GELS Gels Gels are semisolid preparations that contain


























- Slides: 34

GELS

Gels • Gels are semisolid preparations that contain small inorganic particles or large organic molecules interpenetrated by a liquid. • The vehicle may be: Aqueous , hydroalcoholic , alcohol based or non aqueous • In a typical polar gel, a natural or synthetic polymer builds a three-dimensional matrix throughout a hydrophilic liquid. • Gels are attractive delivery systems as they are simple to manufacture and suitable for administering drugs through skin, oral, buccal, ophthalmic, nasal, otic, and vaginal routes • Gels may appear transparent or turbid based on the type of gelling agent used

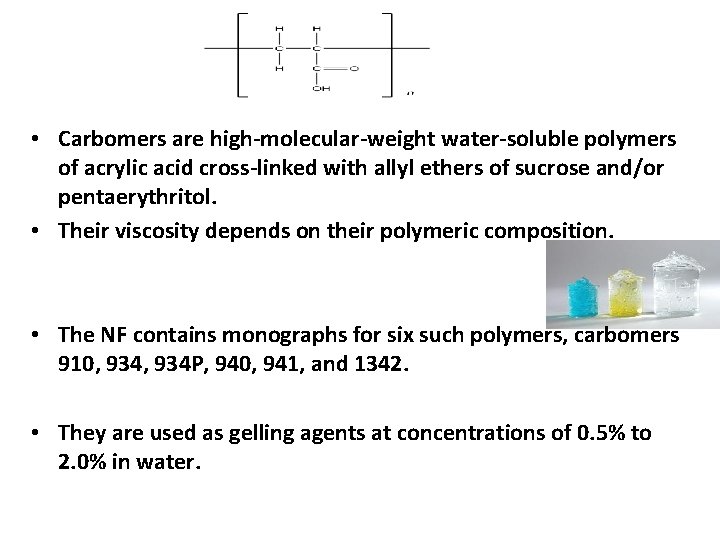
Gels • Gelling Agent : These are substances which, when added to an aqueous mixture, increase its viscosity without substantially modifying its other properties, such as taste. • Types of Gelling Agents: 1. Natural Polymers e. g. proteins, polysaccharides , natural gums, such as tragacanth, carrageenan, pectin, agar and alginic acid 2. Semi synthetic Polymers e. g. , cellulose derivatives methylcellulose, hydroxypropylmethylcellulose and carboxymethylcellulose 3. Synthetic polymers such as carbomer 934

• Carbomers are high-molecular-weight water-soluble polymers of acrylic acid cross-linked with allyl ethers of sucrose and/or pentaerythritol. • Their viscosity depends on their polymeric composition. • The NF contains monographs for six such polymers, carbomers 910, 934 P, 940, 941, and 1342. • They are used as gelling agents at concentrations of 0. 5% to 2. 0% in water.

Properties of Gels 1. Ideally, the gelling agent must be inert, safe and cannot react with other formulation constituents. 2. The gelling agent should produce a sensible solid-like nature at the time of storage which is easily broken when exposed to shear forces produced by squeezing the tube, trembling the bottle or at the time of topical application. 3. It should have suitable anti-microbial agent. 4. The topical gel must not be sticky. 5. The ophthalmic gel must be sterile. 6. The apparent viscosity or gel strength increases with an increase in the effective crosslink density of the gel. However, a rise in temperature may increase or decrease the apparent viscosity, depending on the molecular interactions between the polymer and solvent.

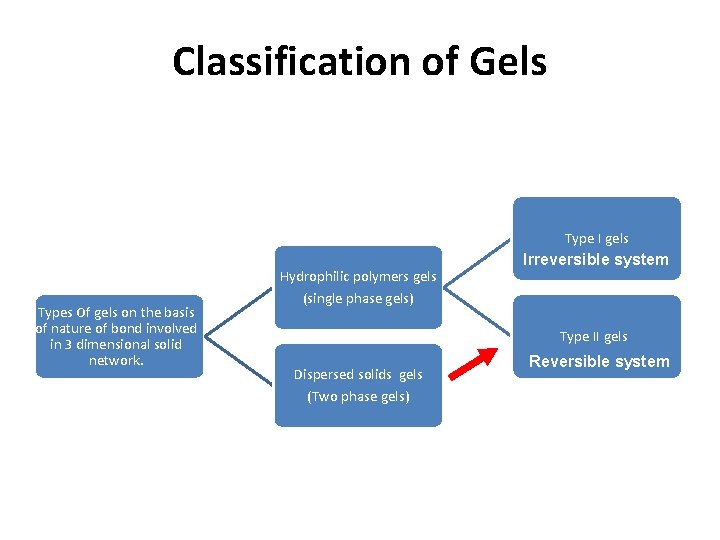
Classification of Gels Type I gels Types Of gels on the basis of nature of bond involved in 3 dimensional solid network. Hydrophilic polymers gels (single phase gels) Irreversible system Type II gels Dispersed solids gels (Two phase gels) Reversible system

GELS • Single-phase gels are gels in which the macromolecules are uniformly distributed throughout a liquid with no apparent boundaries between the dispersed macromolecules and the liquid. • A gel mass consisting of floccules of small distinct particles is termed a two-phase system, often referred to as a magma.

• Classification of Gels can be classified based on nature of continuous phase 1. Hydrogels (Water based) 2. Organogels (With a non-aqueous solvent) 3. Xerogels

Hydrogels (Water based) • A hydrogel is a network of polymer chains that are hydrophilic in which water is dispersion medium. • They are highly absorbent natural or synthetic polymeric networks. They also have a degree of flexibility, due to their significant water content • Uses for hydrogels 1. Sustained-release drug delivery systems 2. Rectal drug delivery and diagnosis 3. Hydrogel-coated wells have been used for cell culture 4. As scaffolds in tissue engineering 5. As environment sensitivity detector 6. Contact lenses (silicone hydrogels, polyacrylamides, polymacon) 7. ECG medical electrode 8. Dressing of healing .

These gels exhibit unique physicochemical properties, including: 1. The ability to absorb a considerable mass of aqueous fluid (often 100 times the original mass) whilst still retaining a three-dimensional structure. 2. Hydrogels exhibit robust mechanical properties, being resistant to fracture following exposure to stresses frequently up to 1 k. Pa 3. Hydrogels exhibit excellent flexibility. • hydrogels are clinically used as wound dressings, as lubricious coatings on urethral catheters and as soft contact lenses. In addition, hydrogels may be used for the controlled delivery of therapeutic agents at the site of implantation.

Organogel • An organogel is a non-crystalline, non-glassy thermoreversible solid material composed of a liquid organic phase trapped in a 3 D cross-linked network. • The liquid can be, E. g. , vegetable oil, an organic solvent or mineral oil. Xerogels • It is a solid formed from a gel by drying with unrestricted shrinkage. It is frequently retains high porosity (15 -50%) and huge surface area (150 -900 m 2/g) • E. g. , Tragacanth ribbons, β-cyclodextrin, dry cellulose and polystyrene, gelatin sheets and acacia tears.

Dispersed Solids Gels ( Two phase gels) • A gel mass consisting of floccules of small distinct particles is termed a two-phase system or dispersed solids gels. • The nature of interaction between particles in network may be: 1. Van der waals , e. g. Al-hydroxide gel USP 2. Electrostatics interaction, examples include kaolin, bentonite and aluminium magnesium silicate

• The bond strength between the particles is weak • Interparticle bonds are broken by the application of relatively low shearing stresses (such as those that occur whenever the product is shaken), thereby liberating the individual particles. • Following removal of the stress the bonds between the particles will reform and hence the rheological structure of these systems is recovered , this is termed thixotropy.

• If the particle size of the dispersed phase is relatively large, the gel mass is sometimes called as a magma. • Milk of magnesia (or magnesia magma), which consists of a gelatinous precipitate of magnesium hydroxide, is such a system.

Formulation considerations for pharmaceutical gels • There are several formulation considerations open to the pharmaceutical scientist concerning the formulation of pharmaceutical gels. These include: (1) The choice of vehicle (2)The inclusion of buffers (3)Preservatives (4) Antioxidants (5) Flavors/sweetening agents; and (6) Colors

Manufacture of Gels • Generally the water soluble excipients are firstly dissolved in vehicle, in a mixing vessel by using mechanical stirrer. • To prevent aggregation, add hydrophilic polymer to the stirred mixture slowly. • Stirring is continued until the dissolution of the polymer has occurred. • The excessive stirring results in entrapment of air. The mixing rate must not be extreme or a mixing vessel may be used to which a vacuum may be pulled, to prevent the entrapment of air.

CREAMS

creams • Pharmaceutical creams are semisolid preparations containing one or more medicinal agents dissolved or dispersed in either a W/O emulsion or an O/W emulsion or in another type of water-washable base. • Creams are more fluid compared to other semisolid dosage forms, such as ointments and pastes, since the bases used in creams are generally o/w emulsions. • Creams have a whitish with creamy appearance. • The use of creams as drug delivery systems is associated with good patient acceptance



• Oil-in-water emulsions are most useful as water washable bases, whereas water-in-oil emulsions are emollient and cleansing.

Cold cream A semisolid white w/o emulsion prepared with cetyl ester wax, white wax, mineral oil, sodium borate, and purified water. • Sodium borate combines with free fatty acids present in the waxes to form sodium salts of fatty acids (soaps) that act as emulsifiers. • These are produced by emulsifying agents of natural origin, e. g. beeswax, wool alcohols or wool fat. • They are useful as softening and cleansing agents ex. A cold cream is used to remove makeup. • The name, cold cream, refers to the cooling sensation associated with the slow evaporation of the dispersed aqueous phase. • Other common cold cream components include mineral oil, jojoba oil, lanolin, glycerin, alcohol, borax, and beeswax in addition to preservatives such as methylparaben and propylparaben. .

creams • Creams find primary application in topical skin products and in products used rectally and vaginally. • Patients often prefer a w/o cream to an ointment because the cream spreads more readily, is less greasy, and the evaporating water soothes the inflamed tissue. • Pharmaceutical manufacturers frequently manufacture topical preparations of a drug in both cream and ointment bases to satisfy the preference of the patient and physician.

Vanishing cream • an o/w emulsion that contains a large percentage of water as well as a humectants (e. g. , sorbitol, glycerin, or propylene glycol) that retards surface evaporation of water. • These are produced by synthetic waxes, e. g. macrogol and cetomacrogol • They are useful as water-washable bases • Examples : Shaving creams , Hand creams , Foundation creams • Upon rubbing this cream on the skin, the external/continuous aqueous phase evaporates, leading to increased concentration of a water-soluble drug in the oily film that adheres to the skin. This increase in the concentration gradient of the drug across the stratum corneum promotes percutaneous absorption

creams • An o/w cream is non-occlusive because it does not deposit a continuous film of waterimpervious liquid. • However, such a cream can deposit lipids and other moisturizers on and into the stratum corneum and so restore the tissue's hydration ability, i. e. the preparation has emollient properties.


Pastes

• Pastes are ointments containing as much as 50% powder dispersed in a fatty base and therefore are stiffer. • They are less greasy than ointments because the powder absorbs some of the fluid hydrocarbons. • Pastes lay down a thick, unbroken, relatively impermeable film.

Pastes • Pastes can be prepared in the same manner as ointments, by direct mixing or the use of heat to soften the base prior to incorporating the solids, which have been comminuted and sieved. • when a levigating agent is to be used to render the powdered component smooth, a portion of the base is often used rather than a liquid, which would soften the paste.

Pastes • Because of the stiffness of pastes, they remain in place after application and are effectively employed to absorb serous secretions. • Because of their stiffness and impenetrability, pastes are not suited for application to hairy parts of the body.

MISCELLANEOUS SEMISOLID PREPARATIONS • PLASTERS • Plasters are solid or semisolid adhesive masses spread on a backing of paper, fabric, or plastic. • The adhesive material is a rubber base or a synthetic resin. • Plasters are applied to the skin to provide prolonged contact at the site. • Unmedicated plasters provide protection or mechanical support at the site of application.

PLASTERS • Medicated plasters provide effects at the site of application. • They may be cut to size to conform to the surface to be covered. • Among the few plasters in use today is salicylic acid plaster used on the toes for the removal of corns. • The horny layers of skin are removed by the keratolytic action of salicylic acid. • The concentration of salicylic acid used in commercial corn plasters ranges from 10% to 40%.

GLYCEROGELATINS • Glycerogelatins are plastic masses containing gelatin (15%), glycerin (40%), water (35%), and an added medicinal substance (10%), such as zinc oxide. • They are prepared by first softening the gelatin in the water for about 10 minutes, heating on a steam bath until the gelatin is dissolved, adding the medicinal substance mixed with the glycerin, and allowing the mixture to cool with stirring until congealed.

GLYCEROGELATINS • Glycerogelatins are applied to the skin for the long term. • They are melted before application, cooled to slightly above body temperature, and applied to the affected area with a fine brush. • Following application, the glycerogelatin hardens, is usually covered with a bandage, and is allowed to remain in place for weeks. • The most recent official glycerogelatin was zinc gelatin, used in the treatment of varicose ulcers.
 Sterile semisolid preparations for ophthalmic use only are
Sterile semisolid preparations for ophthalmic use only are Insidan region jh
Insidan region jh Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block nhĩ thất cấp 1
Block nhĩ thất cấp 1 Tìm vết của đường thẳng
Tìm vết của đường thẳng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thể thơ truyền thống
Thể thơ truyền thống Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Tôn thất thuyết là ai
Tôn thất thuyết là ai Phân độ lown ngoại tâm thu
Phân độ lown ngoại tâm thu Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Preparation of alkynes
Preparation of alkynes Strengthening or concentration preparations can be made by:
Strengthening or concentration preparations can be made by: The cask of amontillado text analysis answers
The cask of amontillado text analysis answers Define monophasic liquid dosage form
Define monophasic liquid dosage form Sterile technique quiz
Sterile technique quiz Why did montresor make sure fortunato is drunk
Why did montresor make sure fortunato is drunk Definition of food preparation
Definition of food preparation Admixture iv
Admixture iv Advantages of solid dosage form
Advantages of solid dosage form Water removable bases
Water removable bases Semisolid gel
Semisolid gel Semisolid dosage form classification
Semisolid dosage form classification Classification of semisolid dosage forms
Classification of semisolid dosage forms Evaluation parameters of semi solid dosage forms
Evaluation parameters of semi solid dosage forms Preparation of ointments
Preparation of ointments Antibody precipitation
Antibody precipitation Stain free gels
Stain free gels Solcoseryl gēls mutes dobumam
Solcoseryl gēls mutes dobumam Which types of gels may be pigmented?
Which types of gels may be pigmented? Monophasic liquid dosage form example
Monophasic liquid dosage form example Pastes definition in pharmacy
Pastes definition in pharmacy Large volume parenterals uses
Large volume parenterals uses Foods high in phosphorus
Foods high in phosphorus