IV ADMIXTURE Introduction The preparation of parenteral admixture


















- Slides: 20

IV ADMIXTURE

Introduction: The preparation of parenteral admixture usually involves the addition of one or more drugs to large volume solutions such as intravenous and nutrients fluids.

Introduction: Components of an IV program Preparation area Policies and procedures Personnel Storage space Admixture systems

Cont’: Components of an IV program Preparation area Ideally in separate room in the pharmacy “clean room” Size vary

Cont”: Components of an IV program Polices and procedures Guidelines for preparing parenteral products should be outlines in the pharmacy’s policy and procedure manual. Detailed information regarding preparation, labeling, storage and expiration dating of parenteral products should be readily available in the pharmacy These policy help to provide quality control for the parenteral products

Cont”: Components of an IV program Polices and procedures Stability Put in your mind that stability is affected by place, environmental condition, diluent used to administer the product, other drugs that may be mixed with Stability and sterility! Gives the expiration date

Cont”: Components of an IV program Polices and procedures incompatibility Physical: visible change e. g. precipitation Chemical: may or not visible change, deterioration or inactivation of an active ingredient. Therapeutic: drug-drug or drug-disease interaction that lead to potentiating of drug effect, drug toxicity, deterioration.

Cont”: Components of an IV program Polices and procedures Aseptic Technique Method of handling sterile products, a sterile parenteral dosage form is free from living microorganisms, particulate matter, and pyrogens.

Cont”: Components of an IV program Polices and procedures Labeling and check systems Reviewed against the patient’s current medication profile.

Cont”: Components of an IV program Personnel Carefully trained Who will prepare? Pharmacist or technician Proper training in aseptic technique and sterile product information is necessary.

Cont”: Components of an IV program Storage space Will depend on the type of system one chooses to use.

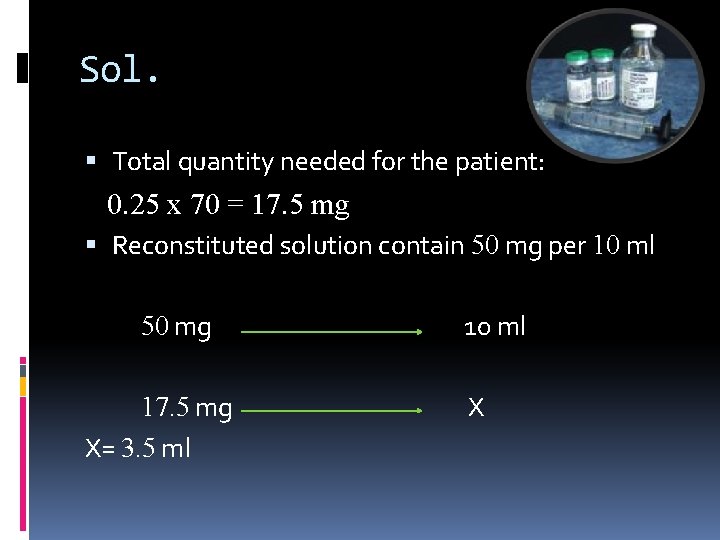
Calculations involving IV admixture: Examples: A medication order for a patient weighing 70 kg calls for 0. 25 mg of amphotericin B per kg of body weight to be added to 500 ml of 5 % dextrose injection. If the amphotericin B is to be obtained from a reconstituted injection that contain 50 mg per 10 ml, how many milliliters should be added to the dextrose injection?

Sol. Total quantity needed for the patient: 0. 25 x 70 = 17. 5 mg Reconstituted solution contain 50 mg per 10 ml 50 mg 17. 5 mg X= 3. 5 ml 10 ml X

Example: An intravenous infusion is to contain 15 m. Eq of potassium ion and 20 m. Eq of sodium ion in 500 ml of 5 % dextrose injection. Using an injection of potassium chloride containing 6 g per 30 ml and 0. 9 % injection of sodium chloride, how many milliliters of each should be used to supply the required ions?

Sol. : 15 m. Eq of K+ will be supplied by 15 m. Eq of KCl And 20 m. Eq of Na+ will supplied by 20 m. Eq of Na. Cl 1 m. Eq of KCl = 74. 5 mg 15 m. Eq of Kcl = 1117. 5 mg or 1. 118 g 6 g 30 ml 1. 118 g X X= 5. 59 ml

Cont”: 1 m. Eq of Na. Cl = 58. 5 mg 20 m. Eq of Na. Cl = 1170 mg or 1. 17 g 0. 9 g 1. 17 g X = 130 ml. 100 ml X

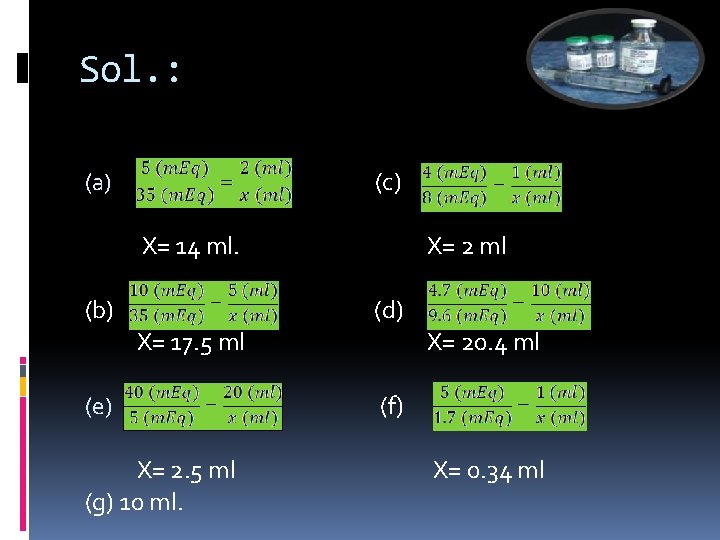
Example: The following is a formula for a desired TPN solution. Using the source of each drug as indicated, Calculate the amount of each component required in preparing solution. TPN solution formula Component Source (a) Sodium Chloride 35 m. Eq (b) Potassium Acetate 35 m. Eq (c) Magnesium Sulphate 8 m. Eq (d) Calcium Gluconte 9. 6 m. Eq (E) Potassium Chloride 5 m. Eq (f) Folic Acid 1. 7 mg (g) Multple vitamin infusion 10 ml To be added to: Amino Acid Infusion (8. 5 %) Dextrose injection (50 %) Vial, 5 m. Eq per 2 ml Vial, 10 m. Eq pre 5 ml Vial, 4 m. Eq per ml Vial, 4. 7 m. Eq per 10 ml Vial, 40 m. Eq per 20 ml Ampul, 5 mg per ml Ampul, 10 ml 500 ml

Sol. : (c) (a) X= 14 ml. (b) X= 2 ml (d) X= 17. 5 ml (e) X= 2. 5 ml (g) 10 ml. X= 20. 4 ml (f) X= 0. 34 ml

Example: A medication order calls for 1000 ml of D 5 W to be administered over an 8 -hour period. Using an IV administration set which delivers 10 drops per ml, how many drops per minute should be delivered to the patient? Sol. : Volume of fluid = 1000 ml 8 hour = 480 minutes 1000/ 480 = 2. 1 ml per min 2. 1 ml/min x 10 (drops per ml) = 21 drops/minute

 Iv admixture examples
Iv admixture examples Intradermal adalah
Intradermal adalah Pipeed
Pipeed Drycast admixtures
Drycast admixtures Steel ratio
Steel ratio Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worms-breton
Tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới