Green Chemistry A greener future Prof ngela Gonzlez


































- Slides: 50

“Green Chemistry” A greener future… Prof. Ángela González Department of Biology, Chemistry and Env. Sciences Interamerican University of Puerto Rico San Germán Campus agonzal@sg. inter. edu

What to expect from this talk… • Why? – History • What? – Definitions • How? – Principles • Examples Angela González, Ph. D. / UIA-SG

¿WHY? • 70’s – need to improve environmental quality – Control Laws – Contaminants produced and THEN treated before releasing them to the environment – Excellent idea IF there is good control and handling of environmental laws • It is continuous, slow and expensive Angela González, Ph. D. / UIA-SG

Angela González, Ph. D. / UIA-SG

¿WHY? • 1991 – Green Chemistry is promoted by EPA – (Paul Anastas) Angela González, Ph. D. / UIA-SG

What is Green Chemistry? … or sustainable/environmentally benign chemistry is the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances www. epa. gov/greenchemistry Angela González, Ph. D. / UIA-SG

What is Green Chemistry? … principles to reduce or eliminate use or production of dangerous chemicals in the design, manufacture and application of chemical products. Green Chemistry Theory & Practice, P T Anastas & J C Warner, Oxford University Press 1998 Angela González, Ph. D. / UIA-SG

Green Chemistry Goals • Reduction or elimination of – Waste – Toxic chemicals or processes – Energy use Angela González, Ph. D. / UIA-SG

Green chemistry is looking to: Waste Materials Danger Reduce Risk Energy Environmental Impact COST! ($$$$) Angela González, Ph. D. / UIA-SG

Green Chemistry vs. Environmental Chemistry • Environmental Chemistry: – Study of sources, reactions, transport, effects and destruction of chemical compounds in the ground, water and air. Stanley Manahan, Env. Chemistry, 6 th Ed. CRC Press. 2005 Angela González, Ph. D. / UIA-SG

Environmental Regulations Clean up Research Environmental Chemistry Monitoring Angela González, Ph. D. / UIA-SG Prevention n y e e tr r G is m e h

Green Chemistry Benefits: • Reduced waste, eliminating costly endof-the-pipe treatments • Safer products • Reduced use of energy and resources • Improved competitiveness of chemical manufacturers and their customers www. epa. gov/greenchemistry/ Angela González, Ph. D. / UIA-SG

What has happened? • 1996 Presidential Green Chemistry Challenge Awards -promote non contaminant technologies. • 1997 Green Chemistry and Engineering Conference • 1999 Journal “Green Chemistry” • Chemical and Engineering News • 2000 GCI integrated into ACS • 2000 Journal of Chemical Education Angela González, Ph. D. / UIA-SG

and? • Until 2006 all the technologies nominated to the PGCCA had eliminated the use or production of: – 1. 2 billion pounds of chemicals and solvents per year • Enough to fill 5000 train tanks or a 62 miles long train – 57 million pounds of CO 2 has been reduced • equivalent to take out circulation 37000 cars. – 16 billion gallons of water per year have been saved www. epa. gov/greenchemistry/ Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry • Paul Anastas and John Warner in Green Chemistry: Theory and Practice (Oxford University Press: New York, 1998).

Twelve Principles of Green Chemistry: 1. Prevent waste • Design chemical syntheses to prevent waste, leaving no waste to treat or clean up. • Easier than clean up, transport or store them • Transforming chemical reactions that incorporate the largest amount of the starting materials = Less waste! Angela González, Ph. D. / UIA-SG

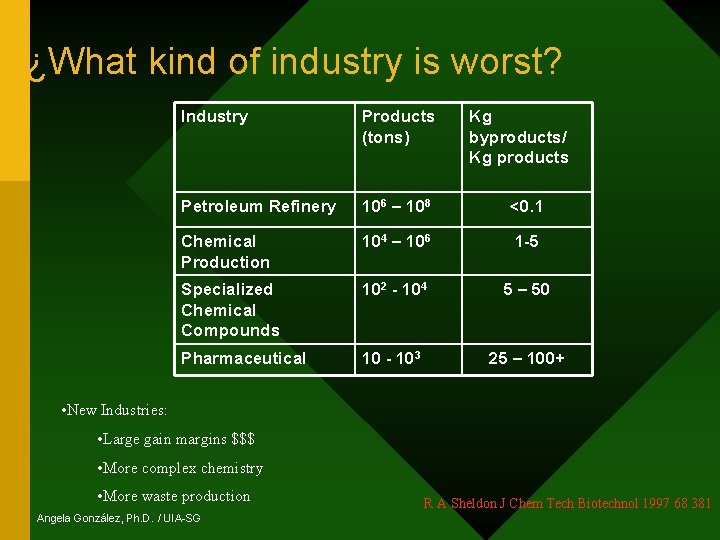
¿What kind of industry is worst? Industry Products (tons) Kg byproducts/ Kg products Petroleum Refinery 106 – 108 <0. 1 Chemical Production 104 – 106 1 -5 Specialized Chemical Compounds 102 - 104 5 – 50 Pharmaceutical 10 - 103 25 – 100+ • New Industries: • Large gain margins $$$ • More complex chemistry • More waste production Angela González, Ph. D. / UIA-SG R A Sheldon J Chem Tech Biotechnol 1997 68 381

Possible sources of waste reduction: Inputs “eco-friendly” solvents, high purity reagents, solvent recycling. Production Optimization of reaction time, temperature and pressure. New synthesis pathways. Discharges Reduce water usage, improve filtering procedures and reuse waste products. Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry: 2. Design safer chemicals and products: • Design chemical products to be fully effective, yet have little or no toxicity. • …chemicals that are less hazardous to human health and the environment are: – Less toxic to organisms and ecosystems – Not persistent or bioaccumulative in organisms or the environment – Inherently safer with respect to handling and use Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry: 3. Design less hazardous chemical syntheses: • Use and generate substances with little or no toxicity to humans and the environment. • Avoid reactions that give dangerous byproducts Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry: 4. Use renewable feedstocks: • Use raw materials and feedstocks that are renewable rather than depleting. • Agricultural products • Wastes of other processes; • Reduce dependency from fossil fuels (petroleum, natural gas, or coal) or are mined. Angela González, Ph. D. / UIA-SG

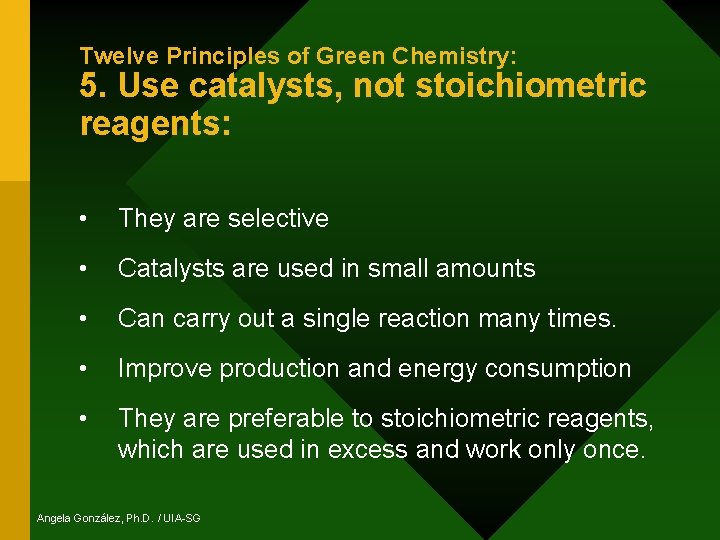
Twelve Principles of Green Chemistry: 5. Use catalysts, not stoichiometric reagents: • They are selective • Catalysts are used in small amounts • Can carry out a single reaction many times. • Improve production and energy consumption • They are preferable to stoichiometric reagents, which are used in excess and work only once. Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry: 6. Avoid chemical derivatives: • Avoid: • blocking or protecting groups or any temporary modifications if possible. • Derivatives use additional reagents and generate waste. Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry: 7. Maximize atom economy: • Final product contains the maximum proportion of the starting materials. There should be few, if any, wasted atoms. • Relation between atoms in the products and atoms in the reagents – Addition – good atom efficiency – Elimination – not so good… Angela González, Ph. D. / UIA-SG

Atom Economy – Barry Trost, Stanford University – Evaluate the efficiency of the chemical transformation “Because an Atom is a Terrible Thing to Waste” Angela González, Ph. D. / UIA-SG

Atom Economy How many of the atoms of the reactant are incorporated into the final product and how many are wasted? Angela González, Ph. D. / UIA-SG

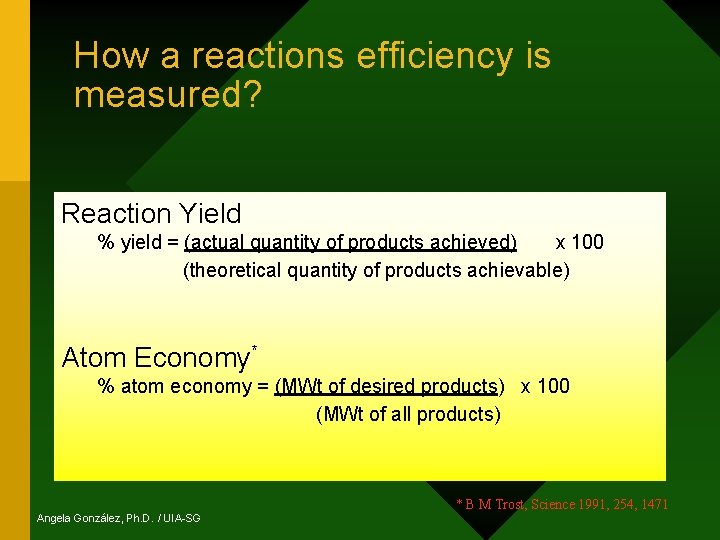
How a reactions efficiency is measured? Reaction Yield % yield = (actual quantity of products achieved) x 100 (theoretical quantity of products achievable) Atom Economy* % atom economy = (MWt of desired products) x 100 (MWt of all products) Angela González, Ph. D. / UIA-SG * B M Trost, Science 1991, 254, 1471

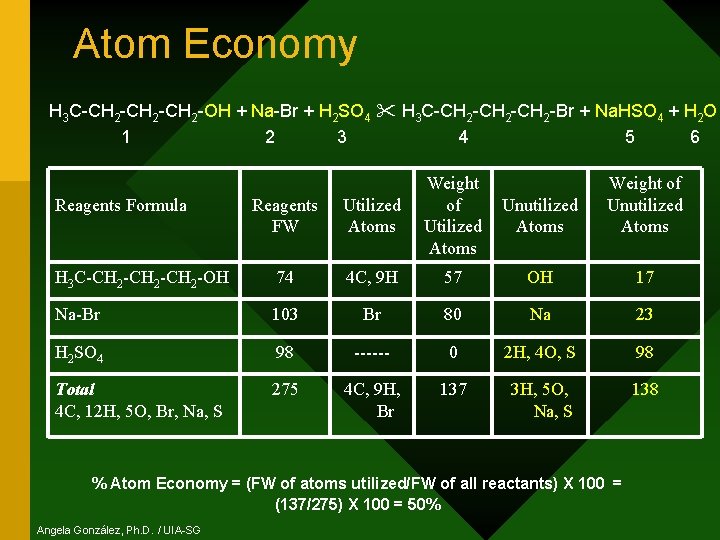
Atom Economy H 3 C-CH 2 -CH 2 -OH + Na-Br + H 2 SO 4 H 3 C-CH 2 -CH 2 -Br + Na. HSO 4 + H 2 O 1 2 3 4 5 6 Reagents FW Utilized Atoms Weight of Utilized Atoms H 3 C-CH 2 -CH 2 -OH 74 4 C, 9 H 57 OH 17 Na-Br 103 Br 80 Na 23 H 2 SO 4 98 ------ 0 2 H, 4 O, S 98 Total 4 C, 12 H, 5 O, Br, Na, S 275 4 C, 9 H, Br 137 3 H, 5 O, Na, S 138 Reagents Formula Unutilized Atoms Weight of Unutilized Atoms % Atom Economy = (FW of atoms utilized/FW of all reactants) X 100 = (137/275) X 100 = 50% Angela González, Ph. D. / UIA-SG

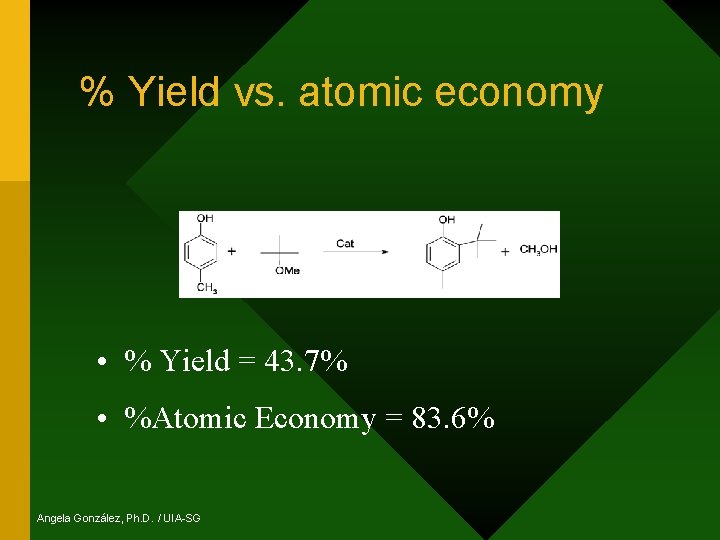
% Yield vs. atomic economy • % Yield = 43. 7% • %Atomic Economy = 83. 6% Angela González, Ph. D. / UIA-SG

Chemical Reactions and Atomic Economy • Addition > Substitutions > Eliminations • Re-arrangements: 100 % efficient – Ex: Diels-Alder Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry: 8. Use safer solvents and reaction conditions • Avoid: – solvents, – separation agents, – other auxiliary chemicals. • USE: innocuous chemicals. Angela González, Ph. D. / UIA-SG

Solvents • Organic solvents = high VOC’s • Alternatives – – Synthesis without solvents Water Supercritical fluids (CO 2) Ionic Liquids Angela González, Ph. D. / UIA-SG

Dry Cleaning – Greener… • Initially gasoline and kerosene were used • Now use PERC • Future use Supercritical CO 2 and CO 2 surfactants Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry: 9. Increase energy efficiency: • Run chemical reactions at ambient temperature and pressure whenever possible. Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry: 10. Design chemicals and products to degrade after use: • Design chemical products to break down to innocuous substances after use so that they do not accumulate in the environment. Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry: 11. Analyze in real time to prevent pollution: • Include in-process realtime monitoring and control during syntheses to minimize or eliminate the formation of byproducts. Angela González, Ph. D. / UIA-SG

PAT: Process Analytical Technologies • Measure quality and execution properties DURING manufacturing • Information is gather continuously to improve process Angela González, Ph. D. / UIA-SG http: //www. fda. gov/Cder/OPS/PAT. htm

Pre - PAT • Analysis of raw materials, intermediates and final products • In case of problems: WHOLE lot rejected = A LOT OF WASTE!. . . A LOT OF $$$$!! Angela González, Ph. D. / UIA-SG

Like you do while cooking… • While you cook you test the food, and apply the needed corrections to improve it: – If it is too salty: … – If it is too bland: …. – If you have extra guest… – If it is too acidic: stop there… Angela González, Ph. D. / UIA-SG

PAT: Process Analytical Technologies • Helps to: – Understand the process – Make corrections in the moment, without waiting for the final product – Develop mitigation strategies. Angela González, Ph. D. / UIA-SG

Twelve Principles of Green Chemistry: 12. Minimize the potential for accidents: • Design chemicals and their forms (solid, liquid, or gas) to minimize the potential for chemical accidents: • explosions, • fires, and • releases to the environment. Angela González, Ph. D. / UIA-SG

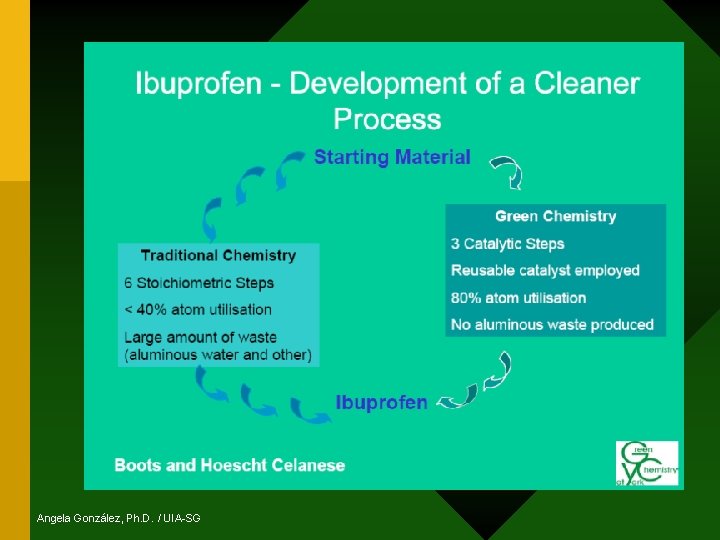
Example of Green Chemistry Synthesis Ibuprofen

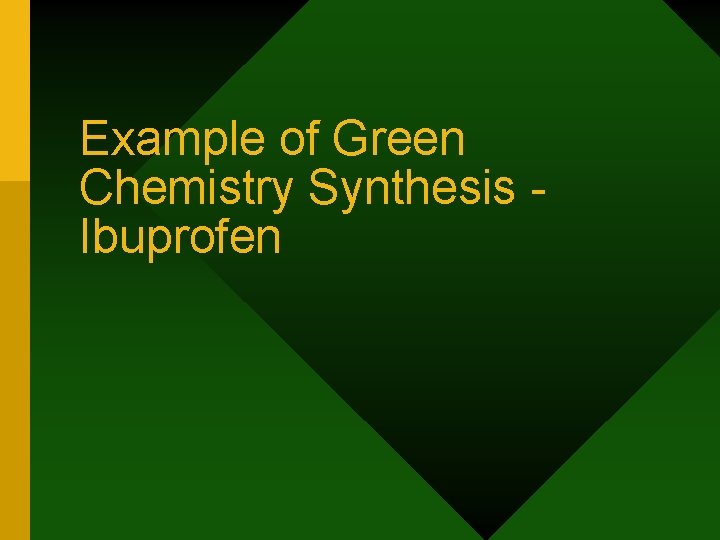
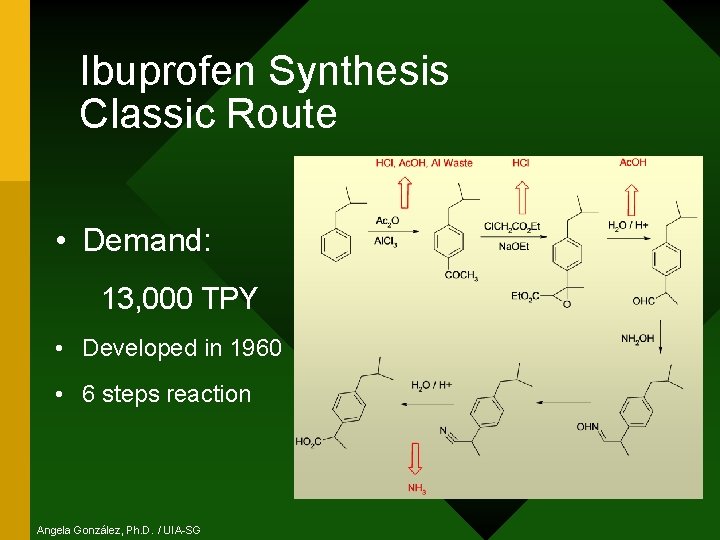
Ibuprofen Synthesis Classic Route • Demand: 13, 000 TPY • Developed in 1960 • 6 steps reaction Angela González, Ph. D. / UIA-SG

Ibuprofen Synthesis Classic Route • Atomic Economy: 32% • If this synthesis were to be used today, the amount of by-products per year: Angela González, Ph. D. / UIA-SG MORE WASTE THAN PRODUCT!!!

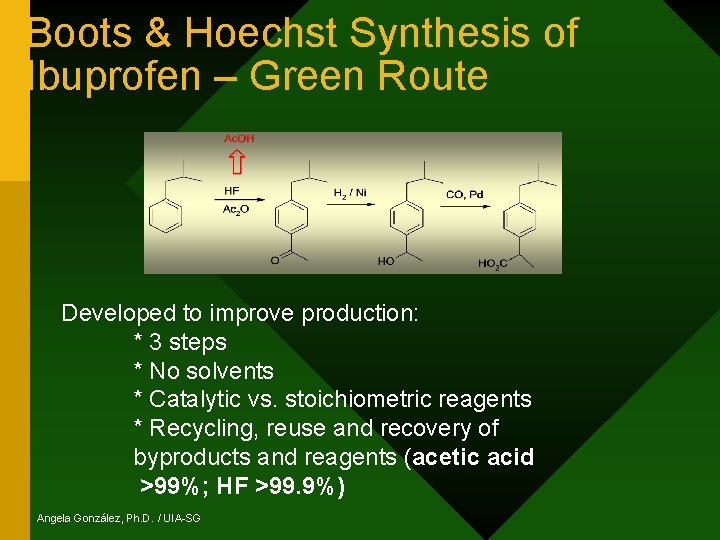
Boots & Hoechst Synthesis of Ibuprofen – Green Route Developed to improve production: * 3 steps * No solvents * Catalytic vs. stoichiometric reagents * Recycling, reuse and recovery of byproducts and reagents (acetic acid >99%; HF >99. 9%) Angela González, Ph. D. / UIA-SG

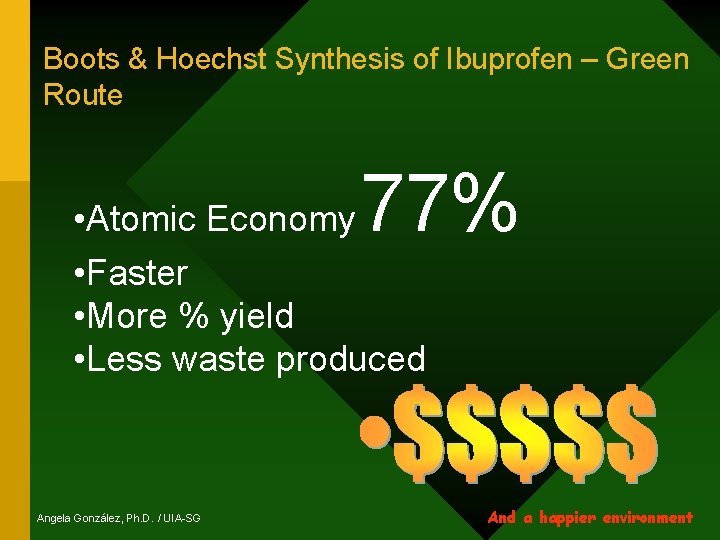
Boots & Hoechst Synthesis of Ibuprofen – Green Route 77% • Atomic Economy • Faster • More % yield • Less waste produced Angela González, Ph. D. / UIA-SG And a happier environment

Angela González, Ph. D. / UIA-SG

Angela González, Ph. D. / UIA-SG

What is green chemistry looking for? • Waste production minimization from the source • Use of catalysts • Use of non-toxic reagents • Use of renewable sources • Improvement of atomic economy • Use of non-solvent or environmental benign solvents Angela González, Ph. D. / UIA-SG

• Anastas, P. T. ; Warner, J. C. Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998 • B M Trost, Science 1991, 254, 1471 • PAT: – http: //www. fda. gov/Cder/OPS/PAT. htm • EPA: – http: //www. epa. gov/greenchemistry/index. html • ACS Green Chemistry Institute: – http: //acs. org • Michael Cann, University of Scranton: – http: //academic. scranton. edu/faculty/CANNM 1/greench emistry. html Angela González, Ph. D. / UIA-SG
 Ngela
Ngela Building a greener world
Building a greener world Gonzlez
Gonzlez Carlos gonzlez
Carlos gonzlez Green green green red
Green green green red Future perfect vs future continuous
Future perfect vs future continuous Future perfect e future continuous
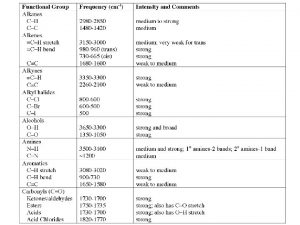
Future perfect e future continuous Functional groups ib chemistry
Functional groups ib chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Atom economy green chemistry
Atom economy green chemistry 12 principles of green chemistry with examples
12 principles of green chemistry with examples Antifoulant green chemistry
Antifoulant green chemistry Auxiliary substances
Auxiliary substances Nature mace
Nature mace E factor green chemistry
E factor green chemistry Acs gci pharmaceutical roundtable
Acs gci pharmaceutical roundtable Green chemistry book by chandrakanta bandyopadhyay
Green chemistry book by chandrakanta bandyopadhyay Ionic liquids green chemistry ppt
Ionic liquids green chemistry ppt Green chemistry
Green chemistry Conclusion of green chemistry
Conclusion of green chemistry Acs green chemistry
Acs green chemistry Peter dunn pfizer
Peter dunn pfizer Lernpyramide von green & green (2005)
Lernpyramide von green & green (2005) Red green yellow blue
Red green yellow blue Frc control system
Frc control system Perfect future tense
Perfect future tense Facts about tenses
Facts about tenses See future continuous
See future continuous Future nurse future midwife
Future nurse future midwife Past future continous tense
Past future continous tense Present progressive of plan
Present progressive of plan Summary tenses
Summary tenses Future plans and finished future actions
Future plans and finished future actions Future perfect future perfect continuous
Future perfect future perfect continuous Kondicional 1 engleski
Kondicional 1 engleski Prof. dr. marcus eckert
Prof. dr. marcus eckert Prof agamenon
Prof agamenon Prof david toback
Prof david toback Dr ali hossain
Dr ali hossain Prof suganda tanuwidjaja
Prof suganda tanuwidjaja Th
Th Tracce svolte tfa sostegno secondaria pdf
Tracce svolte tfa sostegno secondaria pdf Conclusion partielle exemple
Conclusion partielle exemple Syzyfowe prace epika liryka czy dramat
Syzyfowe prace epika liryka czy dramat Prof. grace schneider
Prof. grace schneider Sonnet 29 by edna st vincent millay
Sonnet 29 by edna st vincent millay Prof maya devita
Prof maya devita Radkal
Radkal Obsatar sinaga
Obsatar sinaga Pem nedir tıp
Pem nedir tıp Sfi research professorship
Sfi research professorship