Transcatheter Tricuspid Valve Repair and Replacement Devices Unmet








- Slides: 22

Transcatheter Tricuspid Valve Repair and Replacement Devices Unmet Clinical Need and Opportunity Industry Perspective Neil Moat MB BS MS FRCS CMO Abbott (Structural Heart)

Neil Moat MB BS MS FRCS Employee (CMO) Abbott (Structural Heart)

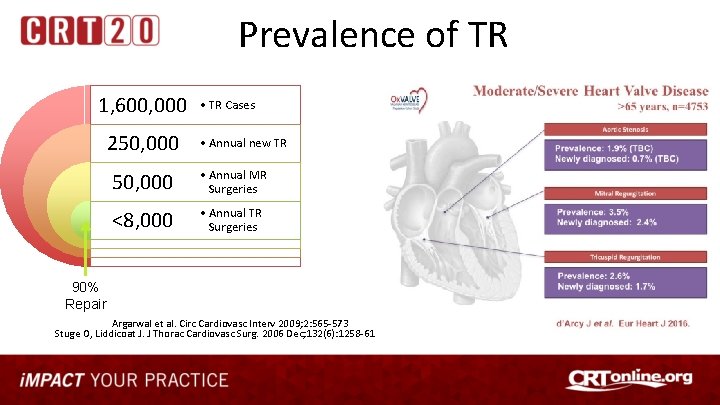
Prevalence of TR 1, 600, 000 250, 000 • TR Cases • Annual new TR 50, 000 • Annual MR Surgeries <8, 000 • Annual TR Surgeries 90% Repair Argarwal et al. Circ Cardiovasc Interv 2009; 2: 565 -573 Stuge O, Liddicoat J. J Thorac Cardiovasc Surg. 2006 Dec; 132(6): 1258 -61

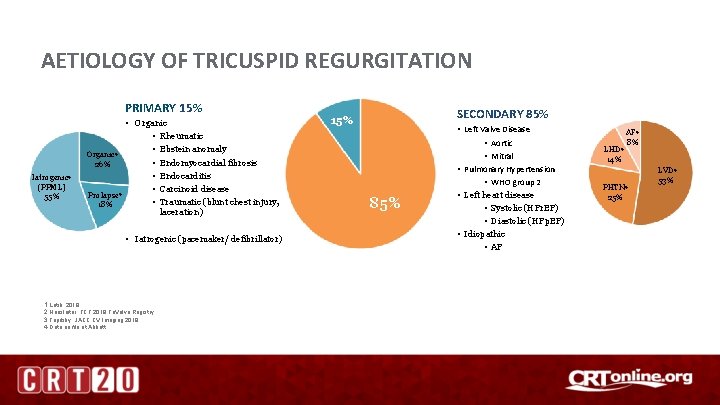
AETIOLOGY OF TRICUSPID REGURGITATION PRIMARY 15% Iatrogenic 4 (PPML) 55% • Organic • Rheumatic • Ebstein anomaly Organic 4 • Endomyocardial fibrosis 26% • Endocarditis • Carcinoid disease Prolapse 4 • Traumatic (blunt chest injury, 18% laceration) • Iatrogenic (pacemaker/ defibrillator) 1. Latib, 2018 2. Hausleiter, TCT 2018 Tri. Valve Registry 3. Topilsky, JACC CV Imaging 2018 4. Data on file at Abbott SECONDARY 85% 15% 85% • Left Valve Disease • Aortic • Mitral • Pulmonary Hypertension • WHO group 2 • Left heart disease • Systolic (HFr. EF) • Diastolic (HFp. EF) • Idiopathic • AF LHD 4 14% AF 4 8% PHTN 4 25% LVD 4 53%

Prognosis of TR Tricuspid valve disease is a severe condition with impact on long term survival, esp in patients with chronic heart failure & LV dysfunction Retrospective analysis of 5, 223 patients (age 66. 5 ± 12. 8 years) adjusted for age, LVEF, inferior vena cava size, and RV size and function Combined endpoint (death, heart transplantation, left ventricular-assist device implantation) 1. 0 No TR 0. 9 Prospective analysis of 576 consecutive patients with CHF (age 56. 4+ 11. 2 years) P < 0. 0001 0. 9 0. 8 0. 7 Surviving 0. 6 0. 5 Moderate TR 0. 5 P <0. 001 0. 4 0. 2 0. 1 300 500 Severe TR 0. 2 0. 1 0 100 Moderate TR 0. 3 0 0 No/Mild TR 0. 4 Severe TR 1 -year survival rate: No TR 91. 7% Mild TR 90. 3% Moderate TR 78. 9% Severe TR 63. 9% 0. 3 Days 5 -year event freedom: No/Mild TR 51. 5 Moderate TR 20. 8 Severe TR 19. 0 0. 8 Mild TR 0. 7 Retrospective analysis of 291 patients with LVEF<50% and Functional TR (age 70 ± 12 yrs; EF 31± 10%) 700 900 1100 Nath et al. J Am Coll Cardiol 2004; 43: 405– 09 1300 1500 0 1 2 3 4 5 6 7 8 Years 9 10 11 12 13 14 Neuhold et al. Eur Heart J 2013; 34: 844– 52 Topilsky et al. Eur Heart J. 2018 Jul 27

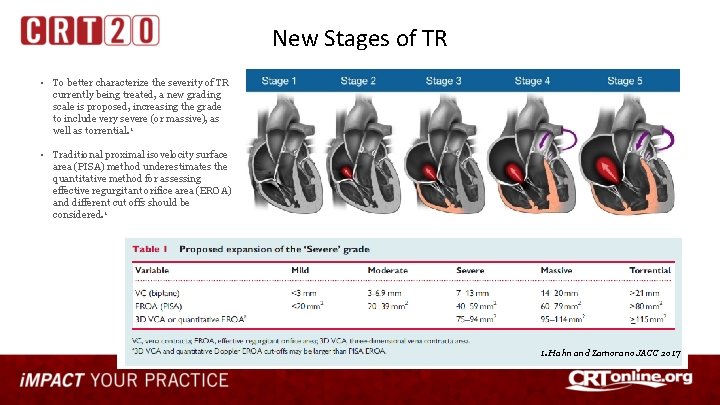
New Stages of TR • To better characterize the severity of TR currently being treated, a new grading scale is proposed, increasing the grade to include very severe (or massive), as well as torrential. 1 • Traditional proximal isovelocity surface area (PISA) method underestimates the quantitative method for assessing effective regurgitant orifice area (EROA) and different cut offs should be considered. 1 1. Hahn and Zamorano JACC 2017

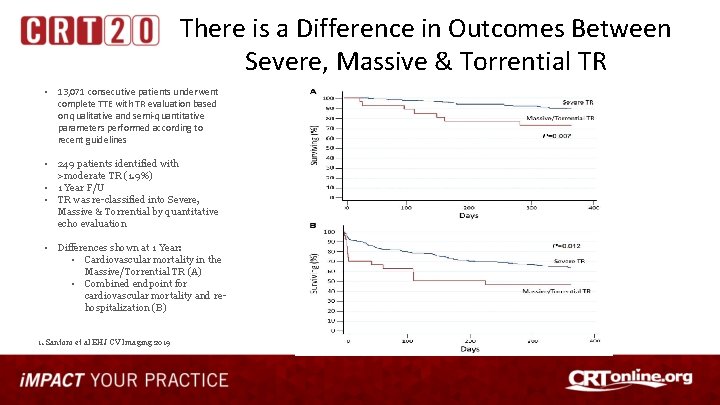
There is a Difference in Outcomes Between Severe, Massive & Torrential TR • 13, 071 consecutive patients underwent complete TTE with TR evaluation based on qualitative and semi-quantitative parameters performed according to recent guidelines • 249 patients identified with >moderate TR (1. 9%) • 1 Year F/U • TR was re-classified into Severe, Massive & Torrential by quantitative echo evaluation • Differences shown at 1 Year: • Cardiovascular mortality in the Massive/Torrential TR (A) • Combined endpoint for cardiovascular mortality and rehospitalization (B) 1. Santoro et al EHJ CV Imaging 2019

TR and Symptoms Decreased CO Fatigue, decreased exercise tolerance “Right-sided” Heart Failure Ascites, edema, decreased appetite, fullness/bloating, nausea FTR - feel terrible ! Valve repair for functional tricuspid valve regurgitation: anatomical and surgical considerations Courtesy Steve Bolling Rogers JH, Bolling SF Semin Thorac Cardiovasc Surg. 2010 ; 22(1): 84 -9

Development of a Risk Prediction Model and Clinical Risk Score for Isolated Tricuspid Valve Surgery Damien J. La. Par, MD The Annals of Thoracic Surgery Volume 106, Issue 1, Pages 129 -136 (July 2018) • VCSQI and MSTCVS Registries (50 hospitals) • 2, 050 isolated TV repair and replacement operations for any etiology (2002 to 2014) • Operative mortality = 9% • Composite major morbidity rate = 42%

Tricuspid Regurgitation A CLEAR UNMET CLINICAL NEED !!!

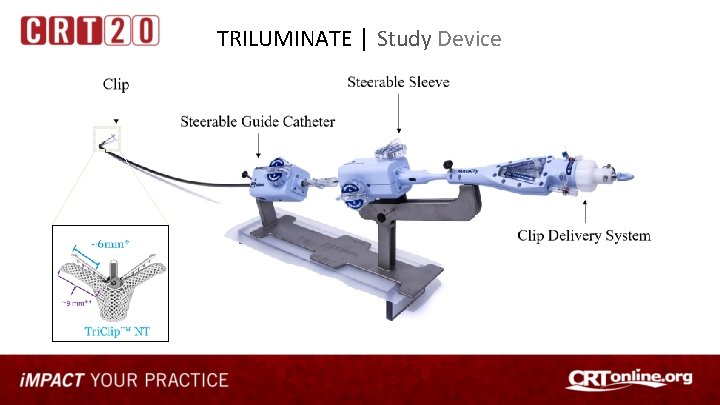
TRILUMINATE │ Study Device

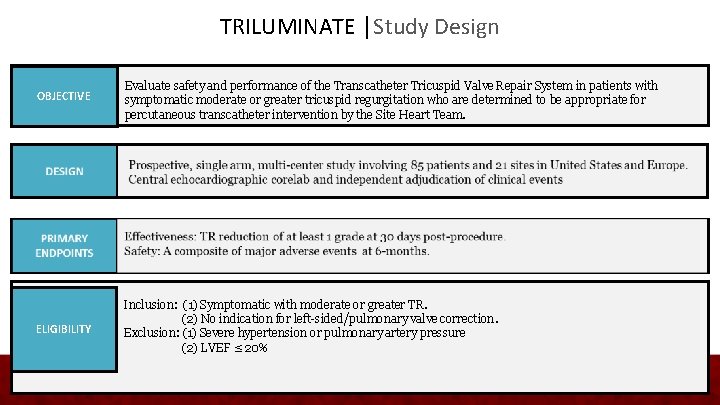
TRILUMINATE │Study Design OBJECTIVE Evaluate safety and performance of the Transcatheter Tricuspid Valve Repair System in patients with symptomatic moderate or greater tricuspid regurgitation who are determined to be appropriate for percutaneous transcatheter intervention by the Site Heart Team. ELIGIBILITY Inclusion: (1) Symptomatic with moderate or greater TR. (2) No indication for left-sided/pulmonary valve correction. Exclusion: (1) Severe hypertension or pulmonary artery pressure (2) LVEF ≤ 20%

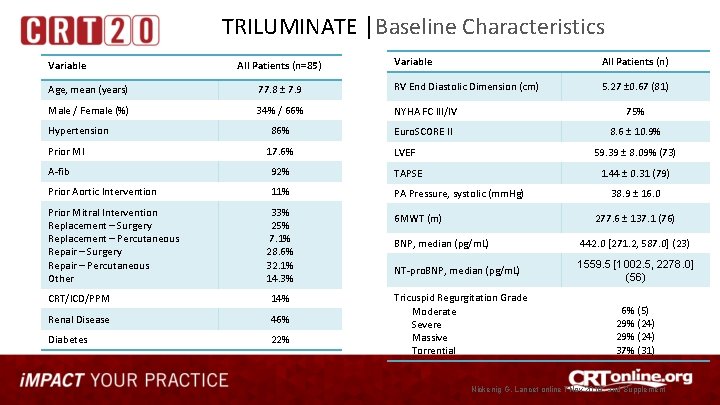
TRILUMINATE │Baseline Characteristics Variable All Patients (n=85) Variable All Patients (n) 5. 27 ± 0. 67 (81) Age, mean (years) 77. 8 ± 7. 9 RV End Diastolic Dimension (cm) Male / Female (%) 34% / 66% NYHA FC III/IV 75% 86% Euro. SCORE II 8. 6 ± 10. 9% Hypertension Prior MI 17. 6% LVEF 59. 39 ± 8. 09% (73) A-fib 92% TAPSE 1. 44 ± 0. 31 (79) Prior Aortic Intervention 11% PA Pressure, systolic (mm. Hg) Prior Mitral Intervention Replacement – Surgery Replacement – Percutaneous Repair – Surgery Repair – Percutaneous Other 33% 25% 7. 1% 28. 6% 32. 1% 14. 3% CRT/ICD/PPM 14% Renal Disease 46% Diabetes 22% 6 MWT (m) 38. 9 ± 16. 0 277. 6 ± 137. 1 (76) BNP, median (pg/m. L) 442. 0 [271. 2, 587. 0] (23) NT-pro. BNP, median (pg/m. L) 1559. 5 [1002. 5, 2278. 0] (56) Tricuspid Regurgitation Grade Moderate Severe Massive Torrential 6% (5) 29% (24) 37% (31) Nickenig G, Lancet online 7 Nov 2019, and Supplement

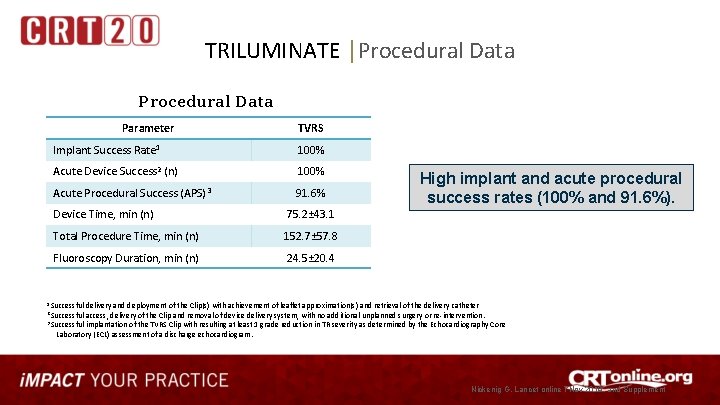
TRILUMINATE │Procedural Data Parameter TVRS Implant Success Rate 1 100% Acute Device Success 2 (n) 100% Acute Procedural Success (APS) 3 91. 6% Device Time, min (n) 75. 2± 43. 1 Total Procedure Time, min (n) 152. 7± 57. 8 Fluoroscopy Duration, min (n) 24. 5± 20. 4 High implant and acute procedural success rates (100% and 91. 6%). 1 Successful delivery and deployment of the Clip(s) with achievement of leaflet approximation(s) and retrieval of the delivery catheter 2 Successful access, delivery of the Clip and removal of device delivery system, with no additional unplanned surgery or re-intervention. 3 Successful implantation of the TVRS Clip with resulting at least 1 grade reduction in TR severity as determined by the Echocardiography Core Laboratory (ECL) assessment of a discharge echocardiogram. Nickenig G, Lancet online 7 Nov 2019, and Supplement

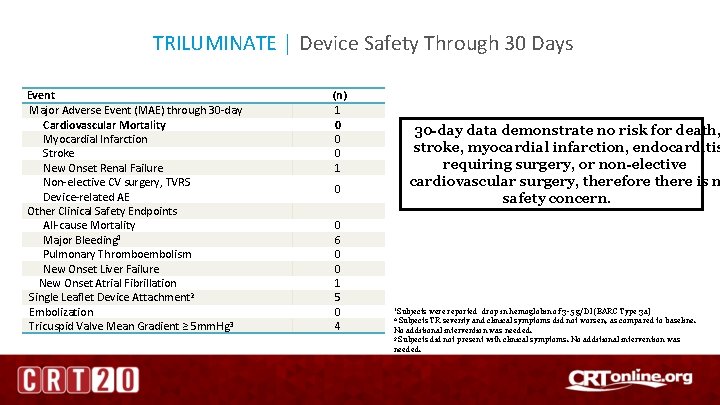
TRILUMINATE │ Device Safety Through 30 Days Event Major Adverse Event (MAE) through 30 -day Cardiovascular Mortality Myocardial Infarction Stroke New Onset Renal Failure Non-elective CV surgery, TVRS Device-related AE Other Clinical Safety Endpoints All-cause Mortality Major Bleeding 1 Pulmonary Thromboembolism New Onset Liver Failure New Onset Atrial Fibrillation Single Leaflet Device Attachment 2 Embolization Tricuspid Valve Mean Gradient ≥ 5 mm. Hg 3 (n) 1 0 0 0 1 30 -day data demonstrate no risk for death, stroke, myocardial infarction, endocarditis requiring surgery, or non-elective cardiovascular surgery, therefore there is n safety concern. 0 0 6 0 0 1 5 0 4 1 Subjects were reported drop in hemoglobin of 3 -5 g/Dl (BARC Type 3 a) Subjects TR severity and clinical symptoms did not worsen, as compared to baseline. No additional intervention was needed. 3 Subjects did not present with clinical symptoms. No additional intervention was needed. 2

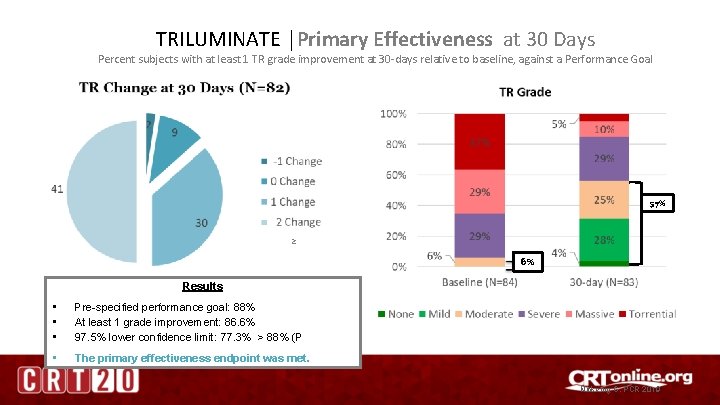
TRILUMINATE │Primary Effectiveness at 30 Days Percent subjects with at least 1 TR grade improvement at 30 -days relative to baseline, against a Performance Goal 57% ≥ 6% Results • • • Pre-specified performance goal: 88% At least 1 grade improvement: 86. 6% 97. 5% lower confidence limit: 77. 3% > 88% (P • The primary effectiveness endpoint was met. Nickenig G, PCR 2019

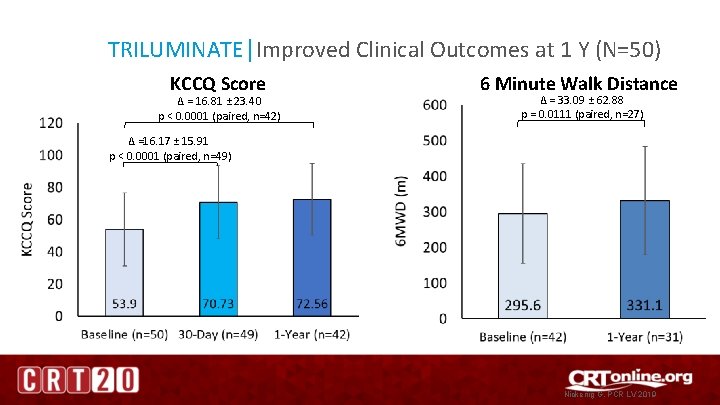
TRILUMINATE│Improved Clinical Outcomes at 1 Y (N=50) KCCQ Score ∆ = 16. 81 ± 23. 40 p < 0. 0001 (paired, n=42) 6 Minute Walk Distance ∆ = 33. 09 ± 62. 88 p = 0. 0111 (paired, n=27) ∆ =16. 17 ± 15. 91 p < 0. 0001 (paired, n=49) Nickenig G, PCR LV 2019

TRIAL DESIGN Clinical TRIal to Eva. LUate Cardiovascular Outco. Mes IN PAtients Treated with the Tricuspid Valv. E Repair System Pivotal (TRILUMINATE Pivotal) Proprietary and confidential — do not distribute

Trial Objectives and Design Evaluate the safety and effectiveness of the Tri. Clip™ device in improving clinical outcomes in symptomatic patients with severe tricuspid regurgitation (TR) who have been determined by the site’s local heart team to be at intermediate or greater estimated risk for mortality with tricuspid valve surgery Scientific Objective • • Trial Design • • Prospective, randomized, controlled, multi-center trial Approximately 700 subjects enrolled at up to 80 sites in United States, Canada, and Europe • Randomized Arm: 450 Subjects • Single Arm: 100 Subjects • Roll-Ins: Up to 3 per site Principal Investigator: Dr. David Adams (Mt. Sinai), Dr. Paul Sorajja (Abbott Northwestern) Echo Core-Laboratory: Dr. Rebecca Hahn (CRF)

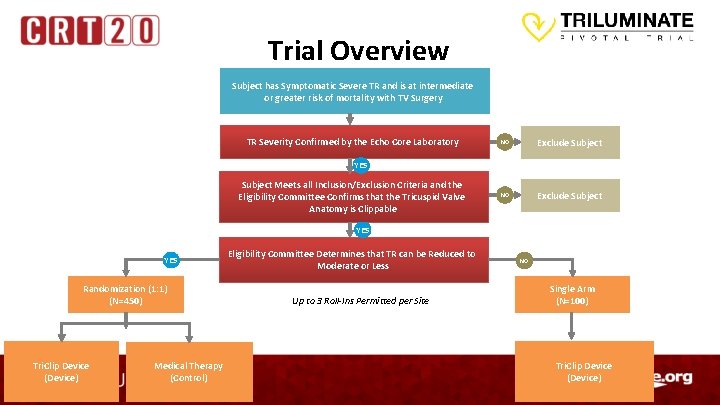
Trial Overview Subject has Symptomatic Severe TR and is at intermediate or greater risk of mortality with TV Surgery TR Severity Confirmed by the Echo Core Laboratory NO Exclude Subject YES Subject Meets all Inclusion/Exclusion Criteria and the Eligibility Committee Confirms that the Tricuspid Valve Anatomy is Clippable YES Randomization (1: 1) (N=450) Tri. Clip Device (Device) Medical Therapy (Control) Eligibility Committee Determines that TR can be Reduced to Moderate or Less Up to 3 Roll-Ins Permitted per Site NO Single Arm (N=100) Tri. Clip Device (Device)

Trial Endpoints Primary Endpoint Secondary Endpoints (Randomized Arm Only) Randomized Arm A composite of mortality or tricuspid valve surgery, heart failure hospitalizations, and quality of life improvement, evaluated at 12 months in a hierarchical fashion. Single Arm: Survival and quality of life at 12 months compared to baseline. To be assessed after the first 350 randomized subjects complete 12 -month follow-up: • TR Reduction to moderate or less at 30 -day post procedure • Freedom from major adverse events (MAE) after procedure attempt at 30 days • Change in KCCQ at 12 months • Change in 6 MWD at 12 months To be assessed after the first 450 randomized subjects complete 24 -month follow-up: • Recurrent HF hospitalizations at 24 months • All-cause mortality or need for tricuspid valve surgery or intervention at 24 months

Transcatheter Tricuspid Valve Repair and Replacement Devices Unmet Clinical Need and Opportunity THANK YOU
 Tricuspid regurgitation echo assessment
Tricuspid regurgitation echo assessment Cincin anulus
Cincin anulus Posterior view of sheep heart labeled
Posterior view of sheep heart labeled Chordae tendineae
Chordae tendineae Parts of the heart
Parts of the heart Tricuspid valve
Tricuspid valve Unmet financial need
Unmet financial need Unmet needs in severe asthma
Unmet needs in severe asthma Nucleotide excision repair
Nucleotide excision repair Base excision repair
Base excision repair Example of combustion reaction
Example of combustion reaction Servo valve control circuit
Servo valve control circuit Nrga in echo
Nrga in echo Triluminate pivotal trial
Triluminate pivotal trial What are literary devices and techniques
What are literary devices and techniques Input and output of computer
Input and output of computer Lagging strand
Lagging strand Brake system diagnosis and repair
Brake system diagnosis and repair Chapter 76 suspension system diagnosis and repair answers
Chapter 76 suspension system diagnosis and repair answers Peak and hold injector waveform
Peak and hold injector waveform Chapter 44 automotive wiring and wire repair
Chapter 44 automotive wiring and wire repair Context diagram for for bus garage and repair system
Context diagram for for bus garage and repair system Form 337 faa
Form 337 faa