Portico Transcatheter Aortic Valve Replacement Program CRT 2013









- Slides: 30

Portico™ Transcatheter Aortic Valve Replacement Program CRT 2013 Hasan Jilaihawi, M. D. Raj R. Makkar, M. D. Cedars-Sinai Heart Institute Los Angeles, CA

Hasan Jilaihawi, MD, MRCP Consulting: Edwards Lifesciences, LLC, St. Jude Medical, Inc. , Venus Medtech Off-Label: Discussion pertaining to transcatheter valve therapies

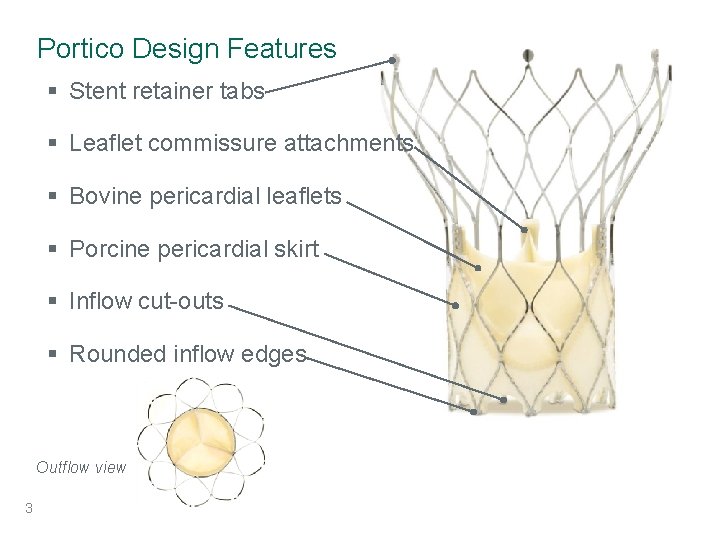
Portico Design Features § Stent retainer tabs § Leaflet commissure attachments § Bovine pericardial leaflets § Porcine pericardial skirt § Inflow cut-outs § Rounded inflow edges Outflow view 3

Portico Valve Design Features § Both leaflets and cuff are treated with Linx. TM AC treatment* § Same anticalcification technology used on Epic and Trifecta surgical valves § Nonflared annulus section of the stent Portico 23 mm Valve *There is no clinical data currently available that evaluates the long-term impact of anticalcification tissue treatment in humans.

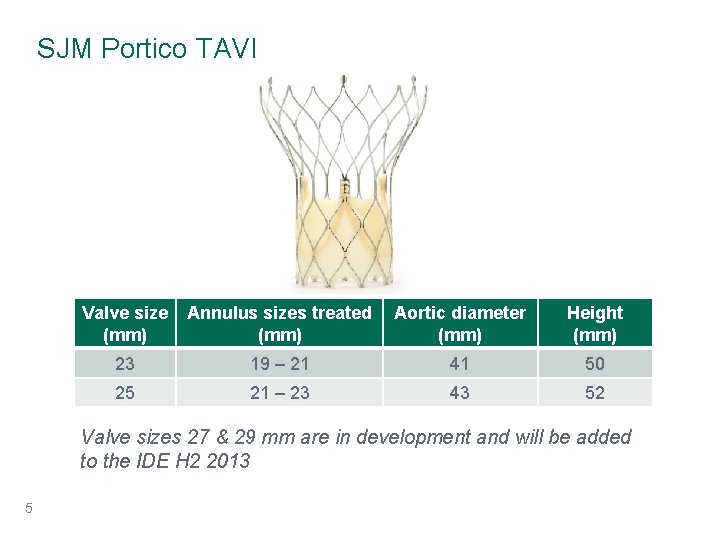
SJM Portico TAVI Valve size (mm) Annulus sizes treated (mm) Aortic diameter (mm) Height (mm) 23 19 – 21 41 50 25 21 – 23 43 52 Valve sizes 27 & 29 mm are in development and will be added to the IDE H 2 2013 5

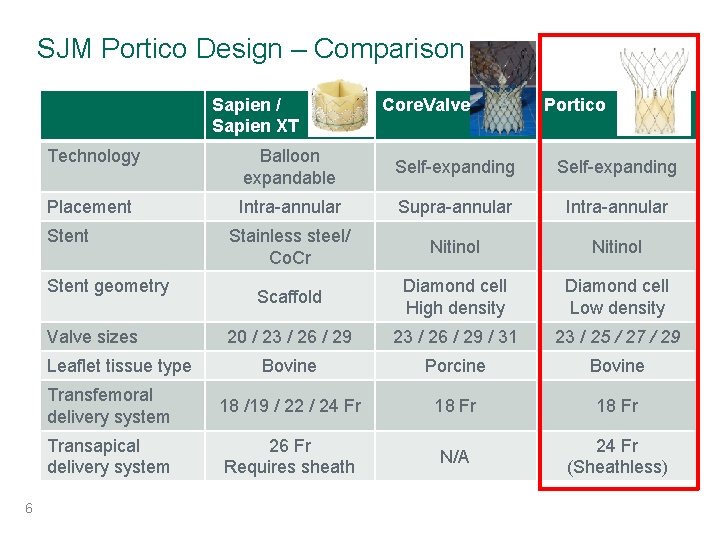
SJM Portico Design – Comparison Sapien / Sapien XT Portico Technology Balloon expandable Self-expanding Placement Intra-annular Supra-annular Intra-annular Stainless steel/ Co. Cr Nitinol Scaffold Diamond cell High density Diamond cell Low density 20 / 23 / 26 / 29 / 31 23 / 25 / 27 / 29 Bovine Porcine Bovine Transfemoral delivery system 18 /19 / 22 / 24 Fr 18 Fr Transapical delivery system 26 Fr Requires sheath N/A 24 Fr (Sheathless) Stent geometry Valve sizes Leaflet tissue type 6 Core. Valve

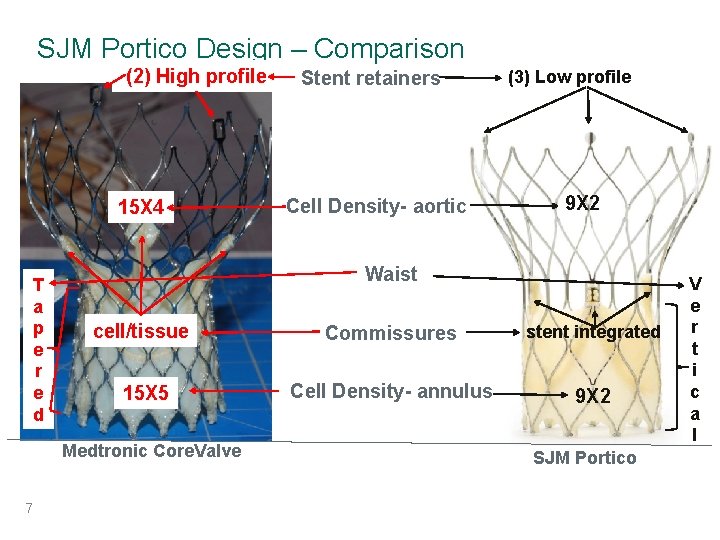
SJM Portico Design – Comparison (2) High profile 15 X 4 T a p e r e d Cell Density- aortic (3) Low profile 9 X 2 Waist cell/tissue Commissures stent integrated 15 X 5 Cell Density- annulus 9 X 2 Medtronic Core. Valve 7 Stent retainers SJM Portico V e r t i c a l

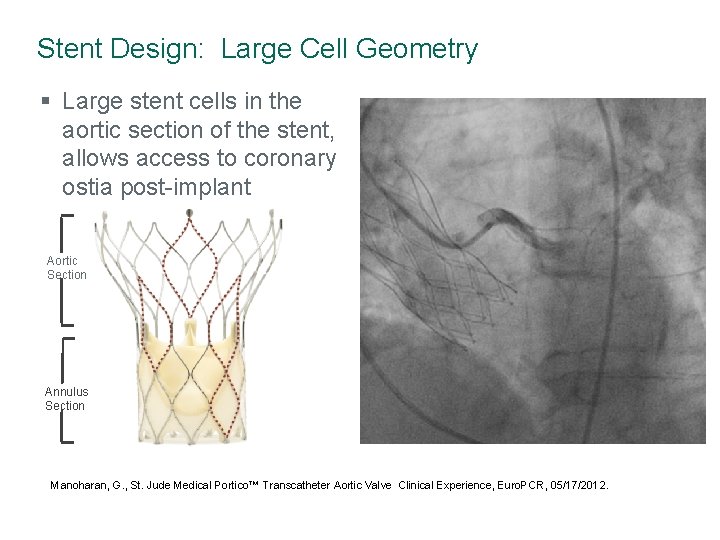
Stent Design: Large Cell Geometry § Large stent cells in the aortic section of the stent, allows access to coronary ostia post-implant Aortic Section Annulus Section Manoharan, G. , St. Jude Medical Portico™ Transcatheter Aortic Valve Clinical Experience, Euro. PCR, 05/17/2012.

Stent Design: Large Cell Geometry § Large stent cells within annulus section of the stent § Less metal § Minimizes risk of stent strut resting against a calcific nodule § More tissue § Allows for tissue to conform around calcific nodules Calcific nodules

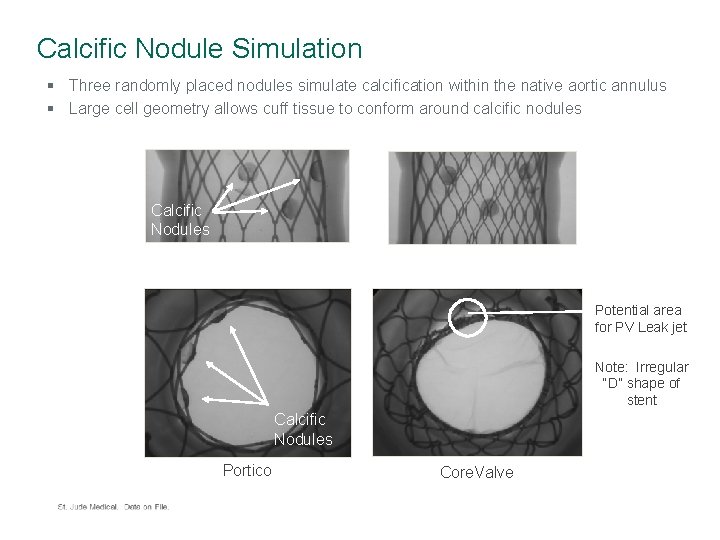
Calcific Nodule Simulation § Three randomly placed nodules simulate calcification within the native aortic annulus § Large cell geometry allows cuff tissue to conform around calcific nodules Calcific Nodules Potential area for PV Leak jet Note: Irregular “D” shape of stent Calcific Nodules Portico Core. Valve

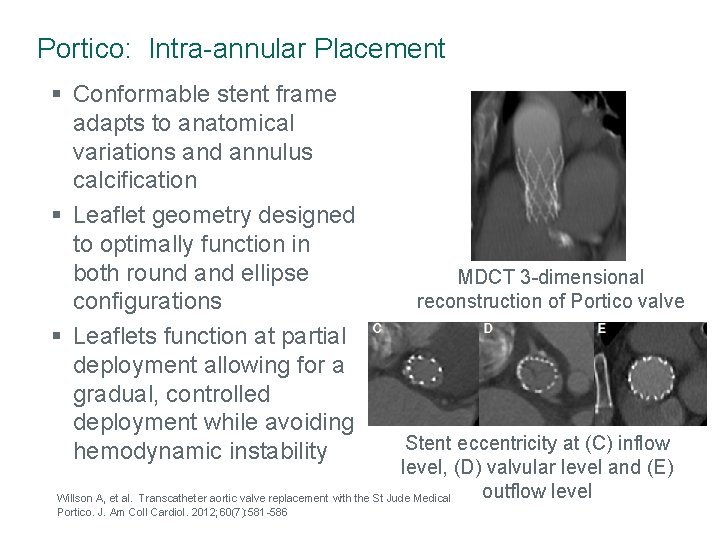
Portico: Intra-annular Placement § Conformable stent frame adapts to anatomical variations and annulus calcification § Leaflet geometry designed to optimally function in both round and ellipse configurations § Leaflets function at partial deployment allowing for a gradual, controlled deployment while avoiding hemodynamic instability MDCT 3 -dimensional reconstruction of Portico valve Stent eccentricity at (C) inflow level, (D) valvular level and (E) outflow level Willson A, et al. Transcatheter aortic valve replacement with the St Jude Medical Portico. J. Am Coll Cardiol. 2012; 60(7): 581 -586

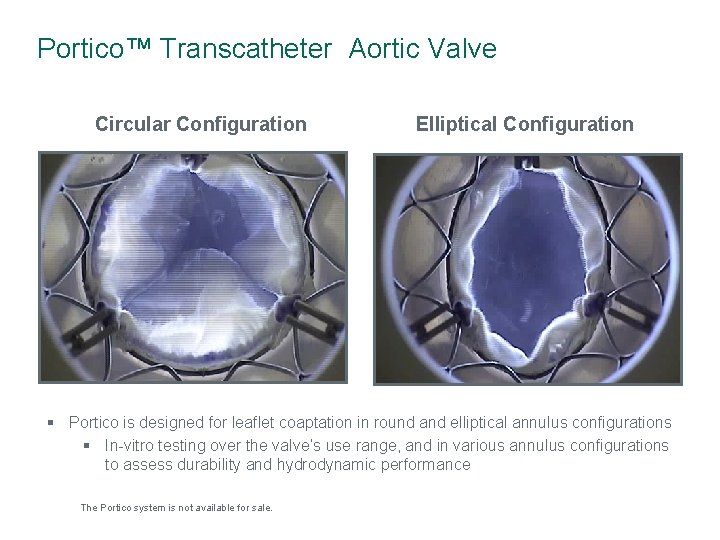
Portico™ Transcatheter Aortic Valve Circular Configuration Elliptical Configuration § Portico is designed for leaflet coaptation in round and elliptical annulus configurations § In-vitro testing over the valve’s use range, and in various annulus configurations to assess durability and hydrodynamic performance The Portico system is not available for sale.

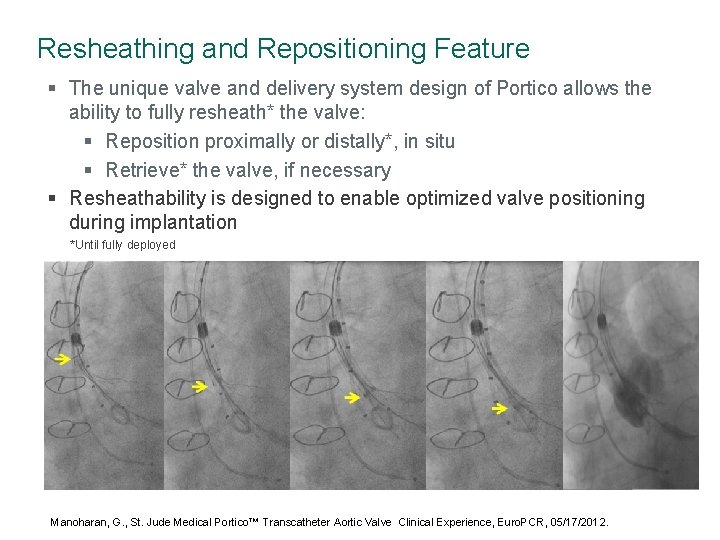
Resheathing and Repositioning Feature § The unique valve and delivery system design of Portico allows the ability to fully resheath* the valve: § Reposition proximally or distally*, in situ § Retrieve* the valve, if necessary § Resheathability is designed to enable optimized valve positioning during implantation *Until fully deployed Manoharan, G. , St. Jude Medical Portico™ Transcatheter Aortic Valve Clinical Experience, Euro. PCR, 05/17/2012.

Portico Valve Loading System § Loading Tools include: § § § Loading tube Loading funnel Loading base Base insert Leaflet tester § Preparation and valve loading performed at room temperature Portico TF Loading Tools

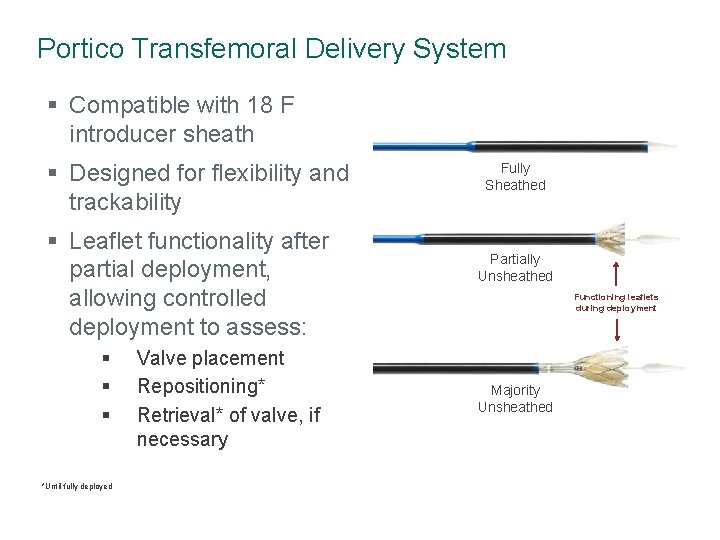
Portico Transfemoral Delivery System § Compatible with 18 F introducer sheath § Designed for flexibility and trackability § Leaflet functionality after partial deployment, allowing controlled deployment to assess: § § § *Until fully deployed Valve placement Repositioning* Retrieval* of valve, if necessary Fully Sheathed Partially Unsheathed Functioning leaflets during deployment Majority Unsheathed

Portico Transfemoral Delivery System Distal End Protective Sheath Retainer Receptacle Protective Sheath Marker Inner Shaft Radiopaque Tip Inner Shaft Marker Band

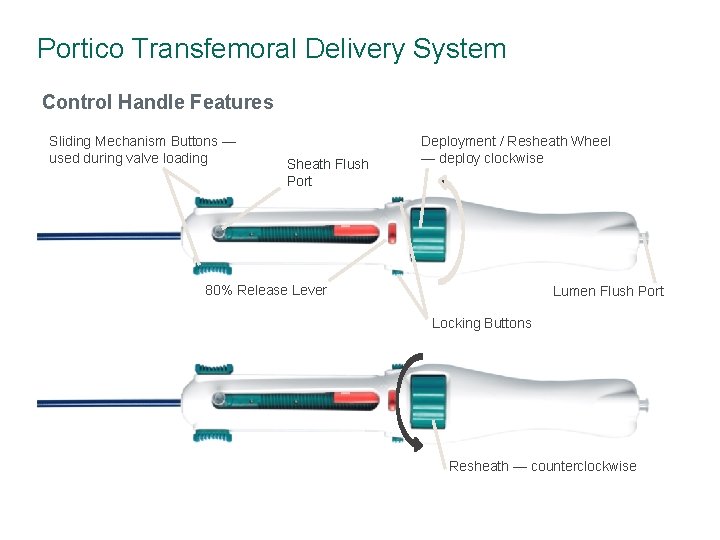
Portico Transfemoral Delivery System Control Handle Features Sliding Mechanism Buttons — used during valve loading Sheath Flush Port Deployment / Resheath Wheel — deploy clockwise 80% Release Lever Lumen Flush Port Locking Buttons Resheath — counterclockwise

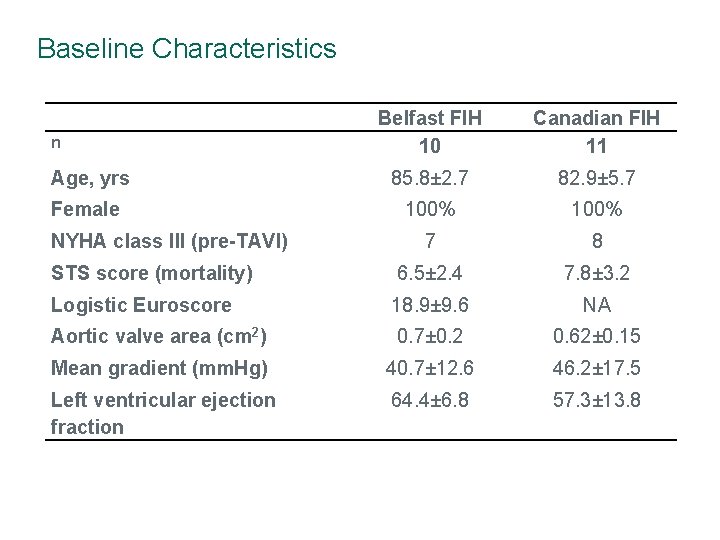
Baseline Characteristics Belfast FIH 10 Canadian FIH 11 Age, yrs 85. 8± 2. 7 82. 9± 5. 7 Female 100% 7 8 STS score (mortality) 6. 5± 2. 4 7. 8± 3. 2 Logistic Euroscore 18. 9± 9. 6 NA Aortic valve area (cm 2) 0. 7± 0. 2 0. 62± 0. 15 Mean gradient (mm. Hg) 40. 7± 12. 6 46. 2± 17. 5 Left ventricular ejection fraction 64. 4± 6. 8 57. 3± 13. 8 n NYHA class III (pre-TAVI)

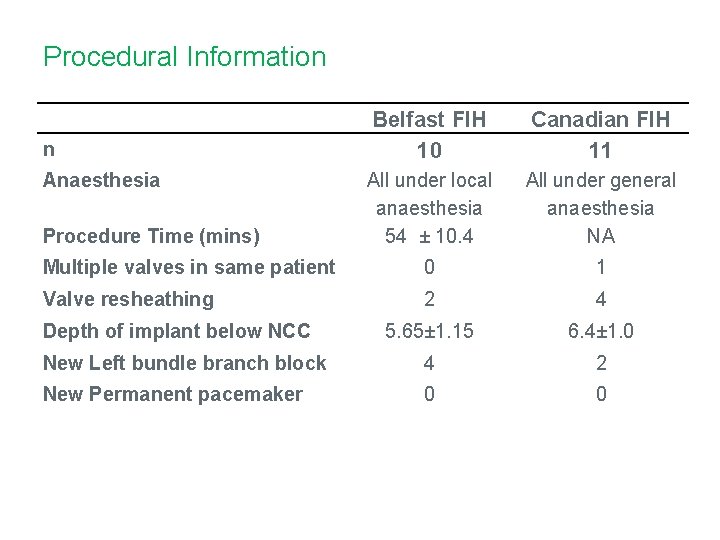
Procedural Information Belfast FIH 10 Canadian FIH 11 All under local anaesthesia 54 ± 10. 4 All under general anaesthesia NA Multiple valves in same patient 0 1 Valve resheathing 2 4 5. 65± 1. 15 6. 4± 1. 0 New Left bundle branch block 4 2 New Permanent pacemaker 0 0 n Anaesthesia Procedure Time (mins) Depth of implant below NCC

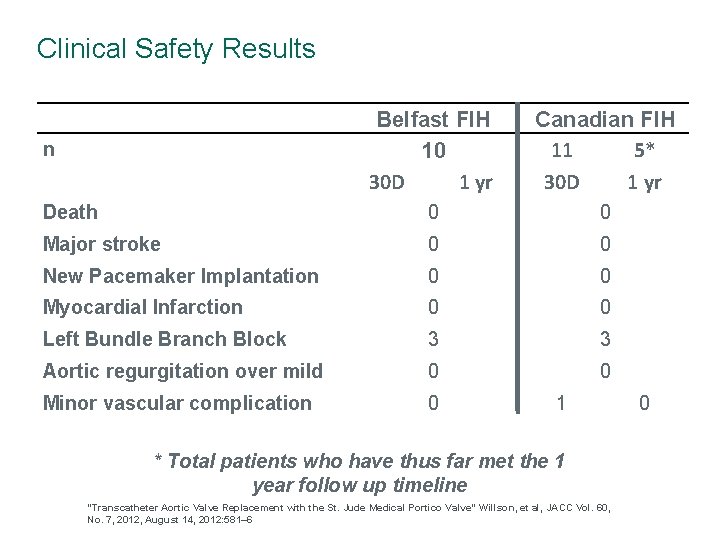
Clinical Safety Results Belfast FIH 10 30 D 1 yr Canadian FIH 11 5* 30 D 1 yr Death 0 0 Major stroke 0 0 New Pacemaker Implantation 0 0 Myocardial Infarction 0 0 Left Bundle Branch Block 3 3 Aortic regurgitation over mild 0 0 Minor vascular complication 0 n 1 * Total patients who have thus far met the 1 year follow up timeline “Transcatheter Aortic Valve Replacement with the St. Jude Medical Portico Valve” Willson, et al, JACC Vol. 60, No. 7, 2012, August 14, 2012: 581– 6 0

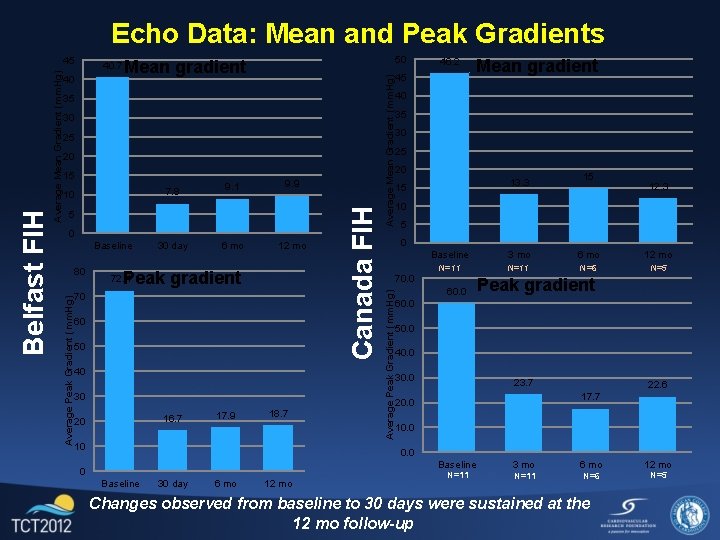
Echo Data: Mean and Peak Gradients 30 25 20 15 7. 8 10 9. 1 9. 9 5 0 Baseline 80 30 day 6 mo 12 mo Peak gradient 72. 4 70 60 50 40 30 16. 7 20 17. 9 18. 7 10 46. 2 45 Mean gradient 40 35 30 25 20 13. 3 15 15 12. 3 10 5 0 70. 0 Average Peak Gradient (mm. Hg) 35 Average Mean Gradient (mm. Hg) 50 Mean gradient Canada FIH Average Mean Gradient (mm. Hg) 40. 7 40 Average Peak Gradient (mm. Hg) Belfast FIH 45 Baseline 3 mo 6 mo 12 mo N=11 N=6 N=5 60. 0 Peak gradient 60. 0 50. 0 40. 0 30. 0 23. 7 22. 6 17. 7 20. 0 10. 0 Baseline 30 day 6 mo 12 mo N=11 3 mo 6 mo N=11 N=6 Changes observed from baseline to 30 days were sustained at the 12 mo follow-up 12 mo N=5

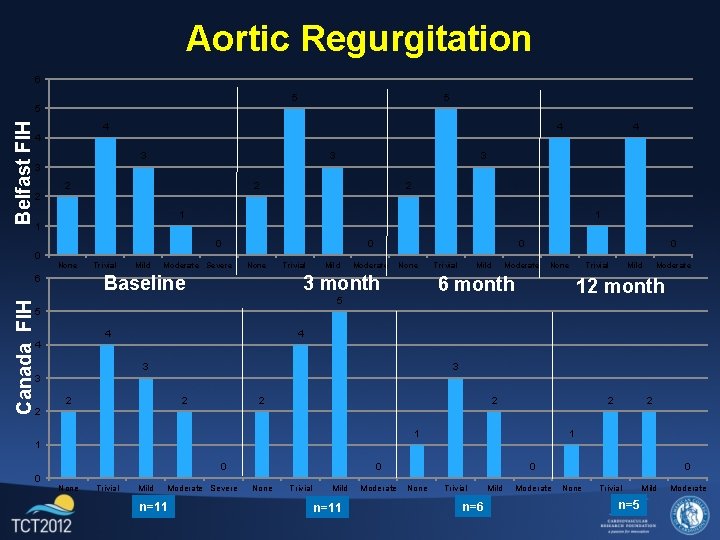
Aortic Regurgitation 6 5 5 Belfast FIH 5 4 4 3 3 2 2 1 1 1 0 0 None Mild Moderate Severe None Baseline 6 Canada FIH Trivial 0 Trivial Mild 0 Moderate None 3 month Trivial Mild 0 Moderate None 6 month 5 Trivial Mild Moderate 12 month 5 4 4 4 3 3 3 2 2 2 2 1 1 1 0 0 None Trivial Mild Moderate Severe n=11 0 None Trivial Mild n=11 Moderate 0 None Trivial n=6 Mild Moderate 0 None Trivial n=5 Mild Moderate

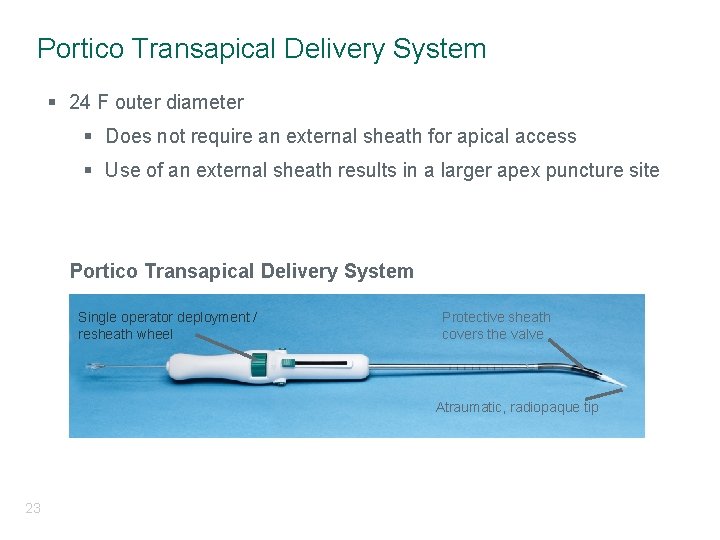
Portico Transapical Delivery System § 24 F outer diameter § Does not require an external sheath for apical access § Use of an external sheath results in a larger apex puncture site Portico Transapical Delivery System Single operator deployment / resheath wheel Protective sheath covers the valve Atraumatic, radiopaque tip 23

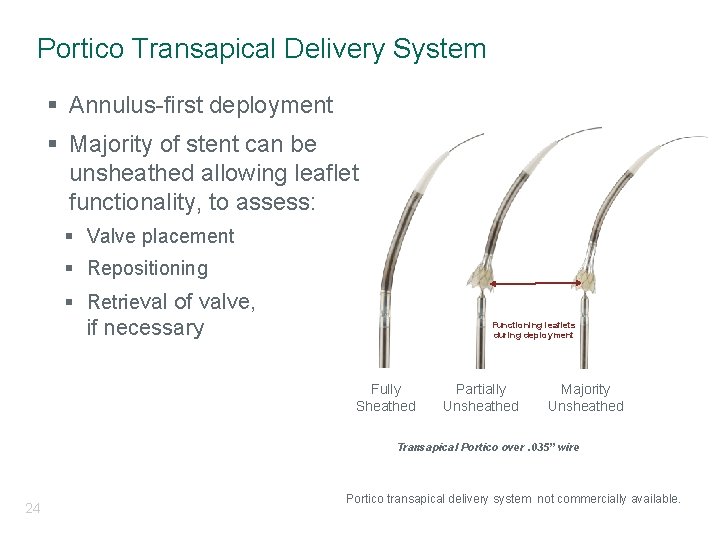
Portico Transapical Delivery System § Annulus-first deployment § Majority of stent can be unsheathed allowing leaflet functionality, to assess: § Valve placement § Repositioning § Retrieval of valve, if necessary Functioning leaflets during deployment Fully Sheathed Partially Unsheathed Majority Unsheathed Transapical Portico over. 035” wire 24 Portico transapical delivery system not commercially available.

Portico TA First-in-Human Case, November 15, 2012 Drs. Cheung and Webb, St Paul’s Hospital, Vancouver 73 yo Asian female § 37 KG, 154 cm (82 lbs, 5’ 1”) § Severe Aortic Stenosis, Preserved LV Function § Prior CABG, patent grafts § Moderate MR Failed TF, Sapien XT § Unable to pass valve through 14 F e. Sheath § Vascular injury Esophageal Injury Recovered, considered for Transapical TAVR

Portico TA First-in-Human Case, November 15, 2012 Drs. Cheung and Webb, St Paul’s Hospital, Vancouver

Portico Transapical FIH Extubated early post operatively Discharged POD 4 Pre-Discharge TTE Trace PVL Mean Gradient 10 mm. Hg Doing well 3 weeks later

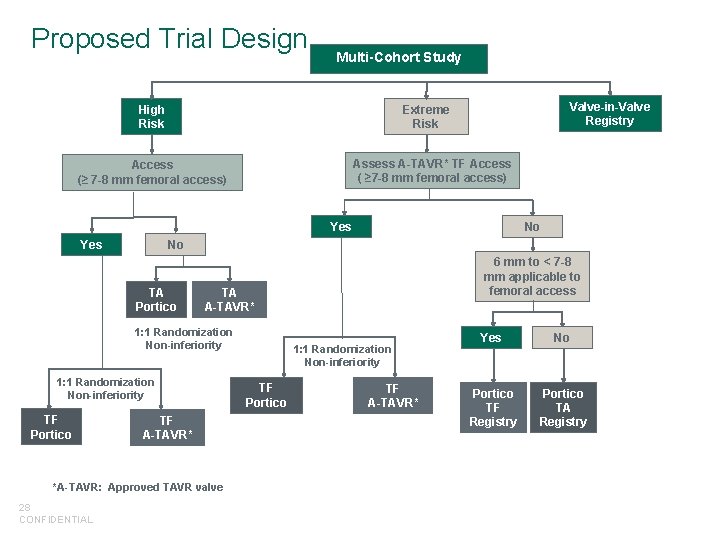
Proposed Trial Design Multi-Cohort Study High Risk Valve-in-Valve Registry Extreme Risk Assess A-TAVR* TF Access ( ≥ 7 -8 mm femoral access) Access (≥ 7 -8 mm femoral access) Yes No TA Portico 1: 1 Randomization Non-inferiority TF A-TAVR* *A-TAVR: Approved TAVR valve 28 CONFIDENTIAL 6 mm to < 7 -8 mm applicable to femoral access TA A-TAVR* 1: 1 Randomization Non-inferiority TF Portico No 1: 1 Randomization Non-inferiority TF Portico TF A-TAVR* Yes No Portico TF Registry Portico TA Registry

Portico Program Status 23 mm TF: CE Mark approved 23 mm TA: FIH series completed 25 mm TF: Enrollment in EU clinical trial underway 27/29 mm: EU clinical trial enrollment 1 H 2014 US IDE: Submitted Q 4 2012 US clinical trial to commence in 2 H 2013

Thank You