FiveYear Outcomes of Transcatheter Aortic Valve Replacement TAVR






- Slides: 25

Five-Year Outcomes of Transcatheter Aortic Valve Replacement (TAVR) in “Inoperable” Patients With Severe Aortic Stenosis: The PARTNER Trial Samir R. Kapadia, MD On behalf of The PARTNER Trial Investigators TCT 2014 | September 13, 2014

Background • Transcatheter aortic valve replacement (TAVR) is the recommended treatment for “inoperable” patients with severe aortic stenosis (AS). • Long term clinical benefit and valve performance in this population remain unknown.

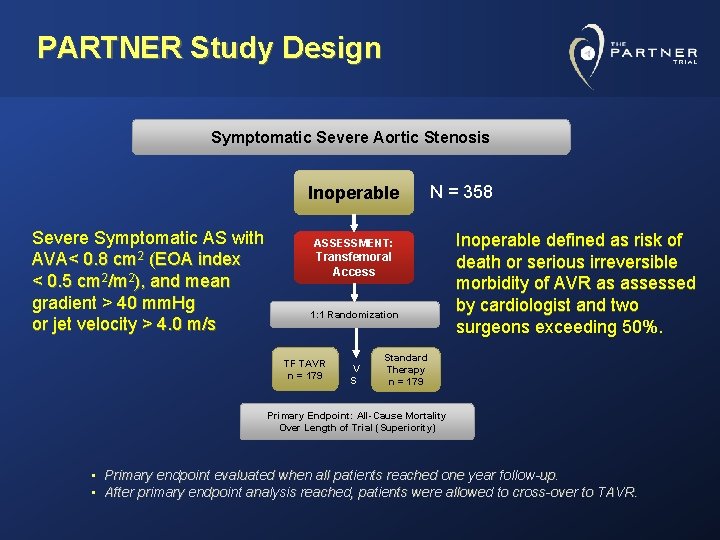
PARTNER Study Design Symptomatic Severe Aortic Stenosis Inoperable Severe Symptomatic AS with AVA< 0. 8 cm 2 (EOA index < 0. 5 cm 2/m 2), and mean gradient > 40 mm. Hg or jet velocity > 4. 0 m/s N = 358 ASSESSMENT: Transfemoral Access 1: 1 Randomization TF TAVR n = 179 V S Inoperable defined as risk of death or serious irreversible morbidity of AVR as assessed by cardiologist and two surgeons exceeding 50%. Standard Therapy n = 179 Primary Endpoint: All-Cause Mortality Over Length of Trial (Superiority) • Primary endpoint evaluated when all patients reached one year follow-up. • After primary endpoint analysis reached, patients were allowed to cross-over to TAVR.

Key End-Points for 5 Year Analysis • All-Cause Mortality • Cardiac Mortality • Re-hospitalization • Stroke • NYHA functional class • Echo-derived valve areas, transvalvular gradients, and paravalvular leak. • Mortality outcomes stratified by STS score, paravalvular leak and age.

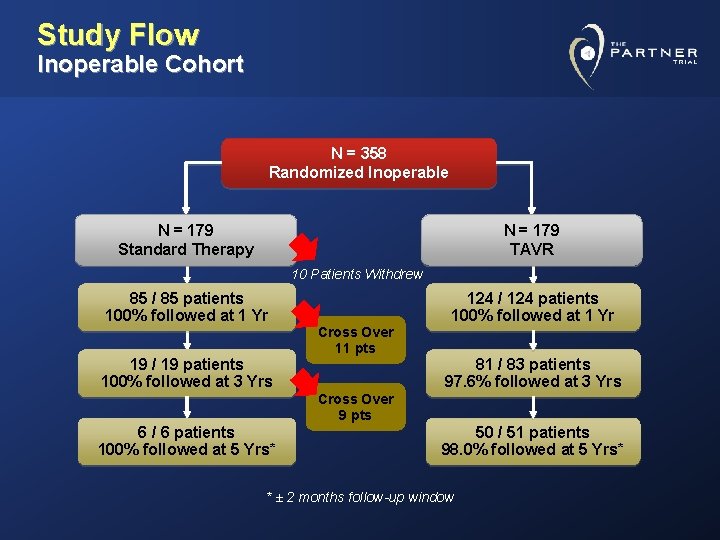
Study Flow Inoperable Cohort N = 358 Randomized Inoperable N = 179 Standard Therapy N = 179 TAVR 10 Patients Withdrew 85 / 85 patients 100% followed at 1 Yr 19 / 19 patients 100% followed at 3 Yrs Cross Over 11 pts 124 / 124 patients 100% followed at 1 Yr 81 / 83 patients 97. 6% followed at 3 Yrs Cross Over 9 pts 6 / 6 patients 100% followed at 5 Yrs* 50 / 51 patients 98. 0% followed at 5 Yrs* * ± 2 months follow-up window

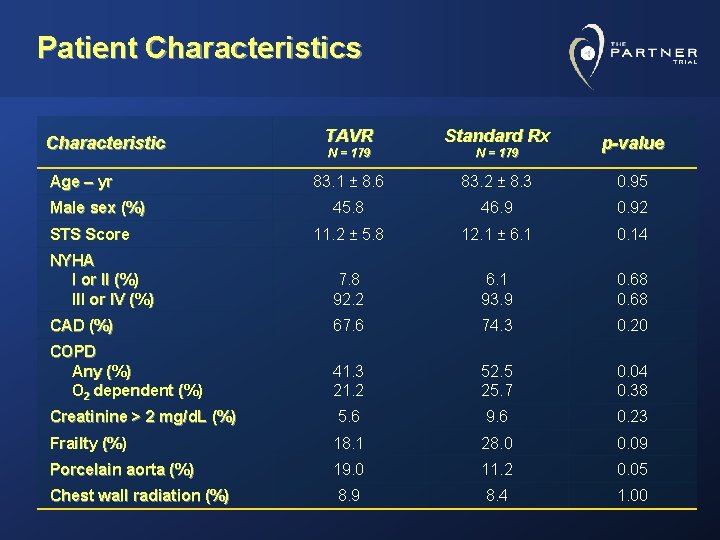
Patient Characteristics TAVR Standard Rx N = 179 83. 1 ± 8. 6 83. 2 ± 8. 3 0. 95 45. 8 46. 9 0. 92 11. 2 ± 5. 8 12. 1 ± 6. 1 0. 14 NYHA I or II (%) III or IV (%) 7. 8 92. 2 6. 1 93. 9 0. 68 CAD (%) 67. 6 74. 3 0. 20 COPD Any (%) O 2 dependent (%) 41. 3 21. 2 52. 5 25. 7 0. 04 0. 38 Creatinine > 2 mg/d. L (%) 5. 6 9. 6 0. 23 Frailty (%) 18. 1 28. 0 0. 09 Porcelain aorta (%) 19. 0 11. 2 0. 05 Chest wall radiation (%) 8. 9 8. 4 1. 00 Characteristic Age – yr Male sex (%) STS Score p-value

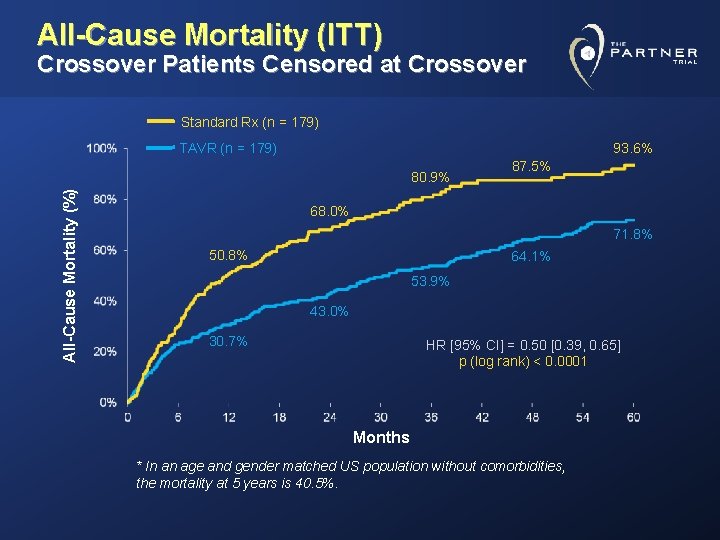
All-Cause Mortality (ITT) Crossover Patients Censored at Crossover Standard Rx (n = 179) TAVR (n = 179) 93. 6% All-Cause Mortality (%) 80. 9% 87. 5% 68. 0% 71. 8% 50. 8% 64. 1% 53. 9% 43. 0% 30. 7% HR [95% CI] = 0. 50 [0. 39, 0. 65] p (log rank) < 0. 0001 Months * In an age and gender matched US population without comorbidities, the mortality at 5 years is 40. 5%.

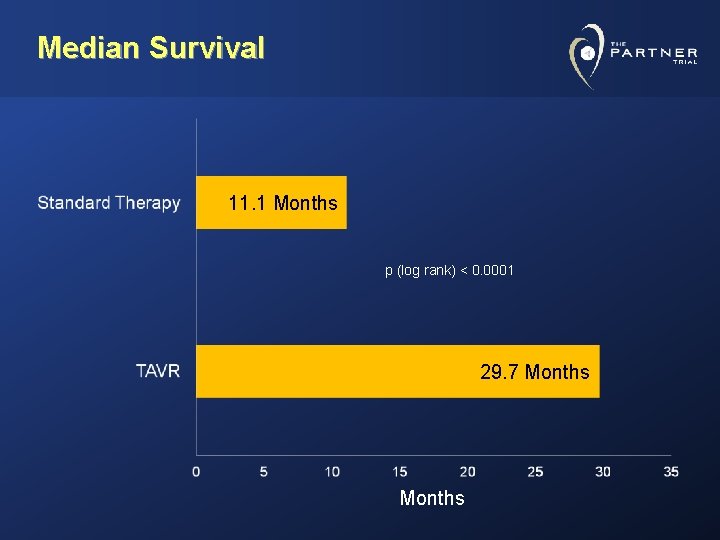
Median Survival 11. 1 Months p (log rank) < 0. 0001 29. 7 Months

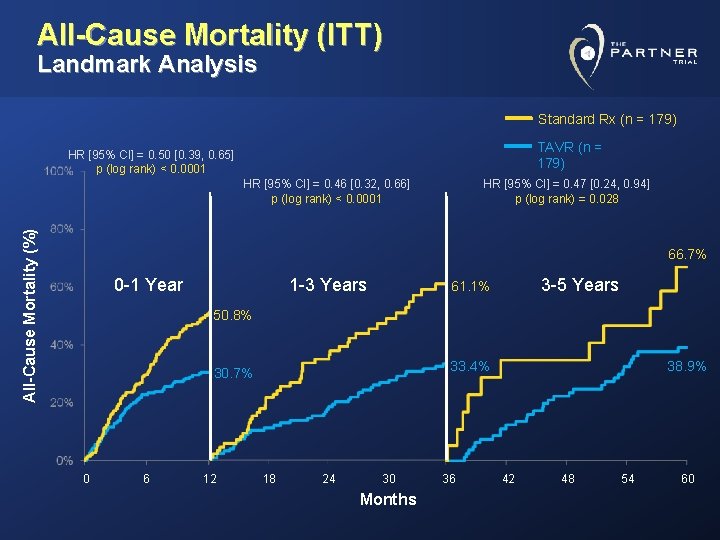
All-Cause Mortality (ITT) Landmark Analysis Standard Rx (n = 179) TAVR (n = 179) HR [95% CI] = 0. 50 [0. 39, 0. 65] p (log rank) < 0. 0001 All-Cause Mortality (%) HR [95% CI] = 0. 46 [0. 32, 0. 66] p (log rank) < 0. 0001 HR [95% CI] = 0. 47 [0. 24, 0. 94] p (log rank) = 0. 028 66. 7% 0 -1 Year 1 -3 Years 3 -5 Years 61. 1% 50. 8% 0 6 12 38. 9% 33. 4% 30. 7% 18 24 30 Months 36 42 48 54 60

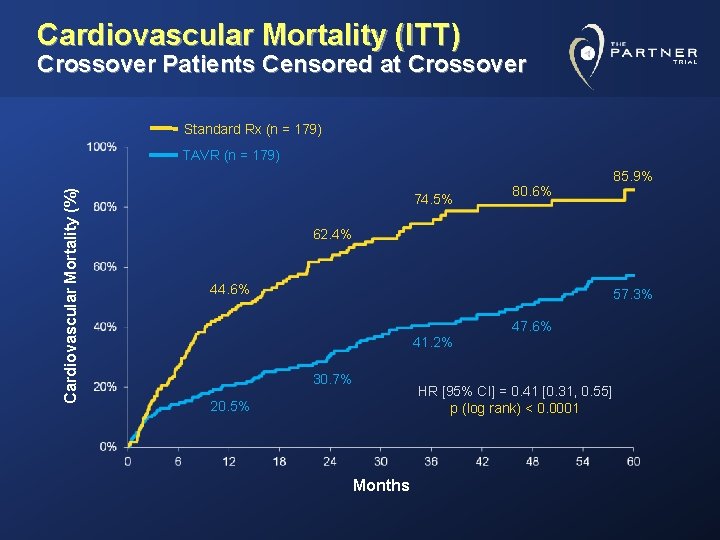
Cardiovascular Mortality (ITT) Crossover Patients Censored at Crossover Standard Rx (n = 179) Cardiovascular Mortality (%) TAVR (n = 179) 74. 5% 80. 6% 85. 9% 62. 4% 44. 6% 57. 3% 47. 6% 41. 2% 30. 7% HR [95% CI] = 0. 41 [0. 31, 0. 55] p (log rank) < 0. 0001 20. 5% Months

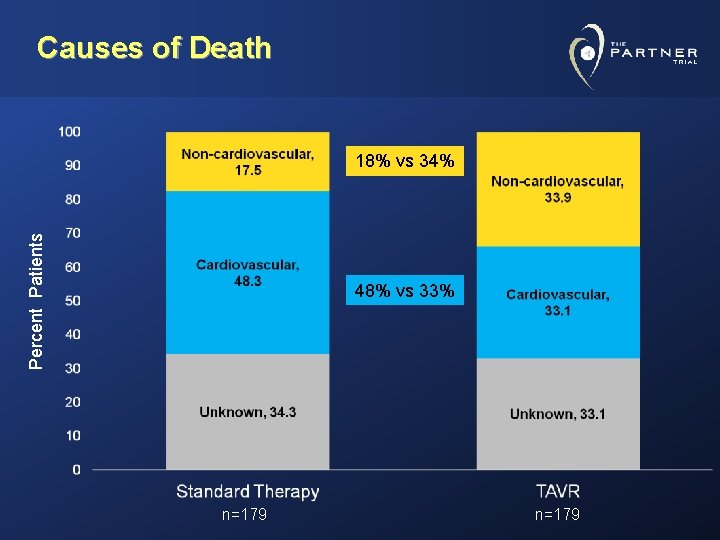
Causes of Death Percent Patients 18% vs 34% 48% vs 33% n=179

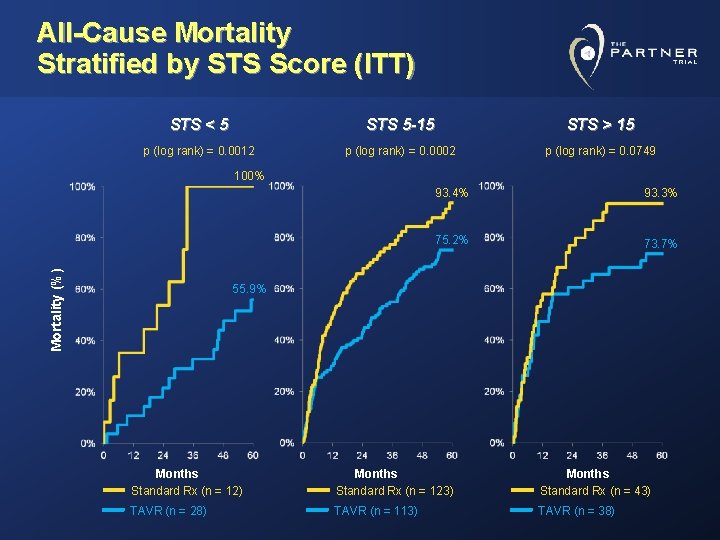
All-Cause Mortality Stratified by STS Score (ITT) STS < 5 STS 5 -15 STS > 15 p (log rank) = 0. 0012 p (log rank) = 0. 0002 p (log rank) = 0. 0749 Mortality (%) 100% 93. 4% 93. 3% 75. 2% 73. 7% 55. 9% Months Standard Rx (n = 12) Months Standard Rx (n = 123) Months Standard Rx (n = 43) TAVR (n = 28) TAVR (n = 113) TAVR (n = 38)

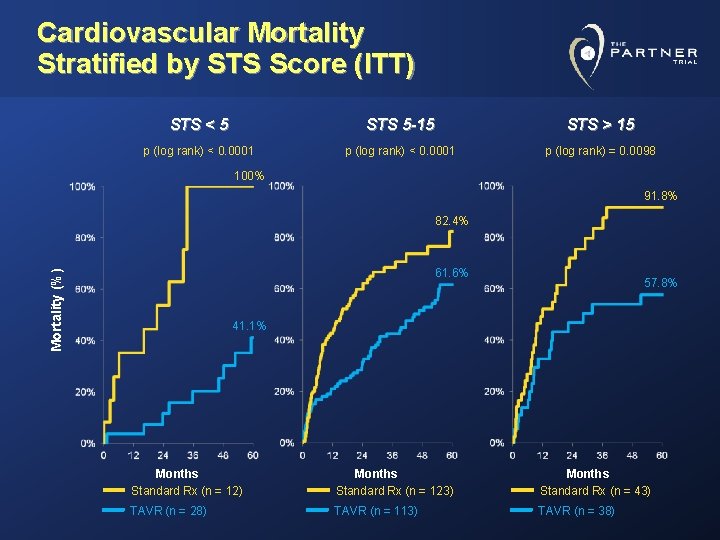
Cardiovascular Mortality Stratified by STS Score (ITT) STS < 5 STS 5 -15 STS > 15 p (log rank) < 0. 0001 p (log rank) = 0. 0098 100% 91. 8% 82. 4% Mortality (%) 61. 6% 57. 8% 41. 1% Months Standard Rx (n = 12) Months Standard Rx (n = 123) Months Standard Rx (n = 43) TAVR (n = 28) TAVR (n = 113) TAVR (n = 38)

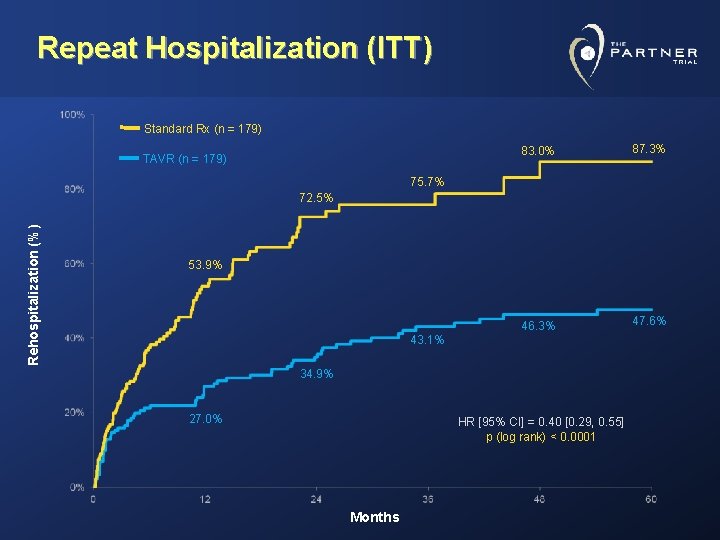
Repeat Hospitalization (ITT) Standard Rx (n = 179) TAVR (n = 179) 83. 0% 87. 3% 46. 3% 47. 6% 75. 7% Rehospitalization (%) 72. 5% 53. 9% 43. 1% 34. 9% 27. 0% HR [95% CI] = 0. 40 [0. 29, 0. 55] p (log rank) < 0. 0001 Months

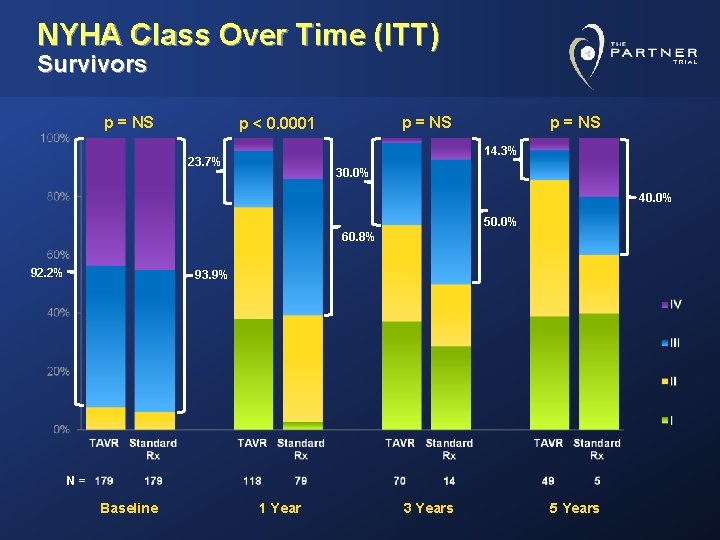
NYHA Class Over Time (ITT) Survivors p = NS p < 0. 0001 p = NS 14. 3% 23. 7% 30. 0% 40. 0% 50. 0% 60. 8% 92. 2% 93. 9% N= Baseline 1 Year 3 Years 5 Years

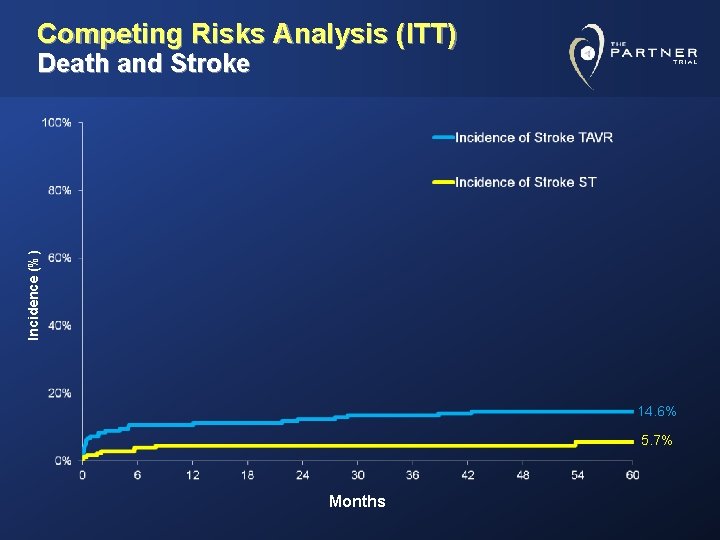
Competing Risks Analysis (ITT) Incidence (%) Death and Stroke 14. 6% 5. 7% Months

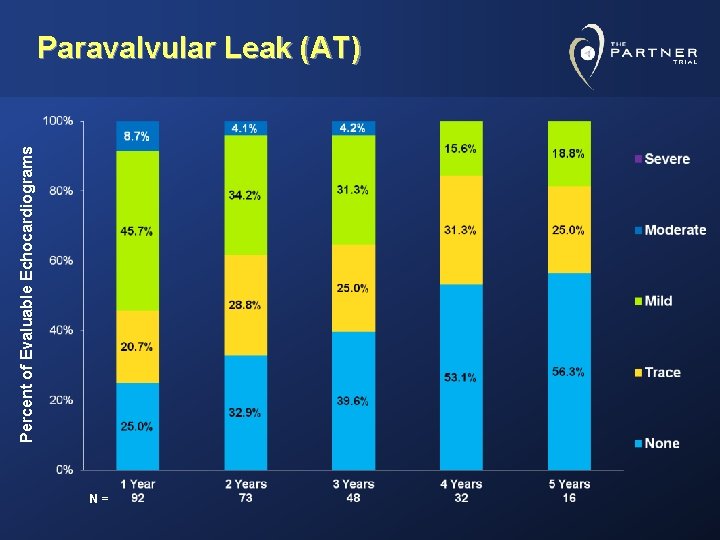
Percent of Evaluable Echocardiograms Paravalvular Leak (AT) N=

Mortality by Paravalvular Leak All-Cause Mortality Cardiovascular Mortality None-Mild (n = 142) Moderate-Severe (n = 23) 78. 3% 74. 6% 69. 2% 51. 3% p (log rank) = 0. 510 p (log rank) = 0. 043

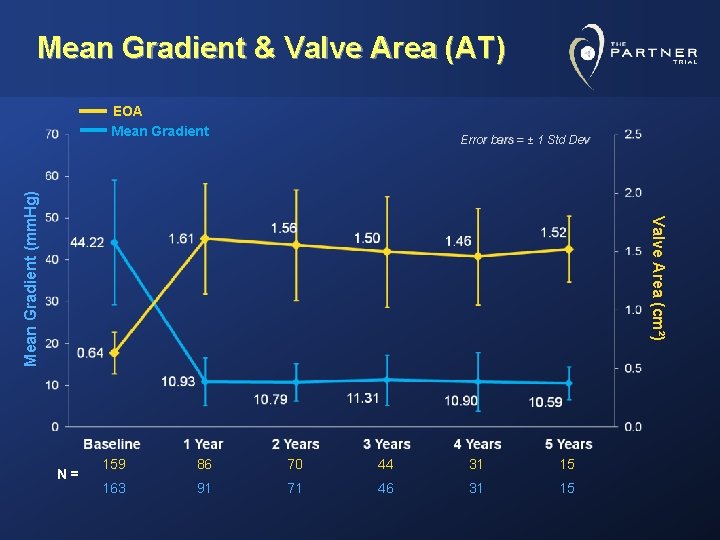
Mean Gradient & Valve Area (AT) EOA Mean Gradient Valve Area (cm²) Mean Gradient (mm. Hg) Error bars = ± 1 Std Dev N= 159 86 70 44 31 15 163 91 71 46 31 15

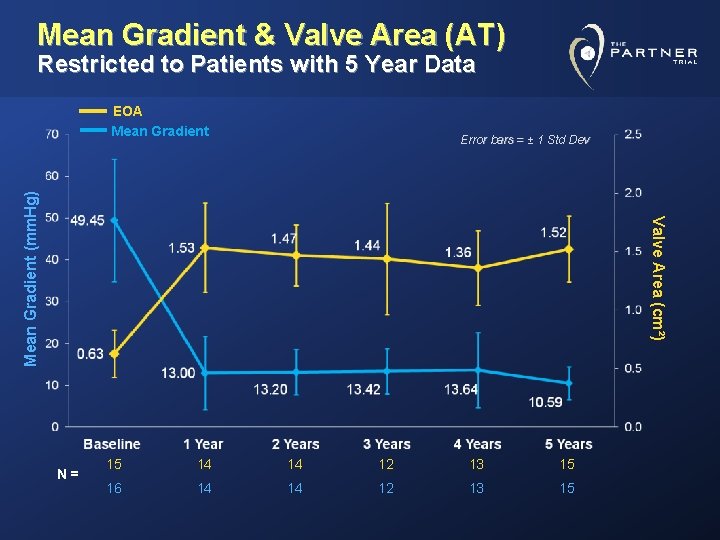
Mean Gradient & Valve Area (AT) Restricted to Patients with 5 Year Data EOA Mean Gradient Valve Area (cm²) Mean Gradient (mm. Hg) Error bars = ± 1 Std Dev N= 15 14 14 12 13 15 16 14 14 12 13 15

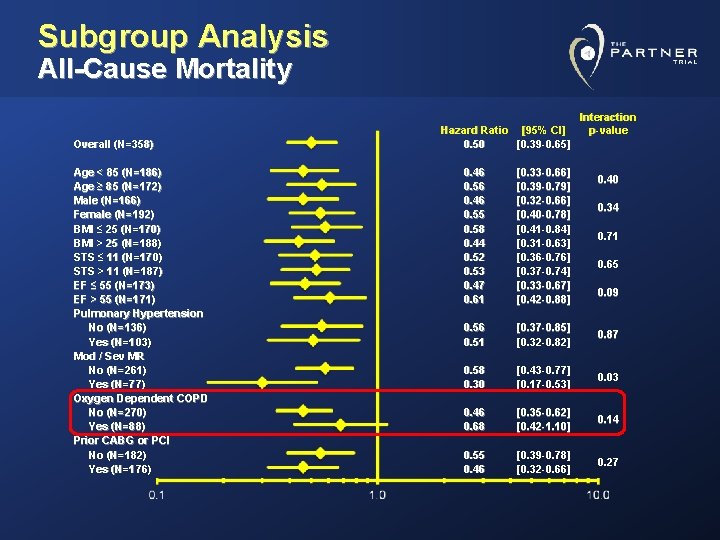
Subgroup Analysis All-Cause Mortality Overall (N=358) Age < 85 (N=186) Age ≥ 85 (N=172) Male (N=166) Female (N=192) BMI ≤ 25 (N=170) BMI > 25 (N=188) STS ≤ 11 (N=170) STS > 11 (N=187) EF ≤ 55 (N=173) EF > 55 (N=171) Pulmonary Hypertension No (N=136) Yes (N=103) Mod / Sev MR No (N=261) Yes (N=77) Oxygen Dependent COPD No (N=270) Yes (N=88) Prior CABG or PCI No (N=182) Yes (N=176) Hazard Ratio [95% CI] 0. 50 [0. 39 -0. 65] Interaction p-value 0. 46 0. 56 0. 46 0. 55 0. 58 0. 44 0. 52 0. 53 0. 47 0. 61 [0. 33 -0. 66] [0. 39 -0. 79] [0. 32 -0. 66] [0. 40 -0. 78] [0. 41 -0. 84] [0. 31 -0. 63] [0. 36 -0. 76] [0. 37 -0. 74] [0. 33 -0. 67] [0. 42 -0. 88] 0. 56 0. 51 [0. 37 -0. 85] [0. 32 -0. 82] 0. 87 0. 58 0. 30 [0. 43 -0. 77] [0. 17 -0. 53] 0. 03 0. 46 0. 68 [0. 35 -0. 62] [0. 42 -1. 10] 0. 14 0. 55 0. 46 [0. 39 -0. 78] [0. 32 -0. 66] 0. 27 0. 40 0. 34 0. 71 0. 65 0. 09

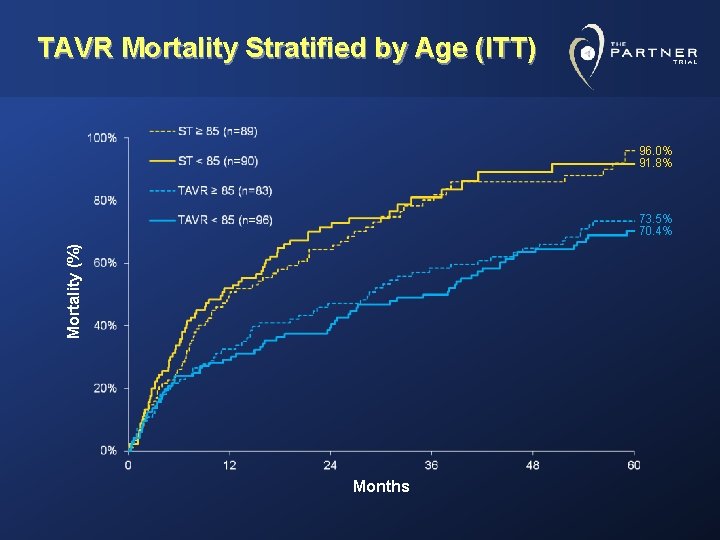
TAVR Mortality Stratified by Age (ITT) 96. 0% 91. 8% Mortality (%) 73. 5% 70. 4% Months

Clinical Observations • Mortality benefit was similar in elderly (>85 yr) patients compared to those ≤ 85 years. • Cardiovascular mortality and all-cause mortality benefit was seen even in patients with high STS score. • Patients with O 2 dependent COPD may have less mortality benefit. • Beyond early procedural risk of stroke there was no persistent risk over 5 -year follow up. • Moderate and severe paravalvular leak is associated with higher cardiovascular mortality particularly in patients with less comorbidities.

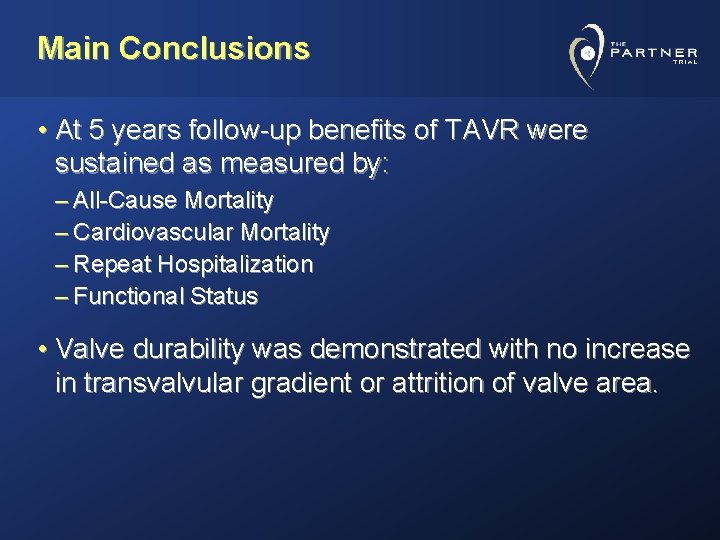
Main Conclusions • At 5 years follow-up benefits of TAVR were sustained as measured by: – All-Cause Mortality – Cardiovascular Mortality – Repeat Hospitalization – Functional Status • Valve durability was demonstrated with no increase in transvalvular gradient or attrition of valve area.

Thank You to the Dedicated Study Teams at All PARTNER Investigational Sites
 Annular rupture tavr
Annular rupture tavr Tavr antiplatelet guidelines
Tavr antiplatelet guidelines Aortic valve leaflets
Aortic valve leaflets Dvi aortic valve
Dvi aortic valve Crista terminalis
Crista terminalis Dvi aortic valve
Dvi aortic valve Transvalvular gradient
Transvalvular gradient Aortic root enlargement
Aortic root enlargement Aortic valve dimensionless index
Aortic valve dimensionless index Endocardium sheep heart
Endocardium sheep heart Dvi aortic valve
Dvi aortic valve Combustion reaction formula
Combustion reaction formula Servo needle valve
Servo needle valve Sternocostal angle
Sternocostal angle Pathophysiology of aortic aneurysm ppt
Pathophysiology of aortic aneurysm ppt Good morning blood
Good morning blood Regurgitatiom
Regurgitatiom Aortic stanosis
Aortic stanosis Horizontal fissure
Horizontal fissure Aorta rupture
Aorta rupture Type b aortic dissection
Type b aortic dissection Right and left aortic sinus
Right and left aortic sinus Mitral regurgitation murmur
Mitral regurgitation murmur True vocal cord
True vocal cord Thorasic aortic aneurysm
Thorasic aortic aneurysm Emedu ecg
Emedu ecg