Heart Failure and Secondary Mitral Regurgitation Echocardiographic Outcomes









- Slides: 27

Heart Failure and Secondary Mitral Regurgitation: Echocardiographic Outcomes from the COAPT trial Federico M. Asch, MD Director, Echocardiographic Core Lab Med. Star Health Research Institute On behalf of Gregg W. Stone, Michael Mack, Neil J Weissman and the COAPT Investigators COAPT (NCT 01626079)

Disclosure Statement Federico M Asch, MD No personal COI Institutional conflict Med. Star Health has Institutional contracts for my work as Director of an Academic Core Lab: Abbott, Boston Scientific, Edwards, Medtronic, Neovasc, Livanova, GDS, Mitralign. COAPT (NCT 01626079) is funded by Abbott

Background (i) • Secondary or functional mitral regurgitation (SMR) is present in >50% of patients with heart failure (HF), and is severe in ~10 -15%. • Prognosis is poor when SMR is severe. • COAPT: Randomized, open-label, multicenter trial in patients with HF and moderate-to-severe (3+) or severe (4+) SMR who remained symptomatic despite maximallytolerated GDMT.

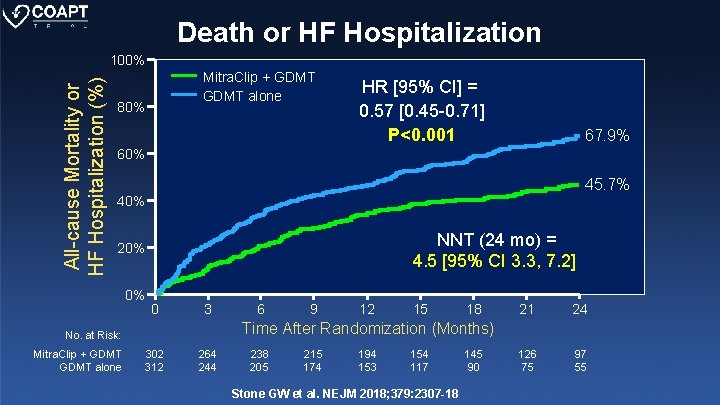
Death or HF Hospitalization All-cause Mortality or HF Hospitalization (%) 100% Mitra. Clip + GDMT alone 80% HR [95% CI] = 0. 57 [0. 45 -0. 71] P<0. 001 67. 9% 60% 45. 7% 40% NNT (24 mo) = 4. 5 [95% CI 3. 3, 7. 2] 20% 0% 0 3 9 12 15 18 21 24 126 75 97 55 Time After Randomization (Months) No. at Risk: Mitra. Clip + GDMT alone 6 302 312 264 244 238 205 215 174 194 153 154 117 Stone GW et al. NEJM 2018; 379: 2307 -18 145 90

Background (ii) • SMR is a consequence of leaflet tethering and incomplete leaflet coaptation. • Evaluation of SMR is challenging, due to asymmetric leaflet anatomy and regurgitant orifice, eccentric jets and enlarged left cardiac chambers. • Expert panels have disagreed on how to define the severity of SMR, resulting in conflicting European and American guidelines.

Objectives COAPT Echo Sub-study 1. To describe the echocardiographic MR grading criteria utilized in COAPT for screening and post-Mitra. Clip 2. To describe the echocardiographic characteristics of the COAPT population 3. To evaluate the serial echocardiographic outcomes 4. To identify baseline echocardiographic predictors of clinical outcomes (responders and non-responders to Mitra. Clip)

Methods • 614 patients with HF and moderate to severe (3+) or severe (4+) secondary MR • Randomized 1: 1 to maximally-tolerated GDMT + Mitra. Clip or GDMT alone. • Transthoracic echocardiograms (TTE) at baseline, 1, 6, 12, 18, 24 months (to continue to year 5) • All echo analysis by an independent echo core lab, adapted from American guidelines (ASE, ACC)

Key Echo Inclusion Criteria 1. LVEF 20% - 50% and LVESD ≤ 70 mm (ischemic or nonischemic) 2. SMR amenable for Mitra. Clip treatment 3. Moderate-to-severe (3+) or severe (4+) SMR confirmed by an independent echo core laboratory prior to enrollment

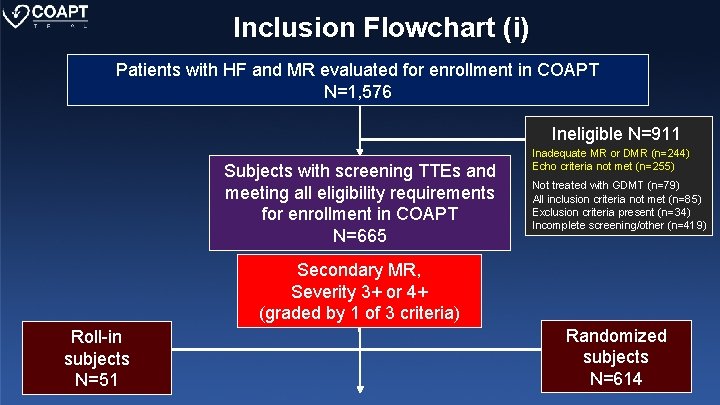
Inclusion Flowchart (i) Patients with HF and MR evaluated for enrollment in COAPT N=1, 576 Ineligible N=911 Subjects with screening TTEs and meeting all eligibility requirements for enrollment in COAPT N=665 Inadequate MR or DMR (n=244) Echo criteria not met (n=255) Not treated with GDMT (n=79) All inclusion criteria not met (n=85) Exclusion criteria present (n=34) Incomplete screening/other (n=419) Secondary MR, Severity 3+ or 4+ (graded by 1 of 3 criteria) Roll-in subjects N=51 Randomized subjects N=614

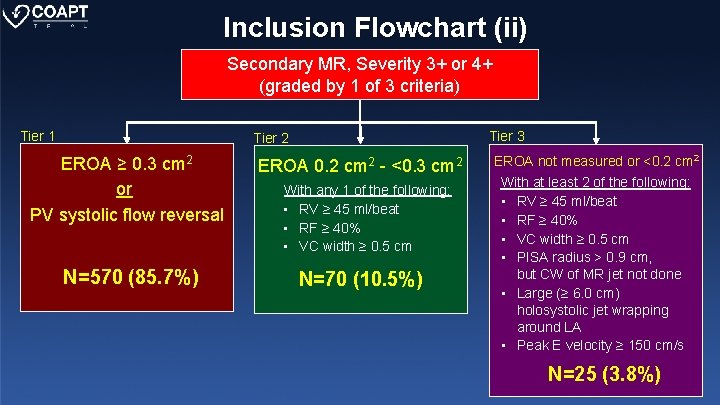
Inclusion Flowchart (ii) Secondary MR, Severity 3+ or 4+ (graded by 1 of 3 criteria) Tier 1 Tier 3 Tier 2 EROA ≥ 0. 3 cm 2 or PV systolic flow reversal EROA 0. 2 cm 2 - <0. 3 cm 2 N=570 (85. 7%) N=70 (10. 5%) With any 1 of the following: • RV ≥ 45 ml/beat • RF ≥ 40% • VC width ≥ 0. 5 cm EROA not measured or <0. 2 cm 2 With at least 2 of the following: • RV ≥ 45 ml/beat • RF ≥ 40% • VC width ≥ 0. 5 cm • PISA radius > 0. 9 cm, but CW of MR jet not done • Large (≥ 6. 0 cm) holosystolic jet wrapping around LA • Peak E velocity ≥ 150 cm/s N=25 (3. 8%)

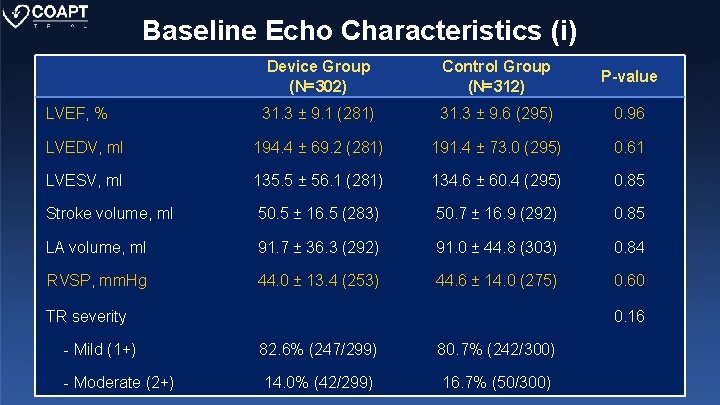
Baseline Echo Characteristics (i) Device Group (N=302) Control Group (N=312) P-value 31. 3 ± 9. 1 (281) 31. 3 ± 9. 6 (295) 0. 96 LVEDV, ml 194. 4 ± 69. 2 (281) 191. 4 ± 73. 0 (295) 0. 61 LVESV, ml 135. 5 ± 56. 1 (281) 134. 6 ± 60. 4 (295) 0. 85 Stroke volume, ml 50. 5 ± 16. 5 (283) 50. 7 ± 16. 9 (292) 0. 85 LA volume, ml 91. 7 ± 36. 3 (292) 91. 0 ± 44. 8 (303) 0. 84 RVSP, mm. Hg 44. 0 ± 13. 4 (253) 44. 6 ± 14. 0 (275) 0. 60 LVEF, % TR severity 0. 16 - Mild (1+) 82. 6% (247/299) 80. 7% (242/300) - Moderate (2+) 14. 0% (42/299) 16. 7% (50/300)

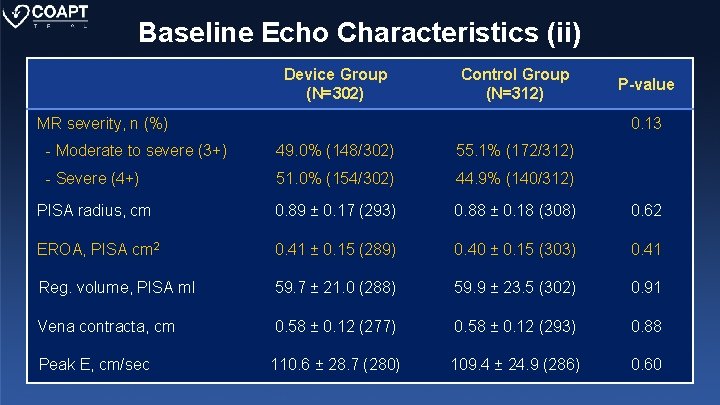
Baseline Echo Characteristics (ii) Device Group (N=302) Control Group (N=312) MR severity, n (%) P-value 0. 13 - Moderate to severe (3+) 49. 0% (148/302) 55. 1% (172/312) - Severe (4+) 51. 0% (154/302) 44. 9% (140/312) PISA radius, cm 0. 89 ± 0. 17 (293) 0. 88 ± 0. 18 (308) 0. 62 EROA, PISA cm 2 0. 41 ± 0. 15 (289) 0. 40 ± 0. 15 (303) 0. 41 Reg. volume, PISA ml 59. 7 ± 21. 0 (288) 59. 9 ± 23. 5 (302) 0. 91 Vena contracta, cm 0. 58 ± 0. 12 (277) 0. 58 ± 0. 12 (293) 0. 88 Peak E, cm/sec 110. 6 ± 28. 7 (280) 109. 4 ± 24. 9 (286) 0. 60

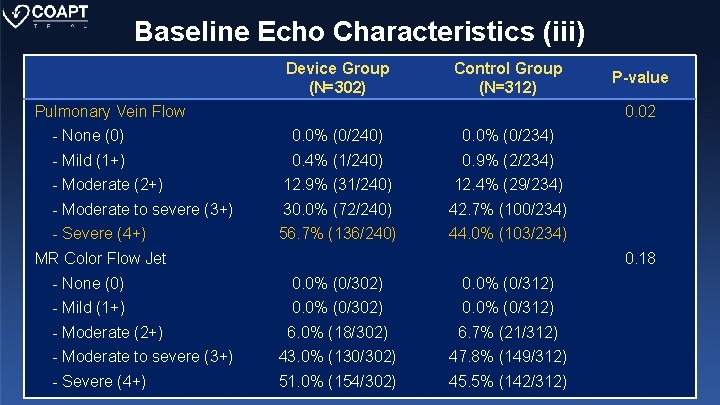
Baseline Echo Characteristics (iii) Device Group (N=302) Control Group (N=312) Pulmonary Vein Flow P-value 0. 02 - None (0) 0. 0% (0/240) 0. 0% (0/234) - Mild (1+) 0. 4% (1/240) 0. 9% (2/234) - Moderate (2+) 12. 9% (31/240) 12. 4% (29/234) - Moderate to severe (3+) 30. 0% (72/240) 42. 7% (100/234) - Severe (4+) 56. 7% (136/240) 44. 0% (103/234) MR Color Flow Jet 0. 18 - None (0) 0. 0% (0/302) 0. 0% (0/312) - Mild (1+) 0. 0% (0/302) 0. 0% (0/312) - Moderate (2+) 6. 0% (18/302) 6. 7% (21/312) - Moderate to severe (3+) 43. 0% (130/302) 47. 8% (149/312) - Severe (4+) 51. 0% (154/302) 45. 5% (142/312)

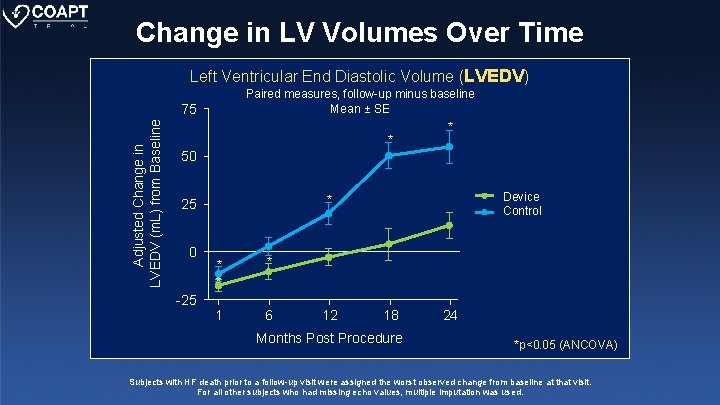
Change in LV Volumes Over Time Left Ventricular End Diastolic Volume (LVEDV) Paired measures, follow-up minus baseline Mean ± SE Adjusted Change in LVEDV (m. L) from Baseline 75 * * 50 0 -25 y (Device) Device * 25 * * * 1 6 12 Control y (Control) 18 Months Post Procedure 24 *p<0. 05 (ANCOVA) Subjects with HF death prior to a follow-up visit were assigned the worst observed change from baseline at that visit. For all other subjects who had missing echo values, multiple imputation was used.

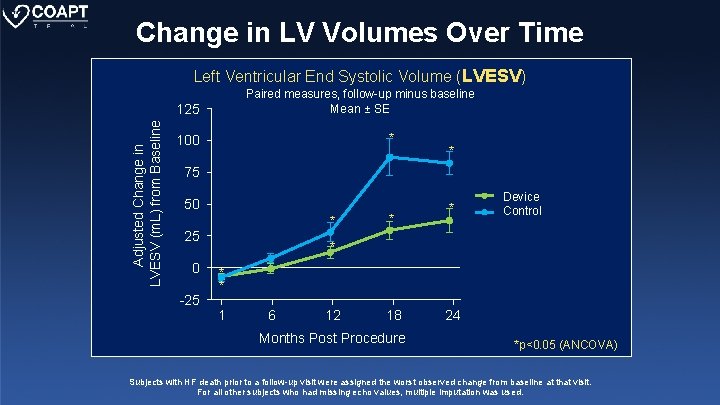
Change in LV Volumes Over Time Left Ventricular End Systolic Volume (LVESV) Paired measures, follow-up minus baseline Mean ± SE Adjusted Change in LVESV (m. L) from Baseline 125 * 100 * 75 50 * 25 0 -25 * * y (Device) Device Control y (Control) * * * 1 6 12 18 Months Post Procedure 24 *p<0. 05 (ANCOVA) Subjects with HF death prior to a follow-up visit were assigned the worst observed change from baseline at that visit. For all other subjects who had missing echo values, multiple imputation was used.

Change in Ejection Fraction Over Time Left Ventricular Ejection Fraction (LVEF) Paired measures, follow-up minus baseline Mean ± SE Adjusted Change in LVEF (%) from Baseline 5 0 * * * -5 * * 12 18 y (Device) Device * * Control y (Control) -10 -15 1 6 Months Post Procedure 24 *p<0. 05 (ANCOVA) Subjects with HF death prior to a follow-up visit were assigned the worst observed change from baseline at that visit. For all other subjects who had missing echo values, multiple imputation was used.

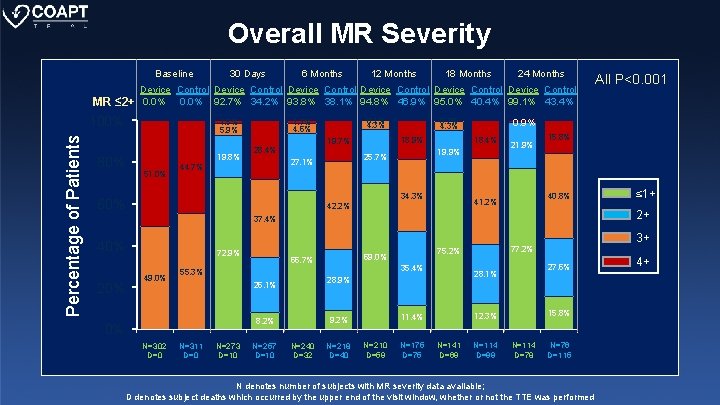
Overall MR Severity Baseline MR ≤ 2+ 6 Months 12 Months 1. 5% 5. 9% 0. 1% 4. 3% 1. 7% 4. 6% 24 Months 19. 8% 51. 0% 28. 4% 44. 7% 18. 4% 19. 9% 25. 7% 27. 1% 34. 3% 60% 21. 9% 15. 8% 40. 8% 41. 2% 42. 2% 20% 72. 9% 49. 0% 55. 3% N=302 D=0 N=311 D=0 N=273 D=10 N=257 D=10 N=240 D=32 N=218 D=40 N=210 D=59 N=175 D=75 15. 8% 12. 3% 11. 4% 9. 2% 8. 2% 27. 6% 28. 1% 28. 9% 26. 1% 0% 35. 4% N=141 D=69 3+ 77. 2% 75. 2% 69. 0% 66. 7% ≤ 1+ 2+ 37. 4% 40% All P<0. 001 0. 9% 0. 7% 4. 3% 18. 9% 19. 7% 80% 18 Months Device Control Device Control 0. 0% 92. 7% 34. 2% 93. 8% 38. 1% 94. 8% 46. 9% 95. 0% 40. 4% 99. 1% 43. 4% 100% Percentage of Patients 30 Days N=114 D=99 N=114 D=78 N=76 D=115 N denotes number of subjects with MR severity data available; D denotes subject deaths which occurred by the upper end of the visit window, whether or not the TTE was performed 4+

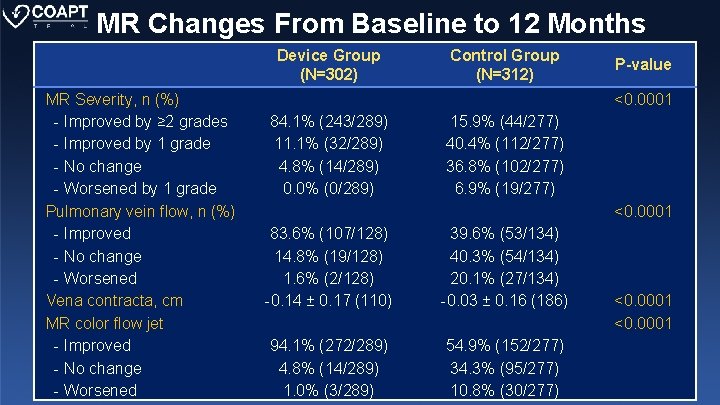
MR Changes From Baseline to 12 Months Device Group (N=302) MR Severity, n (%) - Improved by ≥ 2 grades - Improved by 1 grade - No change - Worsened by 1 grade Pulmonary vein flow, n (%) - Improved - No change - Worsened Vena contracta, cm MR color flow jet - Improved - No change - Worsened Control Group (N=312) P-value <0. 0001 84. 1% (243/289) 11. 1% (32/289) 4. 8% (14/289) 0. 0% (0/289) 15. 9% (44/277) 40. 4% (112/277) 36. 8% (102/277) 6. 9% (19/277) <0. 0001 83. 6% (107/128) 14. 8% (19/128) 1. 6% (2/128) -0. 14 ± 0. 17 (110) 39. 6% (53/134) 40. 3% (54/134) 20. 1% (27/134) -0. 03 ± 0. 16 (186) 94. 1% (272/289) 4. 8% (14/289) 1. 0% (3/289) 54. 9% (152/277) 34. 3% (95/277) 10. 8% (30/277) <0. 0001

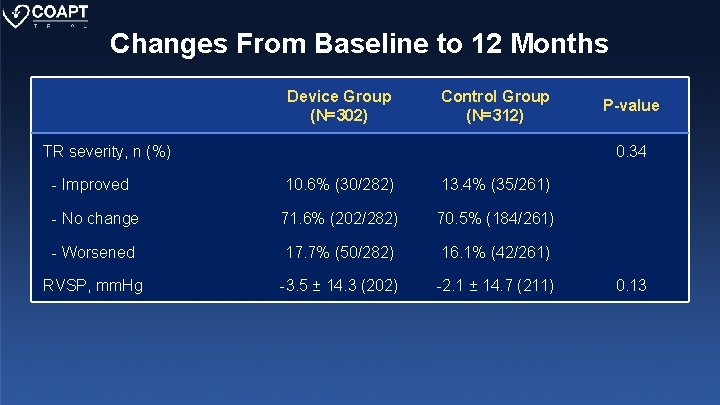
Changes From Baseline to 12 Months Device Group (N=302) Control Group (N=312) TR severity, n (%) P-value 0. 34 - Improved 10. 6% (30/282) 13. 4% (35/261) - No change 71. 6% (202/282) 70. 5% (184/261) - Worsened 17. 7% (50/282) 16. 1% (42/261) RVSP, mm. Hg -3. 5 ± 14. 3 (202) -2. 1 ± 14. 7 (211) 0. 13

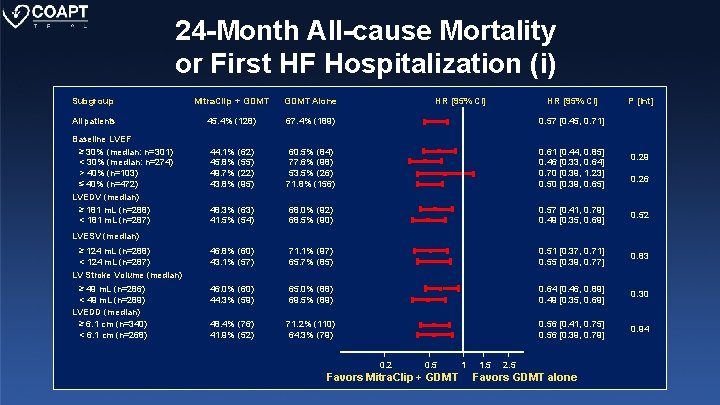
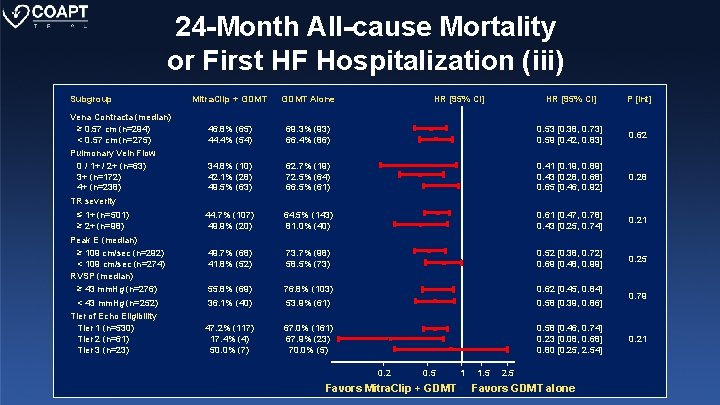
24 -Month All-cause Mortality or First HF Hospitalization (i) Subgroup Mitra. Clip + GDMT Alone All patients 45. 4% (128) 67. 4% (189) 0. 57 [0. 45, 0. 71] 44. 1% (62) 45. 8% (55) 49. 7% (22) 43. 8% (95) 60. 5% (84) 77. 6% (98) 53. 5% (26) 71. 8% (156) 0. 61 [0. 44, 0. 85] 0. 46 [0. 33, 0. 64] 0. 70 [0. 39, 1. 23] 0. 50 [0. 39, 0. 65] 48. 3% (63) 41. 5% (54) 68. 0% (92) 68. 5% (90) 0. 57 [0. 41, 0. 79] 0. 49 [0. 35, 0. 69] 0. 52 46. 8% (60) 43. 1% (57) 71. 1% (97) 65. 7% (85) 0. 51 [0. 37, 0. 71] 0. 55 [0. 39, 0. 77] 0. 83 46. 0% (60) 44. 3% (59) 65. 0% (88) 69. 5% (89) 0. 64 [0. 46, 0. 89] 0. 49 [0. 35, 0. 69] 0. 30 48. 4% (76) 41. 9% (52) 71. 2% (110) 64. 3% (79) 0. 56 [0. 41, 0. 75] 0. 56 [0. 39, 0. 79] 0. 94 Baseline LVEF ≥ 30% (median: n=301) < 30% (median: n=274) > 40% (n=103) ≤ 40% (n=472) LVEDV (median) ≥ 181 m. L (n=288) < 181 m. L (n=287) HR [95% CI] P [Int] 0. 29 0. 26 LVESV (median) ≥ 124 m. L (n=288) < 124 m. L (n=287) LV Stroke Volume (median) ≥ 49 m. L (n=286) < 49 m. L (n=289) LVEDD (median) ≥ 6. 1 cm (n=340) < 6. 1 cm (n=268) 0. 2 0. 5 Favors Mitra. Clip + GDMT 1 1. 5 2. 5 Favors GDMT alone

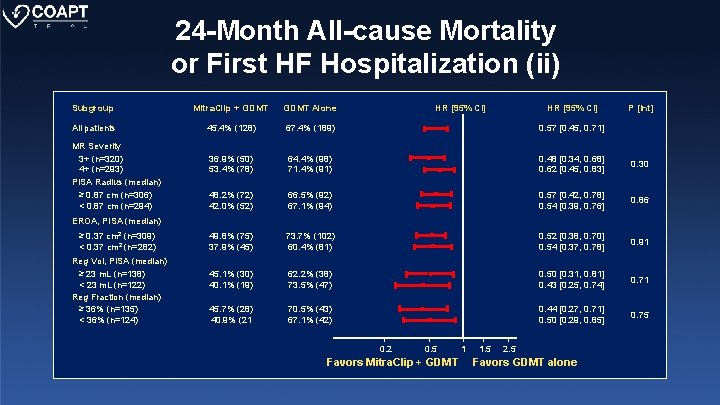
24 -Month All-cause Mortality or First HF Hospitalization (ii) Subgroup Mitra. Clip + GDMT Alone All patients 45. 4% (128) 67. 4% (189) 0. 57 [0. 45, 0. 71] 36. 9% (50) 53. 4% (78) 64. 4% (98) 71. 4% (91) 0. 48 [0. 34, 0. 68] 0. 62 [0. 45, 0. 83] 0. 30 48. 2% (72) 42. 0% (52) 66. 5% (92) 67. 1% (94) 0. 57 [0. 42, 0. 78] 0. 54 [0. 39, 0. 76] 0. 86 49. 8% (75) 37. 9% (45) 73. 7% (102) 60. 4% (81) 0. 52 [0. 38, 0. 70] 0. 54 [0. 37, 0. 78] 0. 91 45. 1% (30) 40. 1% (19) 62. 2% (38) 73. 5% (47) 0. 50 [0. 31, 0. 81] 0. 43 [0. 25, 0. 74] 0. 71 45. 7% (28) 40. 9% (21 70. 5% (43) 67. 1% (42) 0. 44 [0. 27, 0. 71] 0. 50 [0. 29, 0. 85] 0. 75 MR Severity 3+ (n=320) 4+ (n=293) PISA Radius (median) ≥ 0. 87 cm (n=306) < 0. 87 cm (n=294) HR [95% CI] P [Int] EROA, PISA (median) ≥ 0. 37 cm 2 (n=309) < 0. 37 cm 2 (n=282) Reg Vol, PISA (median) ≥ 23 m. L (n=138) < 23 m. L (n=122) Reg Fraction (median) ≥ 36% (n=135) < 36% (n=124) 0. 2 0. 5 Favors Mitra. Clip + GDMT 1 1. 5 2. 5 Favors GDMT alone

24 -Month All-cause Mortality or First HF Hospitalization (iii) Subgroup Vena Contracta (median) ≥ 0. 57 cm (n=294) < 0. 57 cm (n=275) Pulmonary Vein Flow 0 / 1+ / 2+ (n=63) 3+ (n=172) 4+ (n=238) Mitra. Clip + GDMT Alone 46. 8% (65) 44. 4% (54) HR [95% CI] P [Int] 69. 3% (93) 66. 4% (86) 0. 53 [0. 38, 0. 73] 0. 59 [0. 42, 0. 83] 0. 62 34. 8% (10) 42. 1% (28) 49. 5% (63) 62. 7% (19) 72. 5% (64) 66. 5% (61) 0. 41 [0. 19, 0. 89] 0. 43 [0. 28, 0. 68] 0. 65 [0. 46, 0. 92] 0. 28 44. 7% (107) 49. 9% (20) 64. 5% (143) 81. 0% (40) 0. 61 [0. 47, 0. 78] 0. 43 [0. 25, 0. 74] 0. 21 49. 7% (68) 41. 8% (52) 73. 7% (98) 58. 5% (73) 0. 52 [0. 38, 0. 72] 0. 69 [0. 48, 0. 99] 0. 25 55. 8% (69) 36. 1% (40) 76. 8% (103) 53. 9% (61) 0. 62 [0. 45, 0. 84] 0. 58 [0. 39, 0. 86] 0. 79 47. 2% (117) 17. 4% (4) 50. 0% (7) 67. 0% (161) 67. 9% (23) 70. 0% (5) 0. 58 [0. 46, 0. 74] 0. 23 [0. 08, 0. 68] 0. 80 [0. 25, 2. 54] 0. 21 TR severity ≤ 1+ (n=501) ≥ 2+ (n=98) Peak E (median) ≥ 109 cm/sec (n=292) < 109 cm/sec (n=274) RVSP (median) ≥ 43 mm. Hg (n=276) < 43 mm. Hg (n=252) Tier of Echo Eligibility Tier 1 (n=530) Tier 2 (n=61) Tier 3 (n=23) 0. 2 0. 5 Favors Mitra. Clip + GDMT 1 1. 5 2. 5 Favors GDMT alone

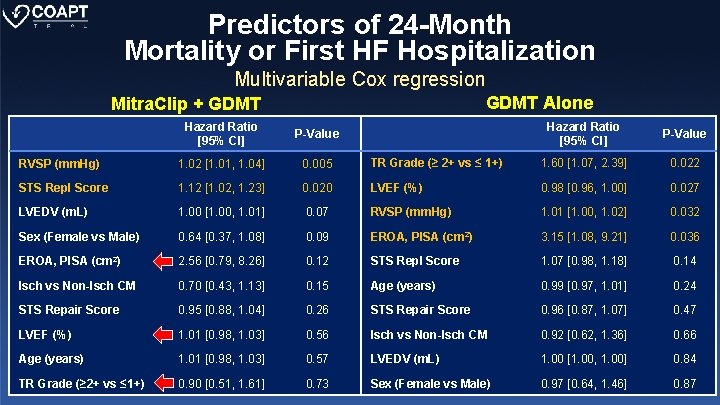
Predictors of 24 -Month Mortality or First HF Hospitalization Multivariable Cox regression GDMT Alone Mitra. Clip + GDMT Hazard Ratio [95% CI] P-Value TR Grade (≥ 2+ vs ≤ 1+) 1. 60 [1. 07, 2. 39] 0. 022 0. 020 LVEF (%) 0. 98 [0. 96, 1. 00] 0. 027 1. 00 [1. 00, 1. 01] 0. 07 RVSP (mm. Hg) 1. 01 [1. 00, 1. 02] 0. 032 Sex (Female vs Male) 0. 64 [0. 37, 1. 08] 0. 09 EROA, PISA (cm 2) 3. 15 [1. 08, 9. 21] 0. 036 EROA, PISA (cm 2) 2. 56 [0. 79, 8. 26] 0. 12 STS Repl Score 1. 07 [0. 98, 1. 18] 0. 14 Isch vs Non-Isch CM 0. 70 [0. 43, 1. 13] 0. 15 Age (years) 0. 99 [0. 97, 1. 01] 0. 24 STS Repair Score 0. 95 [0. 88, 1. 04] 0. 26 STS Repair Score 0. 96 [0. 87, 1. 07] 0. 47 LVEF (%) 1. 01 [0. 98, 1. 03] 0. 56 Isch vs Non-Isch CM 0. 92 [0. 62, 1. 36] 0. 66 Age (years) 1. 01 [0. 98, 1. 03] 0. 57 LVEDV (m. L) 1. 00 [1. 00, 1. 00] 0. 84 TR Grade (≥ 2+ vs ≤ 1+) 0. 90 [0. 51, 1. 61] 0. 73 Sex (Female vs Male) 0. 97 [0. 64, 1. 46] 0. 87 Hazard Ratio [95% CI] P-Value RVSP (mm. Hg) 1. 02 [1. 01, 1. 04] 0. 005 STS Repl Score 1. 12 [1. 02, 1. 23] LVEDV (m. L)

Limitations • Echo analysis was blinded to clinical condition, but not to intervention. • PISA and hemodynamics after Mitra. Clip have major limitations. Color Doppler, PV flow and vena contracta are the most available and reliable methods. • Subgroup predictive analysis was only done based on prespecified plans. • To overcome survivorship bias, worst case scenario and multiple imputation methods were used to account for missing follow-up data (pre-specified).

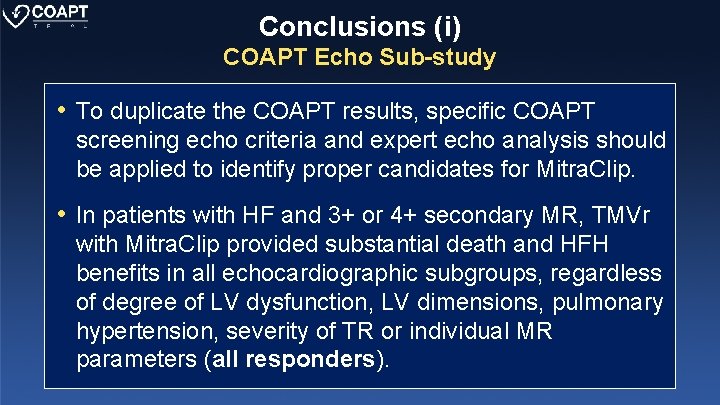
Conclusions (i) COAPT Echo Sub-study • To duplicate the COAPT results, specific COAPT screening echo criteria and expert echo analysis should be applied to identify proper candidates for Mitra. Clip. • In patients with HF and 3+ or 4+ secondary MR, TMVr with Mitra. Clip provided substantial death and HFH benefits in all echocardiographic subgroups, regardless of degree of LV dysfunction, LV dimensions, pulmonary hypertension, severity of TR or individual MR parameters (all responders).

Conclusions (ii) COAPT Echo Sub-study • Baseline LVEF, TR and MR severity predicted poor outcomes in patients with HF treated with GDMT alone, but not after MR was corrected by Mitra. Clip. • RVSP was the only independent echocardiographic predictor of poor outcomes after Mitra. Clip and GDMT treatments.

Acknowledgments • COAPT Principal Investigators and Co-authors ¡ Gregg W. Stone, Michael J. Mack, William T. Abraham, Jo. Ann Lindenfeld, Paul A. Grayburn, Robert J. Siegel, Saibal Kar, D. Scott Lim, Jonathan G. Zaroff, Jacob M. Mishell, Brian Whisenant and Neil J. Weissman • Abbott Structural Heart Global Clinical Affairs ¡ Jeffrey Ellis, Kartik Sundareswaran • Abbott Global Biometrics ¡ Yu Shu, Juanjuan Li, Deepika Morishetti, Hong Nie • All COAPT Sites, Research Coordinators, Heart Teams and Patients • Med. Star Echo Core lab Staff ¡ Vladimir Masati, Valiere Morgan, Ma Therese Tupas-Habib, et al.
 Pathophysiology of valvular heart disease
Pathophysiology of valvular heart disease Mitral regurgitation murmur
Mitral regurgitation murmur Aortic regurgitation murmur
Aortic regurgitation murmur Non conducted pac ecg
Non conducted pac ecg Failure to sense
Failure to sense Brittle failure vs ductile failure
Brittle failure vs ductile failure Primary tricuspid regurgitation
Primary tricuspid regurgitation Jet fazil
Jet fazil Pannus
Pannus Tricuspid regurgitation echo assessment
Tricuspid regurgitation echo assessment Four areas of auscultation
Four areas of auscultation Dicrotic pulse
Dicrotic pulse Chemosis
Chemosis Lacrimal duct
Lacrimal duct Mitrial regurgitation
Mitrial regurgitation Tricuspid regurgitation
Tricuspid regurgitation Forrester classification heart failure
Forrester classification heart failure Chlorpromide
Chlorpromide Diabetes and heart failure
Diabetes and heart failure Heart failure definition
Heart failure definition Heart failure defined
Heart failure defined New york scale heart failure
New york scale heart failure Nursing assessment for congestive heart failure
Nursing assessment for congestive heart failure Right sided heart failure
Right sided heart failure Heart failure complications
Heart failure complications Chapter 24 heart failure drugs
Chapter 24 heart failure drugs Heart failure
Heart failure Congestive heart failure zones for management
Congestive heart failure zones for management