Heat Treatment of Metals Eng Ahmed Afeefy Eng










- Slides: 21

Heat Treatment of Metals Eng. Ahmed Afeefy Eng. Ibrahim Aljaish

Objectives • To study the effect of the cooling rate and the amount of alloying elements on the micro structure and the mechanical properties ( Rockwell hardness ) of medium carbon steel. • To know the several techniques of heat treatment.

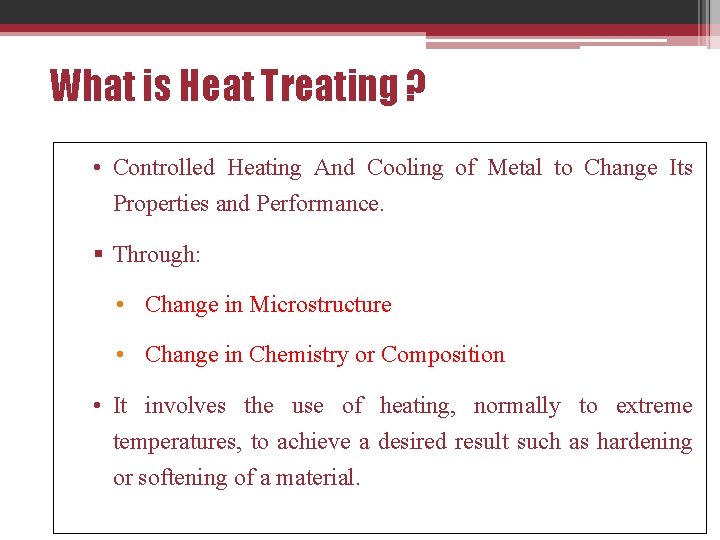
What is Heat Treating ? • Controlled Heating And Cooling of Metal to Change Its Properties and Performance. § Through: • Change in Microstructure • Change in Chemistry or Composition • It involves the use of heating, normally to extreme temperatures, to achieve a desired result such as hardening or softening of a material.

Why Heat Treat? • To improve Toughness • To increase Hardness • To increase Ductility • To improve Machinability • To refine Grain Structure • To remove Residual Stresses

Heat treatment techniques include Case Hardening q Tempering q Annealing q normalizing q Quenching q

Case Hardening • Case Hardening: is the process of hardening the surface of a metal, often a low carbon steel, by infusing elements into the material's surface, forming a thin layer of a harder alloy.

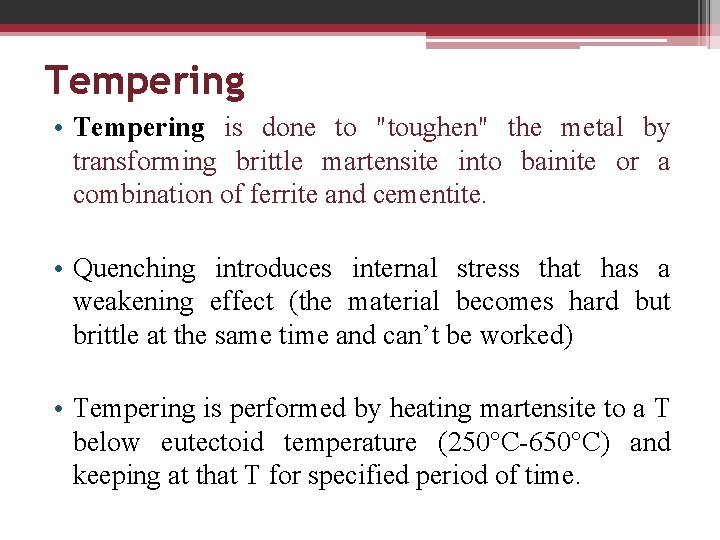
Tempering • Tempering is done to "toughen" the metal by transforming brittle martensite into bainite or a combination of ferrite and cementite. • Quenching introduces internal stress that has a weakening effect (the material becomes hard but brittle at the same time and can’t be worked) • Tempering is performed by heating martensite to a T below eutectoid temperature (250°C-650°C) and keeping at that T for specified period of time.

Annealing • Annealing: is a heat process whereby a metal is heated to a specific temperature and then allowed to cool slowly in the furnace. • The steel becomes soft and ductile depending on the pearlite (ferrite + cementite )percentage means it can be cut and shaped more easily.

• Full annealing is the process of slowly raising the temperature about 50 ºC above the Austenitic temperature line in the case of Hypoeutectoid steels (steels with < 0. 77% Carbon) and 50 ºC into the Austenite-Cementite region in the case of Hypereutectoid steels (steels with > 0. 77% Carbon). • It is held at this temperature for sufficient time for all the material to transform into Austenite or Austenite. Cementite as the case may be. It is then slowly cooled at the rate of about 20 ºC/hr in a furnace to about 50 ºC into the Ferrite-Cementite range. At this point, it can be cooled in room temperature air with natural convection.

Why Annealing? • Annealing is carried out to: q Relieve Stress q Increase softness, ductility and toughness q Produce a specific microstructure

Annealing Stages • The annealing process may be divided into three stages: 1. Recovery : the relief of residual internal stresses 2. Recrystallization : there is a significant drop in tensile strength, hardness and a large increase in the ductility of the material during recrystallization. 3. Grain growth : the tensile strength and hardness continue to decrease but at a much less rate than the recrystallization stage. The major change observed during this stage is the growth of the grain boundaries and reaching the original grain size.

Process Annealing (not required in the exam) • It is used to treat work-hardened parts made out of low-Carbon steels (< 0. 25% Carbon). This allows the parts to be soft enough to undergo further cold working without fracturing. • Process annealing is done by raising the temperature to just below the Ferrite-Austenite region line on the diagram. This temperature is about 727 ºC so heating it to about 700 ºC should suffice. This is held long enough to allow recrystallization of the ferrite phase, and then cooled in still air.

• Since the material stays in the same phase through out the process, the only change that occurs is the size, shape and distribution of the grain structure. This process is cheaper than either full annealing or normalizing since the material is not heated to a very high temperature or cooled in a furnace.

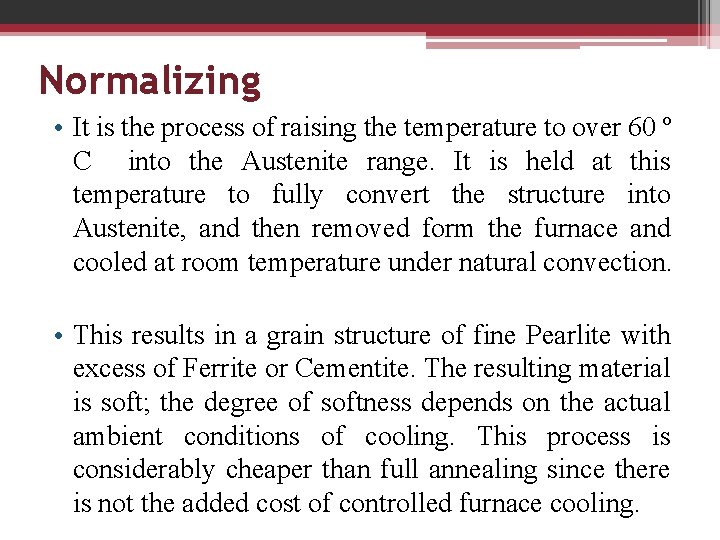
Normalizing • It is the process of raising the temperature to over 60 º C into the Austenite range. It is held at this temperature to fully convert the structure into Austenite, and then removed form the furnace and cooled at room temperature under natural convection. • This results in a grain structure of fine Pearlite with excess of Ferrite or Cementite. The resulting material is soft; the degree of softness depends on the actual ambient conditions of cooling. This process is considerably cheaper than full annealing since there is not the added cost of controlled furnace cooling.

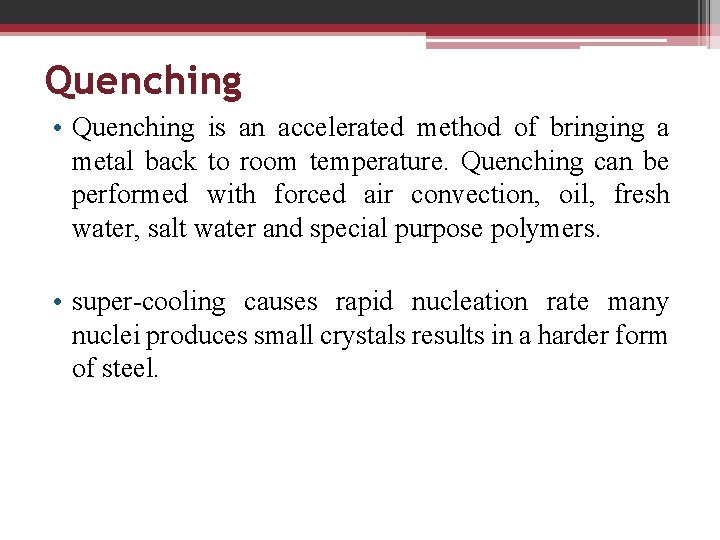
Quenching • Quenching is an accelerated method of bringing a metal back to room temperature. Quenching can be performed with forced air convection, oil, fresh water, salt water and special purpose polymers. • super-cooling causes rapid nucleation rate many nuclei produces small crystals results in a harder form of steel.

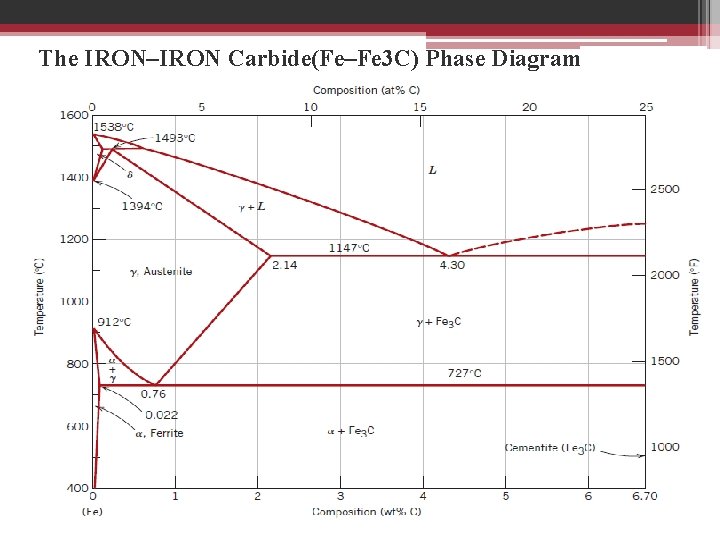
• Ferrite ▫ Known as α-iron ▫ Pure iron at room temperature ▫ Body-centered cubic structure BCC. • Austenite ▫ ▫ Known as γ-iron Face-centered cubic FCC. Much softer than ferrite More easily worked • Pearlite (Perlite) ▫ Most common constituent of steel ▫ Gives steel most of its strength ▫ layered mixture of ferrite and cementite • Cementite(fe 3 c) ▫ Hard, brittle, white ▫ On the phase diagram, cementite corresponds to a vertical line at 6. 7% C

The IRON–IRON Carbide(Fe–Fe 3 C) Phase Diagram

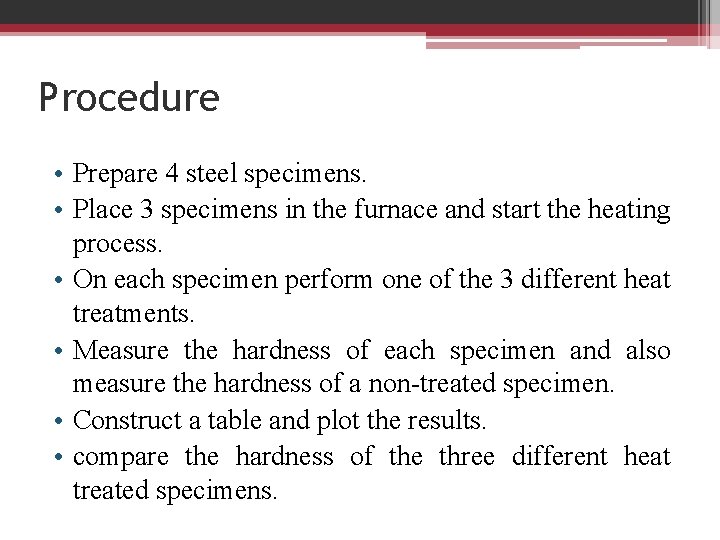
Procedure • Prepare 4 steel specimens. • Place 3 specimens in the furnace and start the heating process. • On each specimen perform one of the 3 different heat treatments. • Measure the hardness of each specimen and also measure the hardness of a non-treated specimen. • Construct a table and plot the results. • compare the hardness of the three different heat treated specimens.

Results • Brinelle hardness test for the 4 specimen

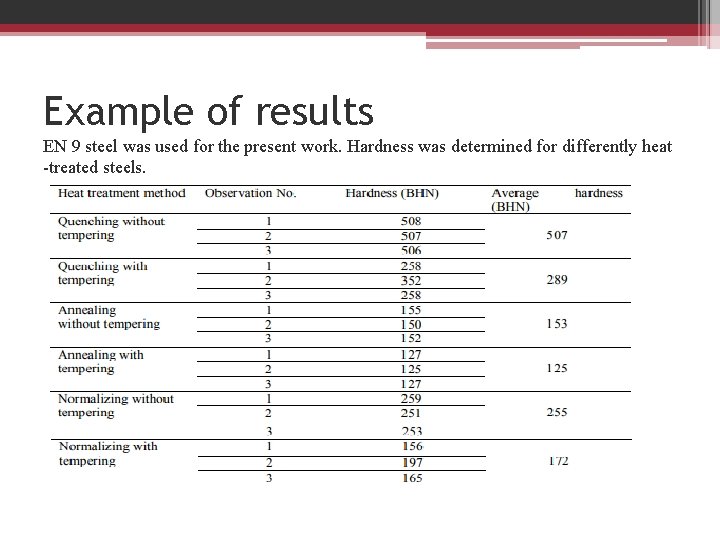
Example of results EN 9 steel was used for the present work. Hardness was determined for differently heat -treated steels.

references • An Experimental Investigation on Hardness and Microstructure of Heat Treated EN 9 Steel
 Ahmed muhudiin ahmed
Ahmed muhudiin ahmed Eng katta va eng kichik qiymatlari
Eng katta va eng kichik qiymatlari Grade 7 natural science separating mixtures
Grade 7 natural science separating mixtures Metals and non metals in the periodic table
Metals and non metals in the periodic table Natural science grade 7 term 3 notes
Natural science grade 7 term 3 notes Ferrous vs non ferrous
Ferrous vs non ferrous Example of metals
Example of metals Compare metals nonmetals and metalloids
Compare metals nonmetals and metalloids Specific heat of metals lab answers
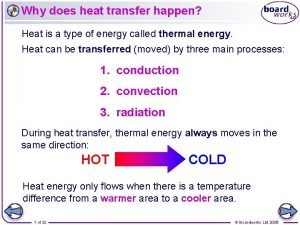
Specific heat of metals lab answers How does heat transfer
How does heat transfer Normalizing heat treatment
Normalizing heat treatment Htc calculator
Htc calculator Sun stroke treatment
Sun stroke treatment Heat treatment cost calculator
Heat treatment cost calculator Non equilibrium heat treatment
Non equilibrium heat treatment Wholesale hardening and tempering
Wholesale hardening and tempering Purpose of heat treatment
Purpose of heat treatment Heat treatment phase diagram
Heat treatment phase diagram Non equilibrium heat treatment
Non equilibrium heat treatment Heat treatment process
Heat treatment process Purpose of heat treatment
Purpose of heat treatment Flame hardening mild steel
Flame hardening mild steel