External gel preparations 1 Gel preparations Gels are
























- Slides: 33

External gel preparations 1

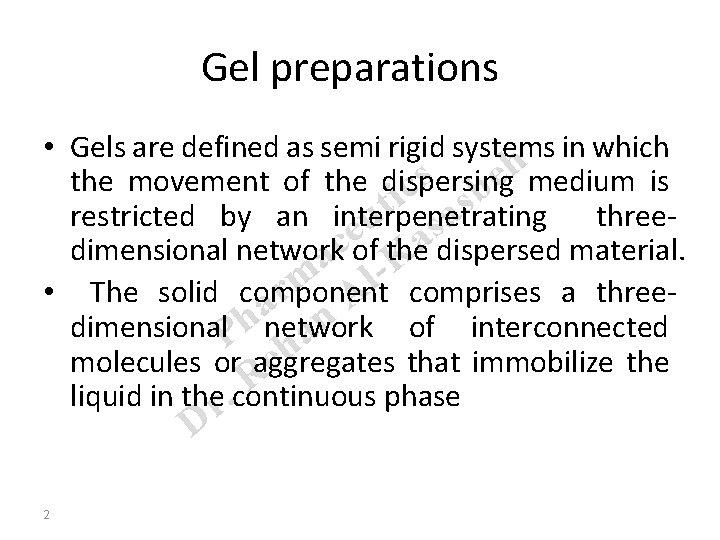
Gel preparations • Gels are defined as semi rigid systems in which h s e the movement of the dispersing medium is c b i s t restricted by an interpenetrating threea u s e a c dimensional network of the a -K dispersed material. m Al comprises a threer • The solid component a n dimensional. P h network of interconnected a h e molecules or aggregates that immobilize the R liquid in ther. continuous phase D 2

Pharmaceutical Gel preparations • • • 3 Gels, sometimes called jellies, are semisolid systems consisting of dispersions of small or large molecules usually in aqueous liquid vehicle. h s e c Gels may thicken on standing, forming a thixotrope, andb must be shaken before i s t use to liquefy the gel and enable pouring. a u s e a c Gels may be formulated to containadrugs, solvents (such as alcohol and/or PG), K preservatives (such as methylparaben andl -propylparaben or chlorhexidine m gluconate), and stabilizers. r A a n administration by various routes, including h afor Medicated gels may P be prepared skin, eye, nose, vagina, and rectum. h e R Aqueous gels are used. for lubrication or applying a drug to the skin. r D Oily gels are also available and used for occlusion.

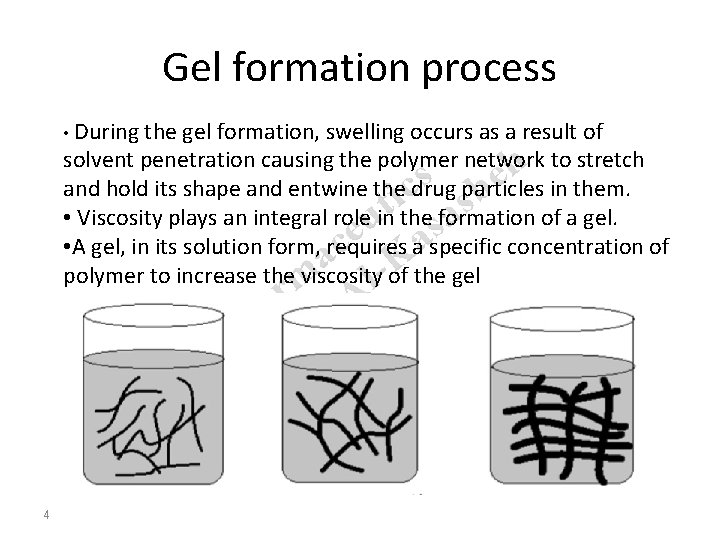
Gel formation process • During the gel formation, swelling occurs as a result of h s e c b i s t a u s e a c a -K m Al r a n h P ha e R. r D solvent penetration causing the polymer network to stretch and hold its shape and entwine the drug particles in them. • Viscosity plays an integral role in the formation of a gel. • A gel, in its solution form, requires a specific concentration of polymer to increase the viscosity of the gel 4

Advantages of Gel Formulations • Some of the major advantages of gel formulations over other semisolid dosage forms are as follows • Gels are easy to formulate as compared to other semisolid dosage forms. • A gel is an elegant non-greasy formulation. • It can be used as controlled release formulation • Gels have good adherence property to the site of application. • They are biodegradable and biocompatible. • They form a protective layer on the application site. • They are washable and nontoxic in nature. • They provide excellent spreadibility and cooling effect because of solvent evaporation. • They can be used to administer both polar and non-polar drugs. h s e c b i s t a u s e a c a -K m Al r a n h P ha e R. r D 5

Disadvantages / Limitations of Gel Formulations • The effect of gels is comparatively slower and sustained • The water content may increase the chances of microbial or fungal attack in gels • Syneresis (expulsion of solvent from the gel matrix) may occur in gels during storage. • Solvent evaporation from the formulation may result in drying of the gel. • Covalent bonds present in some gels may render them unbreakable thus sealing the medicament inside the gel matrix • Flocculation in some gels may produce an unstable gel. • Rheology of some gels may alter due to the effect of temperature, humidity and other environmental factors. • The gelling agents may precipitate and result in salting out. • Some drugs may degrade in gel formulation due to the presence of polymers. h s e c b i s t a u s e a c a -K m Al r a n h P ha e R. r D 6

Uses Some of the common uses of gel formulations are listed h as follows s e c b i s t • Gels are used to produce sustained release dosage a u s e form. a c a and K • They are used as lubricants as carriers for different l m r A pharmaceutical agents. a n h a drugs can be formulated in a • Both polar and. Pnon-polar h e single gel. R. to administer drugs through different • They can be used r routes like D topical, intraocular, intranasal, vaginal, rectal, and in some cases parenteral and intramuscular. 7

Gel types • Single-phase gels: in which the macromolecules h s e are uniformly distributed throughout a liquid with c b i s t a no apparent boundaries the dispersed ubetween s e a c macromolecules andathe liquid. K l m r A • Two-phase system: consisting of floccules of a n h a often referred to as small distinct. Pparticles, h Magmas (milk R ofemagnesia). . r • In a two-phase D system, the particle size of the dispersed phase is relatively large 8

Gel types • The nature of the solvent classify gels into two h s e basic types c b i s t a u s e a • Organogels c a -K • Hydrogels rm l A a n h P ha e R. r D 9

organogels • Solid material composed of liquid organic phase entrapped in three dimensional cross linked network. h s e c • A non-crystalline, non-glassy thermo b i reversible s t (thermoplastic) solid materialucomposed of a liquid organic a s e a phase entrapped in a three-dimensionally cross-linked c a -K network. l m r • The liquid can be, for example, an organic solvent, mineral A a oil, or vegetable oil. n h a on self-assembly of the P based • These systems are h e structuring molecules. R. are currently being studied as the • Only few organogels r organic solvents D are not generally accepted pharmaceutically for drug delivery. (e. g alkanes) 10

Organogels • Organogels may also be referred as oleaginous h s e gels. They are composed iof both polar and c b s t nonpolar groups but the ratio of the non-polar a u s e a c part is very high. a K l m 35%Awater r • They may contain as the gels tend a n h to swell in water. P ha e • Organogelators are usually low molecular R. molecules that have the ability to weight small r thicken in. Dorganic solvents 11

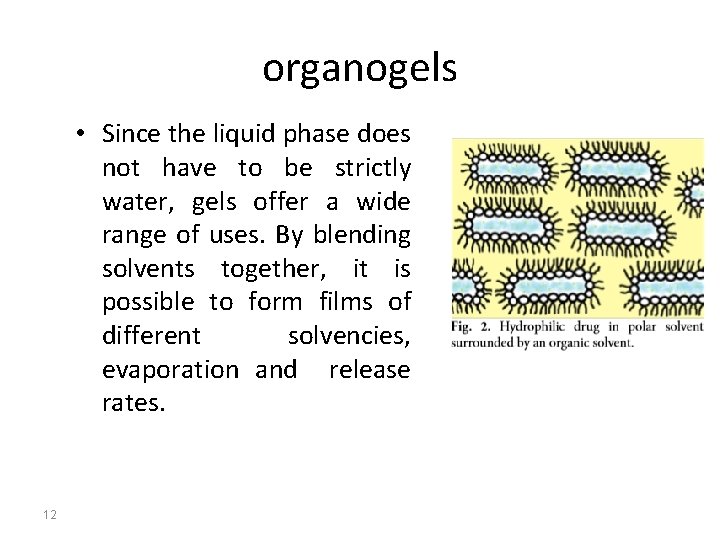
organogels • Since the liquid phase does not have to be strictly water, gels offer a wide range of uses. By blending solvents together, it is possible to form films of different solvencies, evaporation and release rates. 12

Hydrogels • It is a network of polymer chains that are h s e hydrophilic or colloidal gel in which water is c b i s t a u s the dispersion medium e a c a vehicles K • Xerogels: Gels in which has been l m r A a removed, leaving a polymer network (polymer n h P ha film). e R • Xerogel vs aerogel r. D 13

Hydrogel types Hydrogels may also be classified based on the h s e nature of the bonds involved in the three c b i s t dimensional solid network: a u s e a c • chemical gels formawhen strong covalent K l m r A together. bonds hold theanetwork n h a with 3 dimensional • Irreversible P system h e network formed by a covalent bonds between R. macromolecules. r D • Does not reply to any stress effect 14

• physical gels form when hydrogen bonds and h s electrostatic and van der Waals interactions e c b i s t maintain the gel network. a u • • 15 s e a c a -K l Reversible systemr m in which interaction occurred A a n h between polymers by a non-covalent bonding. P ha e R. Temporary destruction of bonds when stress r D applied thus formulation enable to flow.

Uses for hydrogels h s e c • Sustained-release drug delivery systems b i s t a • As scaffolds in tissuecengineering eu as • Contact lenses m a l - K r A a • ECG medical electrode n h P ha • Dressing of healing e R. magma, gelatin, cellulose E. g. , Bentonite r D and poloxamer gel. derivatives 16

Pharmaceutical Consideration Of Pharmaceutical Gels • • • 17 Choice of vehicles h s e c b Inclusion of buffers i s t a u s e a Preservatives c a -K Antioxidants r m A l a n h Flavoring and coloring P ha agent h e R. r D

Preparation of Gels can be prepared by following mechanisms: • Thermal changes : Some gels require temperature manipulation for their formation. Heating the liquid and then incorporating the polymer in it with thorough mixing and then leaving it to cool down for settling is a basic procedure for their formation. In contrast to this method, some gels should be kept away from heating because increasing the temperature will disrupt the bonds (for e. g. hydrogen bonds). • Flocculation: gelatin is triggered by increasing the concentration of the solute to high degree with out reaching precipitation concentration. • Chemical reaction: gelatin is introduced by chemical interactions between solute and solvents. h s e c b i s t a u s e a c a -K m Al r a n h P ha e R. r D 18

h s e c b i s t a u s e a c a -K m Al r a n h P ha e R. r D 19

Formulating gel • • 20 There are 3 methods: h s e c b Fusion Method i s t a u s e a Cold Method c a -K Dispersion Method rm Al a n h P ha e R. r D

Fusion method • In this method various waxy materials h s e employed as gellant in non polar media. c b i s t a u s e • Drug was added when waxy materials melted a c a -K by fusion. m Al r a • stirred slowlyhuntilauniform gel formed n P ha e R. r D 21

Cold method • Gelling agents are slowly added to Cold Water hcomplete. (4 -10°C) with mixing until solution is s e c b i s t a u • Drug is added in solution form slowly with s e a c a -K gentle mixing. m Al r • Heating to warm R. T , hence, liquid becomes a n h P ha clear gel. e R. r D 22

Dispersion method • Gelling agents are dispersed in water with h s e vigorous stirring. c b i s t a u s e awith preservative. • Drug is dissolved in solvent c a -K • Addition of drugrsolution m Al to the gel with a continuous stirring. h an P ha e R. r D 23

Gelling agents • These are substances which, when added to an h s e aqueous mixture, increase iits viscosity without c b s t a substantially modifyingeits properties. u other s a c a stability, K • Provide body, increase and improve l m r A suspension of added ingredient a n h a synthetic macromolecules P include • Gelling agents h e such as carbomers; cellulose derivatives such as R. r CMC or HPMC; D and natural gums such as tragacanth. 24

Gelling agents Tragacanth h s e • Concentrations of 2– 5% of tragacanth are used to c b i s t produce different viscosities. a u s a • Tragacanth is a naturalc eproduct and is therefore a -K liable to microbial m contamination. l r Alumps when added to a form • The gum tendshto n water and therefore P ha most formulae will include a e wetting agent such as ethanol, glycerol or R propylene glycol. . r D polymers must be prepared at RT, as • Tragacanth the material is heat labile. 25

h s e c b i s t a u s e a c a -K m Al r a n h P ha e R. r D 26

Gelling agents Alginates hseaweeds e • Natural polysaccaride extractedc sfrom the b i s t • The viscosity of alginate gels is more standardised than a u s e a that of tragacanth. c a -K • Alginate concentrations l produce fluid gels. m of. A 1. 5% r a • Alginate concentrations of 5– 10% produce n h agels suitable for topical dermatological. Pgrade h e application. R. (such as glycerol) need to be employed r • Wetting agents to prevent D production of a lumpy product. 27

h s e c b i s t a u s e a c a -K m Al r a n h P ha e R. r D 28

Gelling agents Cellulose derivatives h neutral, • Cellulose derivatives are widely used and form s e c stable gels. b i s t a attack. u microbial • They exhibit good resistanceeto s a when dried on the • Form clear gels with gooda c film strength K skin. m Al r a • Methylcellulose 450 is used in strengths of 3– 5% to n h produce gels. P a h • Carmellose sodium (sodium carboxymethylcellulose) is e R used in concentrations of 1. 5– 5% to make lubricating gels. . r the polymer solution until almost • Prepared by. D heating boiling (80 -90 °C), then the solution thickens into gel upon lowering the temperature (ice chilled water is a choice) 29

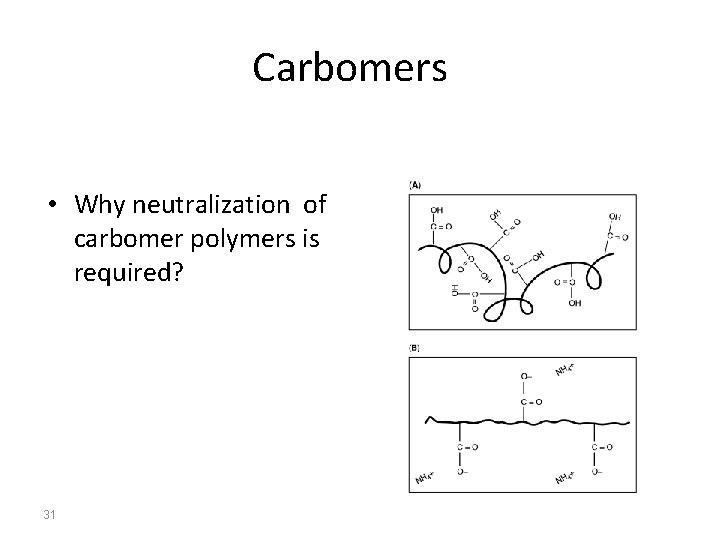
Gelling agents Carbomers • • • 30 h s e c b of acrylic i high-molecular weight water soluble polymers s t a u acid cross with polyalkenyl polyethers s e a c Carbomer is useful in production of clear gels (provided a K - in the gel production) too much air is not incorporated l m r Acarbomer acts as a lubricant. a In concentrations of 0. 3– 1%, n h a Carbomer is used preparations in P in dermatological h concentrations of 0. 5– 5%. e R neutralization increases their viscosity. Acidic in nature. but r D

Carbomers • Why neutralization of carbomer polymers is required? 31

Xanthan gum, gellan gum and pectin • • • 32 Xanthan gum and gellan gum are high molecular weight polysaccharides produced by microbial fermentation. The high viscosity associated with xanthan gum solutions enables products to keep particles suspended or prevent oil droplets from coalescing. Because the viscosity drops when shear is applied, the end-consumer products can be easily scooped, poured, or squeezed from its container. Once the force is removed, the solutions regain their initial viscosity almost immediately. h s e c b i s t a u s e a c a -K m Aat lextremely low use levels, forming solid Gellan gum is a gelling agent, reffective gels at concentrations as low as 0. 1%. a n h a Pectins are a family of. Ppartially methyl esterified polysaccharides produced from h e citrus peel and sugar beet pulp R Pectins are classified as high methoxyl (HM) pectin and low methoxyl (LM) pectin. . HM pectin requires r a narrow p. H range, around 3. 0, to form gels; LM pectin requires the presence of a controlled amount of calcium or other divalent cations D to form a gel.

Example/ Lubricating gelly h s e c b i s t a u s e a c a -K m Al r a n h P ha e R. r D 33
 Antigentest åre
Antigentest åre Gel kim
Gel kim Agarose gel
Agarose gel Agarose gel electrophoresis vs sds page
Agarose gel electrophoresis vs sds page What are stock solutions
What are stock solutions Amontillado is a type of?
Amontillado is a type of? Monophasic liquid dosage form is which type of solution
Monophasic liquid dosage form is which type of solution Why did montresor make sure fortunato is drunk
Why did montresor make sure fortunato is drunk Sterile technique quiz
Sterile technique quiz Basic food preparation meaning
Basic food preparation meaning Sterile semisolid preparations for ophthalmic use only are
Sterile semisolid preparations for ophthalmic use only are Examples of iv admixtures
Examples of iv admixtures Preparation of alkynes
Preparation of alkynes External-external trips
External-external trips Stain free gels
Stain free gels Gels are
Gels are Describe how light cured gels are applied over forms.
Describe how light cured gels are applied over forms. Liquid dosage forms examples
Liquid dosage forms examples Pastes in pharmacy
Pastes in pharmacy Precipitation antibody
Precipitation antibody Preparation of ointments pastes creams and gels
Preparation of ointments pastes creams and gels Pempfigus
Pempfigus Mevlana gel
Mevlana gel Process of gel electrophoresis
Process of gel electrophoresis Advantages of gel filtration chromatography
Advantages of gel filtration chromatography D
D Biorad
Biorad Astroglide side effects
Astroglide side effects Dna sequencing gel
Dna sequencing gel Basic principles of electrophoresis
Basic principles of electrophoresis Tobermonite gel is chemically
Tobermonite gel is chemically Ice power hot warm gel
Ice power hot warm gel Ice power hot
Ice power hot Gel filtration chromatography definition
Gel filtration chromatography definition