Chapter 24 Final preparations for evaluation Completing preparations







- Slides: 9

Chapter 24 Final preparations for evaluation

Completing preparations • Geez, I thought we were all ready… • This chapter dots the i’s and crosses the t’s I guess…

Roles for evaluators • If you’re the only person, you have to do • everything…or, assign, I mean, delegate responsibilities Facilitator: § “flight attendant”, looks after well being of participant § “sportscaster”: data collector, keeps the participant talking (if talk-aloud, presumably) § “scientist”: ensuers data collected in scientific manner (objective, unbiased, etc. )

Other roles • How many roles are there? Lots… § Note-taker: self-explanatory § Equipment operator: self-explanatory § Observer: good for “outsiders” (e. g. , stakeholders) § Meeter and greeter § Recruiter • Lone Evaluator: does all of the above

Use a script • (Seems like I said to use a script several • times already…) Scripts are important for several reasons: § so you don’t forget to say or do something (e. g. , get consent form signed) § so you don’t influence the participant, or if you do, at least the influence is consistent (e. g. , between groups)

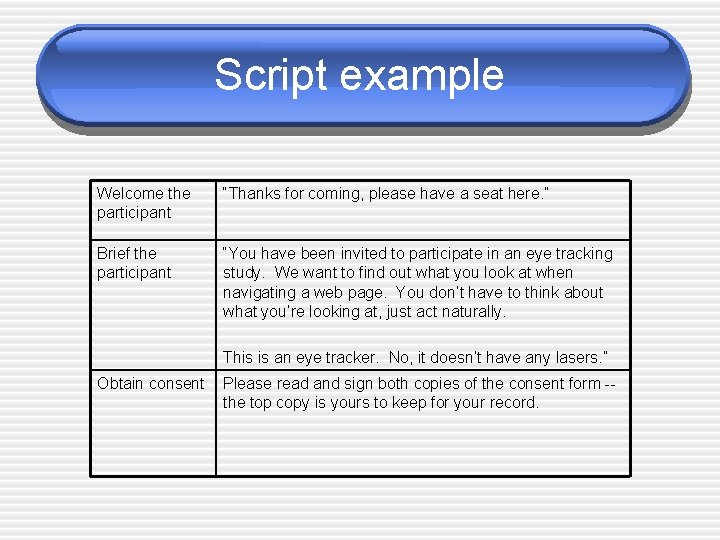
Script example Welcome the participant “Thanks for coming, please have a seat here. ” Brief the participant “You have been invited to participate in an eye tracking study. We want to find out what you look at when navigating a web page. You don’t have to think about what you’re looking at, just act naturally. This is an eye tracker. No, it doesn’t have any lasers. ” Obtain consent Please read and sign both copies of the consent form -the top copy is yours to keep for your record.

Informed Consent • What’s on the Informed Consent Form? § Rights of the participant • • • minimal risk (no harm) procedural info (what is going to happen) comprehension (does user understand) voluntariness (no coercion or pressure) human rights (participants must be informed) § Rights of the company/school • • • nondisclosure (is an NDA needed? ) confidentiality (thought this was subject’s identity kept secret) waivers (for pictures, video recordings, etc. ) legalese (use plain English) expectations (not clear on this…)

Example of IC form • See Clemson’s IRB: § http: //www. clemson. edu/research/orc. Site/orc. I RB. htm

Pilot study • Before running the full experiment, run a pilot study: § this will expose “minor annoyances” that you may have missed, that are easy/quick to fix, but that may hinder collection of the “real data” § you can generally expect to throw away all data collected in the pilot