GLYCOSIDES Definition and properties of glycosides Glycosides are










- Slides: 20

GLYCOSIDES

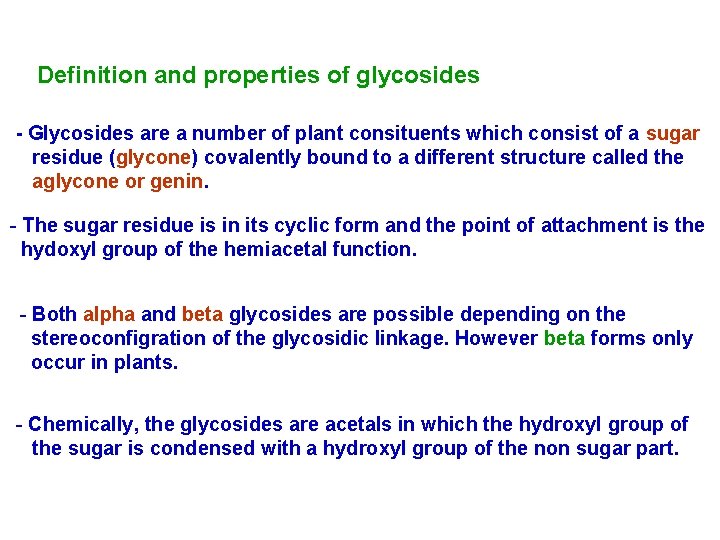
Definition and properties of glycosides - Glycosides are a number of plant consituents which consist of a sugar residue (glycone) covalently bound to a different structure called the aglycone or genin. - The sugar residue is in its cyclic form and the point of attachment is the hydoxyl group of the hemiacetal function. - Both alpha and beta glycosides are possible depending on the stereoconfigration of the glycosidic linkage. However beta forms only occur in plants. - Chemically, the glycosides are acetals in which the hydroxyl group of the sugar is condensed with a hydroxyl group of the non sugar part.

- Glycosides are usually soluble in water and in polar organic solvents, whereas aglycones are normally insoluble or slightly soluble in water. - Glycoside biosynthesis includes the aglycone as well as the linkage between sugar and aglycone. - Most of them are bitter in taste, although some glycosides have sweet taste. - The biosynthesis of the aglycone depends of course on its structure. The coupling of the sugar and aglycone, however, takes place in the same way, whatever the nature of the aglycone. Uridin Tri. Phosphate

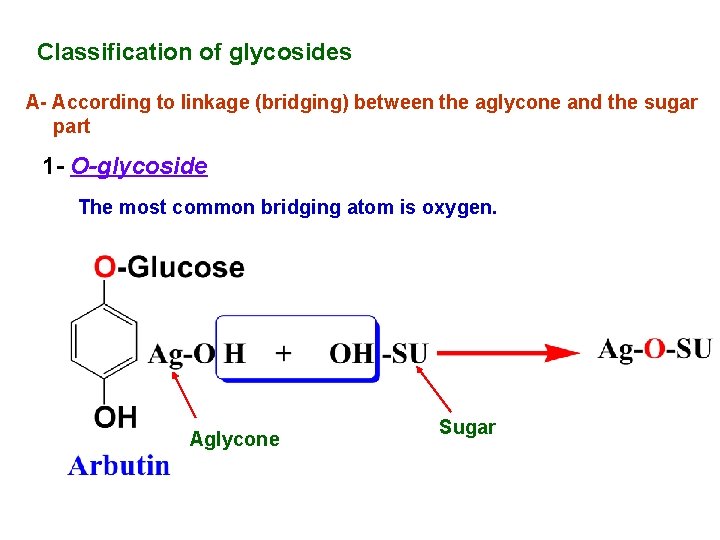
Classification of glycosides A- According to linkage (bridging) between the aglycone and the sugar part 1 - O-glycoside The most common bridging atom is oxygen. Aglycone Sugar

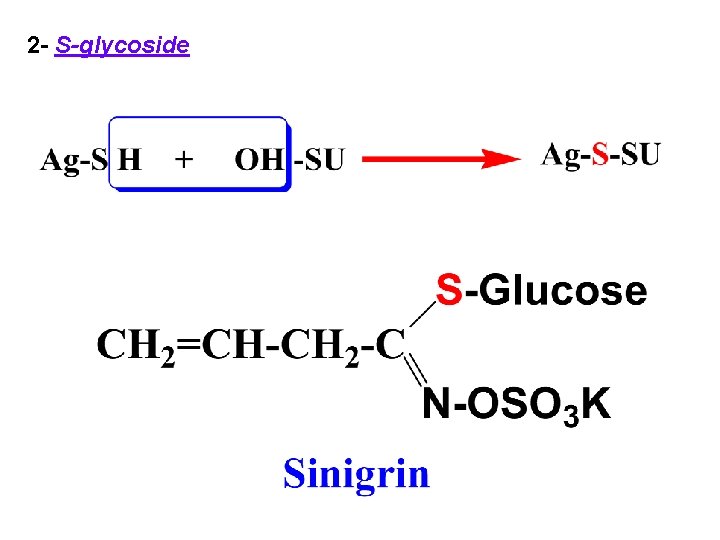
2 - S-glycoside

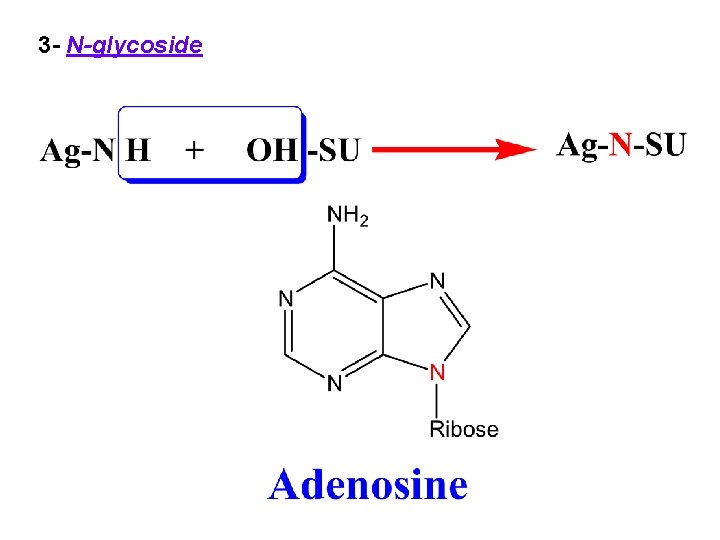
3 - N-glycoside

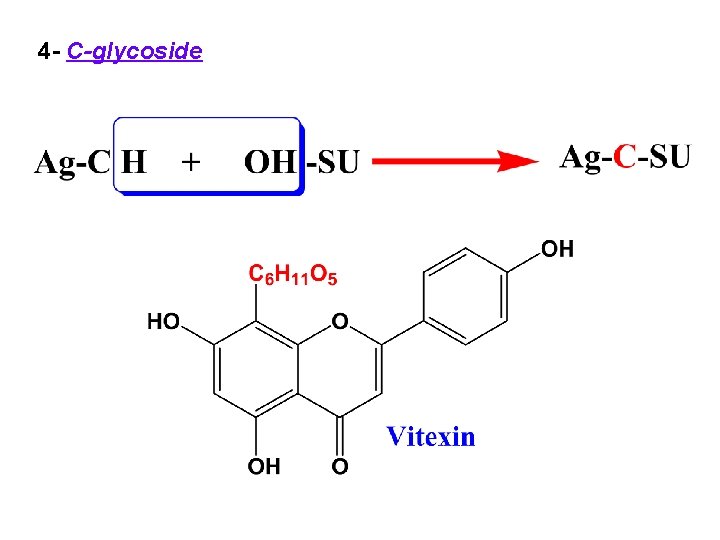
4 - C-glycoside

B- According to configuration of the glycosidic linkage - May be alpha or beta glycoside, but most of naturally occuring glycosides are of the beta type with some exception as sucrose, starch. C- According to the type of sugar - According to the sugar linked to the aglycone, glycosides are classified into the following: 1 - Glucosides: contain glucose sugar. 2 - galactosides: contain galactose sugar. 3 - Rhamnoside: contain rhamnose sugar.

D- According to the chemical nature of the aglycone - They are classified to the following: 1 - Flavonoid glycosides 2 - Anthraquinone glycosides 3 - Cardiac glycosides 4 - saponin glycosides 5 - Thioglycosides (sulphur glycosides) 7 - Phenolic glycoside 6 - Cyanophor glycosides 8 - Coumarins 9 - Terpenoidal glycosides 10 - Complex glycosides: which include, glycosidal tannin and glycosidal resin.

Stability and hydrolytic cleavage of glycosides I- Enzymatic hydrolysis - Almost all plants that contain glycosides also contain enzymes that bring about their hydrolysis (glycosidases). - In order to obtain the glycosides intact, the plant material must be treated so that contact between enzymes and glycosides is avoided as far as possible. - Enzymes require water in order to function. Rapid drying of the plant material may therfore decrease the risk of glycoside hydrolysis.

- Examples for enzymes, myrosin enzyme hydrolyses singrin, emulsin enzyme hydrolyses amygdalin. II- Acidic hydrolysis - Acid hydrolysis of the glycosides leads to the cleavage of the glycosidic linkages. - As a general rule the acetal linkage between the aglycone and sugar is readly split than linkage between the sugars of saccharide portion of the glycoside. - Glycosides containing 2 -deoxy sugars are easily cleaved by weak acids. - C-glycosides are relatively resistnt to acid hydrolysis than O-glycosides and required strong oxidizing agents as Fe. Cl 3

III- Alkaline hydrolysis - It is less common because it may causes modification or break down in the aglycone or the sugar moities. For examples: - Lacton ring of the cardiac glycosides is opened. - Deacetylation of acetylated aglycone or sugar. Function of glycosides in plants 1 - Represent stored food reservers or sugar reservers. 2 - Source of energy as energy released during hydrolysis. 3 - Have regulatory role in the plant, they regulate osmosis and regulate the supply of important substances for metabolism.

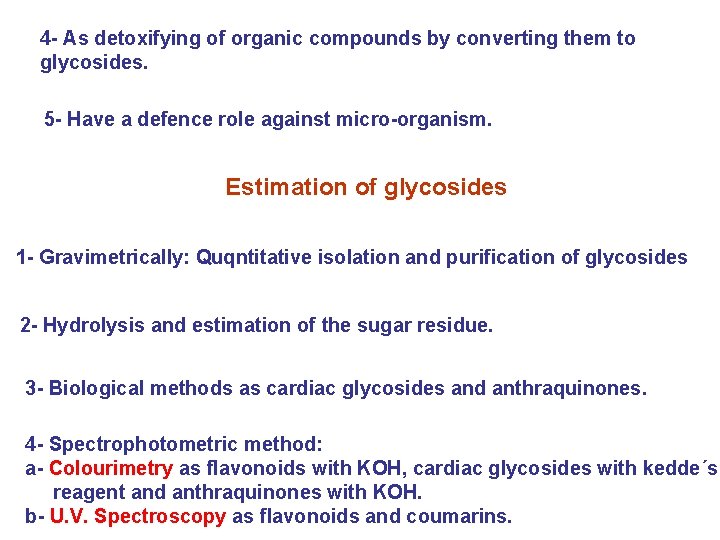
4 - As detoxifying of organic compounds by converting them to glycosides. 5 - Have a defence role against micro-organism. Estimation of glycosides 1 - Gravimetrically: Quqntitative isolation and purification of glycosides 2 - Hydrolysis and estimation of the sugar residue. 3 - Biological methods as cardiac glycosides and anthraquinones. 4 - Spectrophotometric method: a- Colourimetry as flavonoids with KOH, cardiac glycosides with kedde´s reagent and anthraquinones with KOH. b- U. V. Spectroscopy as flavonoids and coumarins.

Phenolic glycosides A- Simple phenolic glycosides B- Anthraquinone glycosides C- Flavonoid glycosides D- Coumarin glycosides

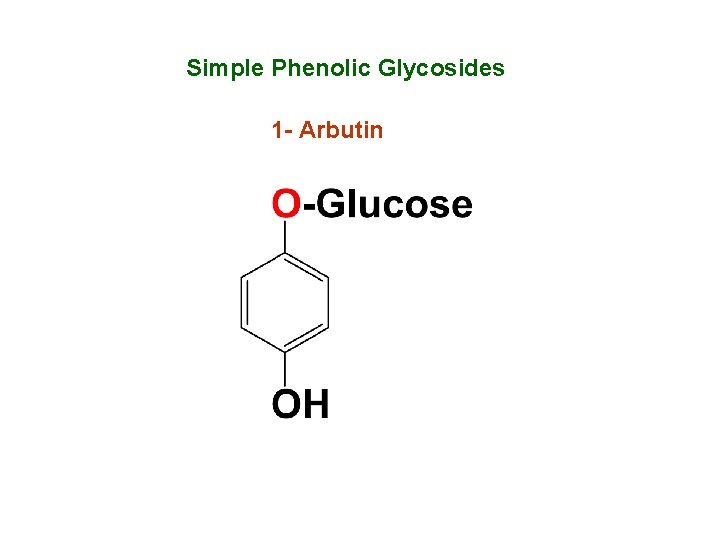
Simple Phenolic Glycosides 1 - Arbutin

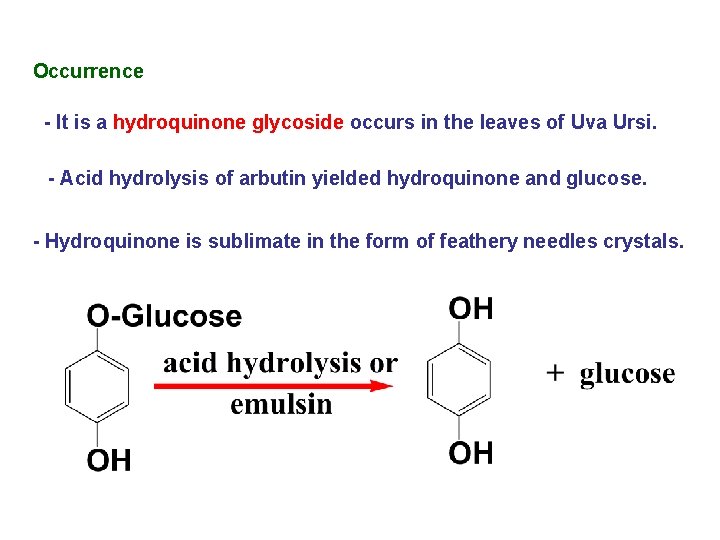
Occurrence - It is a hydroquinone glycoside occurs in the leaves of Uva Ursi. - Acid hydrolysis of arbutin yielded hydroquinone and glucose. - Hydroquinone is sublimate in the form of feathery needles crystals.

Test for identity 1 - With Fe. Cl 3 give blue colour 2 - Microsublimation test. Uses 1 - Mild astringent. 2 - Diuretic and used in urethritis. 3 - Urinary tract antiseptic.

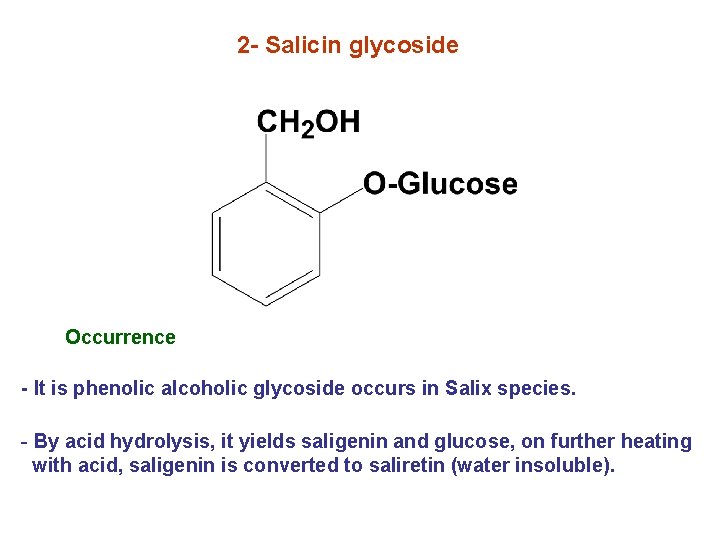
2 - Salicin glycoside Occurrence - It is phenolic alcoholic glycoside occurs in Salix species. - By acid hydrolysis, it yields saligenin and glucose, on further heating with acid, saligenin is converted to saliretin (water insoluble).


Test for identity 1 - Salicin + Conc. H 2 SO 4 give red colour disappear by adding water. 2 - Salicin + Froehd´s reagent give violet colour. 3 - Salicin + Erdmann´s reagent give bright red colour. Uses - It is used as analgesic, antipyretic and antirheumatic (aspirin-like action).
 Antigentest åre
Antigentest åre Chemical test for glycosides
Chemical test for glycosides Glycosides in pharmacognosy
Glycosides in pharmacognosy Glycosides examples
Glycosides examples Anthracene glycoside
Anthracene glycoside Glycosides examples
Glycosides examples Functions of glycosides
Functions of glycosides Glycoside
Glycoside Intensive and extensive properties
Intensive and extensive properties Chemical property of matter
Chemical property of matter Definition of a kite in geometry
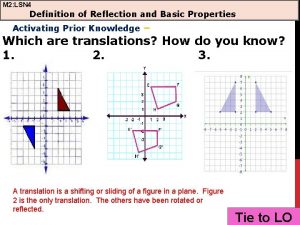
Definition of a kite in geometry Definition of reflection and basic properties
Definition of reflection and basic properties Colligative properties definition
Colligative properties definition Definition of colligative properties
Definition of colligative properties Solubility of matter
Solubility of matter Properties of light definition
Properties of light definition Properties of kite
Properties of kite Segment fg is the angle bisector of efh
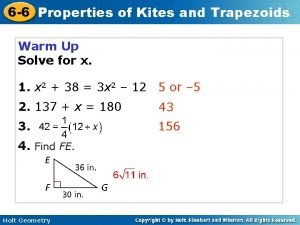
Segment fg is the angle bisector of efh Homework 6 trapezoids
Homework 6 trapezoids What are the characteristics of bases
What are the characteristics of bases Atomic structure and properties ap chemistry
Atomic structure and properties ap chemistry