or reactivation of a previously acquired type-specific HPV infection. An important consideration, however, is that reactivation of previously acquired, latent HPV infection, rather than re-infection, may explain the apparent lack of protection against re-infection from natural antibodies in the older age group. These results suggest that, in the presence of naturally acquired antibodies, reactivation or intermittent viral shedding of a previously acquired infection is a more probable source of new HPV detection than new acquisition.


No HPV test can determine which HPV infection will clear and which will progress. Having HPV does not mean that a person or his/her partner is having sex outside the relationship. Having HPV does not make it harder for a woman to get pregnant or carry a pregnancy to term. However, some of the precancers or cancers that HPV can cause, and the treatments needed to treat them, might lower a woman’s ability to get pregnant or have an uncomplicated delivery. Treatments are available for the conditions caused by HPV, but not for the virus itself.

HPV is found in virgins Study examined the frequency of vaginal HPV and the association with non-coital sexual behavior in longitudinally followed cohort of adolescent women without prior vaginal intercourse HPV was detected in 46% of women prior to first vaginal sex 70% of these women reported non-coital behaviors that may in part explain genital transmission Shew, J Infect Dis. 2012

Wart The average incubation period of visible warts is 3 weeks to one year. Latency periods of years have been reported before the emergence of warts or cervical abnormalities. This can be quite confusing and stressful for partners in long term relationships. An example of this is the person whose immune system, on its own, keeps HPV under control or latent for so many years and then a long-delayed symptom may appear which seems to come from nowhere.

Women with genital warts do not need Pap tests more often than other women. Condoms might lower the chances of transmitting genital warts if used consistently and correctly; however, HPV can infect areas that are not covered by a condom and might not fully protect against HPV. Time of HPV acquisition cannot be definitively determined. Genital warts can develop months or years after getting HPV types that cause genital warts can be passed on to another person even in the absence of visible signs of warts. Sex partners tend to share HPV, even though signs of HPV (e. g. , warts) might occur in only one partner or in neither partner. Although genital warts can be treated, such treatment does not cure the virus itself. For this reason, it is common for genital warts to recur after treatment, especially in the first 3 months

Because genital warts can be sexually transmitted, patients with genital warts benefit from testing for other STDs. Sexual activity should be avoided with new partners until the warts are gone or removed. HPV might remain present and can still be transmitted to partners even after the warts are gone. Virus can remain in surrounding tissue after warts have been destroyed.

Updates on Pap Smear Guidelines 2015

Screening Today When to start? When to stop? How often? HR HPV testing Continue in patients after hysterectomy? No change in women vaccinated against HPV

Possible harms of screening Anxiety over a positive test Stigma of an STI Pain/bleeding from procedures Number of colposcopies is a marker for harms

Principles in Interpreting Guidelines Current strategies cannot eliminate the risk of developing cancer. No screening test has 100% sensitivity. Attempts to eliminate often result in unanticipated harm from excessive evaluation and treatment.

Cervical cancer screening should begin at age 21 Women <21 should not be screened regardless of age of sexual contact. Prevalence of oncogenic HPV types are high among adolescents aged <21 years, and oncogenic HPV and squamous intraepithelial lesions caused by HPV in adolescent girls are more likely to regress than those in older women.

Screening 21 - 29 Every 3 years Co-testing with HPV should NOT be performed (HPV frequent in this population and >90% will spontaneously clear within 2 yrs)

Screening 30 - 65 Every 5 yrs Co-testing of HPV 16/18


Screening a vaccinated cohort Recommended screening practices should not change on the basis of HPV vaccination. Vaccination against HPV 16/18 Reduces CIN 3 by 17 -33% Reduces colposcopy by 10% Reduces treatment by 25%


HPV Vaccines Currently, three HPV vaccines are available that can prevent the most common types of HPV infection, including bivalent Cervarix, targeting HPV types 16 and 18, quadrivalent Gardasil, targeting HPV 16, 18, 6 and 11 and 9 -valent (Gardasil-9), targeting HPV 31, 33, 45, 52 and 58 in addition to HPV 16, 18, 6 and 11. All 3 HPV vaccines protect against HPV types 16 and 18, which cause 70% of cervical cancers. HPV type 16 also causes the majority of other cancers attributable to HPV. Quadrivalent Gardasil also protects against HPV types 6 and 11, which cause >90% of genital warts and recurrent respiratory papillomatosis. Gardasil-9 protects against infection with the HPV types covered by Gardasil (HPV 16, 18, 6 and 11) and also protects against five other HPV types (HPV 31, 33, 45, 52 and 58).

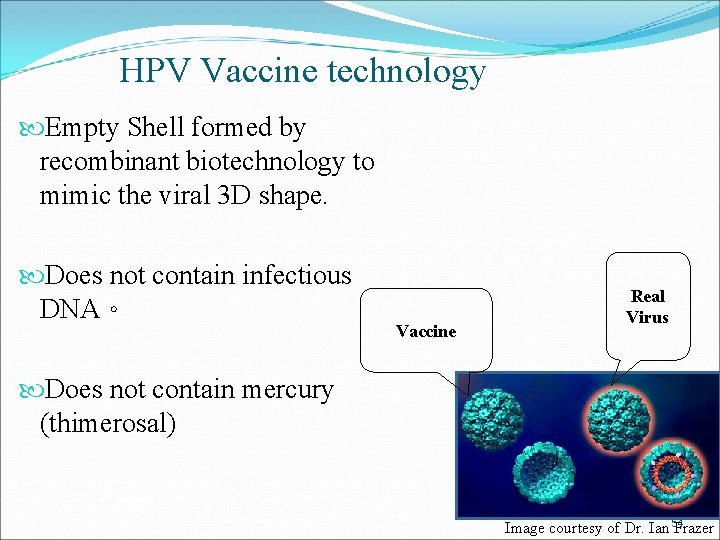
HPV Vaccine technology Empty Shell formed by recombinant biotechnology to mimic the viral 3 D shape. Does not contain infectious DNA。 Vaccine Real Virus Does not contain mercury (thimerosal) GARDASIL is a trademark of Merck & Co. , Inc. , Whitehouse Station, NJ, USA. *VLP = Virus-like particle. 1. Villa LL, Costa RL, Petta CA, et al. Lancet Oncol. 2005; 6: 271– 278. 54 Image courtesy of Dr. Ian Frazer

Vaccination Schedule and Use In October 2016, CDC recommended that healthy adolescents who initiated HPV vaccination before age 15 years should receive two doses of any HPV vaccine. The second dose of HPV vaccine should be given 6 -12 months after the first dose (0, 6 -12 month schedule). Cases that start the HPV vaccine series at ages 15 -26 years, need three doses of HPV vaccine to protect against cancer-causing HPV infections. Three dose series are still recommended for cases with immunocompromising conditions aged 9 - 26 years

The recommendations for adult immunization schedule are as follows: Adult females and males through age 26 years who have not received any HPV vaccine should receive a 3 -dose series of HPV vaccine at 0, 1– 2 and 6 months. Adult females and males through age 26 years who initiated the HPV vaccination series before age 15 years and received 2 doses at least 5 months apart are considered adequately vaccinated and do not need an additional dose of HPV vaccine. Adult females and males through age 26 years who initiated the HPV vaccination series before age 15 years and received only 1 dose or 2 doses less than 5 months apart, are not considered adequately vaccinated and should receive 1 additional dose of HPV vaccine.

Adult females and males through age 26 years with immunocompromising conditions such as HIV infection, malignant neoplasm, transplantation, autoimmune disease and immunosuppressive therapy should receive a 3 -dose series of HPV vaccine at 0, 1– 2, and 6 months. Because HPV vaccines are subunit vaccines, they can be administered to immunosuppressed subjects. However, the immune response and vaccine efficacy might be less than immunocompetent persons. Women who are breastfeeding may receive HPV vaccine.

Although routine vaccination is recommended at age 9 years, older adolescents and young adults through the recommended ages can benefit from vaccination. HPV vaccines are most effective when administered before exposure to HPV. Adolescents and young adults who are not yet sexually active can be expected to receive the full benefit of vaccination. Although sexually active persons in this age group might have been infected with one or more vaccine HPV types, studies suggest that only a small percentage have been infected with both HPV 16 and 18 or all four vaccine types.

Although vaccine effectiveness would be lower when administered to those who are sexually active, and would decrease with older age and likelihood of previous HPV exposure, the majority of persons in the recommended age groups will derive at least partial benefit from vaccination.

Duration of Protection of Vaccine Data from follow-up through 8 to 10 years showed no evidence of waning protection. (ongoing research) There is no known serologic correlative of immunity or minimum titer determined to be protective. The high efficacy found in the clinical trials to date has precluded identification of a minimum protective antibody titer. Modeling studies have shown consistently that the routine vaccination of 12 -year-old girls with either HPV 2 or HPV 4 is a costeffective use of public health resources, as long as vaccine duration of protection is sufficient (e. g. , 30 years).

How long is the duration of protection?Is booster dose needed? US CDC 2012 Feb: Gardasil vaccine information statement Protection from HPV vaccine is expected to be longlasting Additional (booster) doses are not recommended. http: //www. cdc. gov/vaccines/pubs/vis/downloads/vis-hpv-gardasil. pdf 64

HPV Vaccine Safety Most common adverse events reported-considered mild For serious adverse events, no unusual pattern or clustering that suggest events caused by HPV vaccine

Adverse Reactions Following Vaccination The HPV vaccine is very safe, and it is effective at preventing HPV. Vaccines, like any medicine, can have side effects. Many people who get the HPV vaccine have no side effects at all. Some people report having very mild side effects, like a sore arm from the shot. The most common side effects are usually mild. Common Side Effects of HPV Vaccine: Pain, redness, or swelling in the arm where the shot was given Fever Headache or feeling tired Nausea Muscle or joint pain Brief fainting spells and related symptoms (such as jerking movements) can happen after any medical procedure, including vaccination. Sitting or lying down for about 15 minutes after a vaccination can help prevent fainting and injuries caused by falls.

HPV vaccine In the United States, the vaccines are not licensed or recommended for use in men or women aged >26 years. HPV vaccines are not recommended for use in pregnant women. HPV vaccines can be administered regardless of history of anogenital warts, abnormal Pap/HPV tests, or anogenital precancer. Women who have received HPV vaccine should continue routine cervical cancer screening if they are aged ≥ 21 years. Recent results of Phase III vaccination trials have documented that vaccine efficacy among adult women (shown to age 45/55) is excellent, provided they are HPV negative at the time of vaccination. Therefore, the European Medicines Agency (EMA) extended the indication of HPV vaccine to women aged 9+ with no upper limit.

Management of Sex Partners The benefit of disclosing a positive oncogenic HPV test to current and future sex partners is unclear. The following counseling messages can be communicated to sex partners: Sex partners do not need to be tested for HPV. Sex partners tend to share HPV, even though signs of HPV such as an abnormal Pap-test result might occur in only one partner. Sex partners of persons with HPV infection also likely have HPV. When used correctly and consistently, condoms might lower the risk for HPV infection and might decrease the time to clear in women with HPV infection. However, HPV can infect areas not covered by the condom and might not fully protect against HPV.
 Hpv cervical cancer
Hpv cervical cancer Hpv cervical cancer
Hpv cervical cancer Hpv infection
Hpv infection Hpv oral cancer
Hpv oral cancer Hpv cancer prevention
Hpv cancer prevention National breast and cervical cancer early detection program
National breast and cervical cancer early detection program National breast and cervical cancer early detection program
National breast and cervical cancer early detection program Stage 4 cervical cancer
Stage 4 cervical cancer Cervical cancer hcp
Cervical cancer hcp Hpv type 16 and 18
Hpv type 16 and 18 Hpv type 16 and 18
Hpv type 16 and 18 Hpv test for men
Hpv test for men Hpv vaccine schedule adults
Hpv vaccine schedule adults Muzaffer sancı
Muzaffer sancı Ascus hpv negativ
Ascus hpv negativ Does hpv go away
Does hpv go away Hpv tipleri
Hpv tipleri Hpv infektion
Hpv infektion Bivalan opa
Bivalan opa Lsil
Lsil Hpv
Hpv Hpv nic
Hpv nic Low grade squamous intraepithelial lesion
Low grade squamous intraepithelial lesion Hpv testi nasıl yapılır
Hpv testi nasıl yapılır Hpv infektion
Hpv infektion Hpv
Hpv How to get hpv
How to get hpv Prevention hpv
Prevention hpv Hpv
Hpv Epidermal dysplasia verruciformis
Epidermal dysplasia verruciformis Ahcc hpv
Ahcc hpv Hpv discret test
Hpv discret test Hpv
Hpv 3 layers of spinal cord
3 layers of spinal cord Lumbosacral trunk
Lumbosacral trunk Spinal cord and spinal nerves exercise 15
Spinal cord and spinal nerves exercise 15 Difference between cervical thoracic and lumbar vertebrae
Difference between cervical thoracic and lumbar vertebrae Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Chapter 16 infection prevention and control
Chapter 16 infection prevention and control Chapter 16 infection control and standard precautions
Chapter 16 infection control and standard precautions Laws are also called in cosmetology
Laws are also called in cosmetology Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Cbic recertification
Cbic recertification