SAPIEN 3 Transcatheter Aortic Valve Replacement Compared with











- Slides: 32

SAPIEN 3 Transcatheter Aortic Valve Replacement Compared with Surgery in Intermediate-Risk Patients: A Propensity Score Analysis Vinod H. Thourani, MD on behalf of The PARTNER Trial Investigators ACC 2016 | Chicago | April 3, 2016

Disclosure Statement of Financial Interest Vinod H. Thourani, MD Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company • Grant/Research Support • Boston Scientific, Claret Medical, Edwards Lifesciences, Medtronic, St. Jude Medical • Consulting Fees/Honoraria • Abbott Vascular, Edwards Lifesciences, St. Jude Medical • Major Stock Shareholder/Equity • None

Background • The 3 rd generation SAPIEN 3 transcatheter heart valve has demonstrated improved clinical outcomes in high-risk patients at 1 year. • The 30 -day outcomes in intermediate-risk patients treated with SAPIEN 3 in PARTNER 2 were… – All-cause Mortality: 1. 1% – Disabling Stroke: 1. 0% – PVL > Moderate: 3. 8% • There is a paucity of longer-term data in intermediate-risk patients with SAPIEN 3 and there have been no rigorous comparisons with surgery in intermediate-risk patients.

Purpose • To evaluate the 1 -year clinical and echo outcomes of SAPIEN 3 TAVR in intermediate-risk patients. • To compare these intermediate-risk patient outcomes using SAPIEN 3 TAVR with surgery results in similar intermediate-risk patients from the PARTNER 2 A trial using a rigorous pre-specified propensity score analysis.

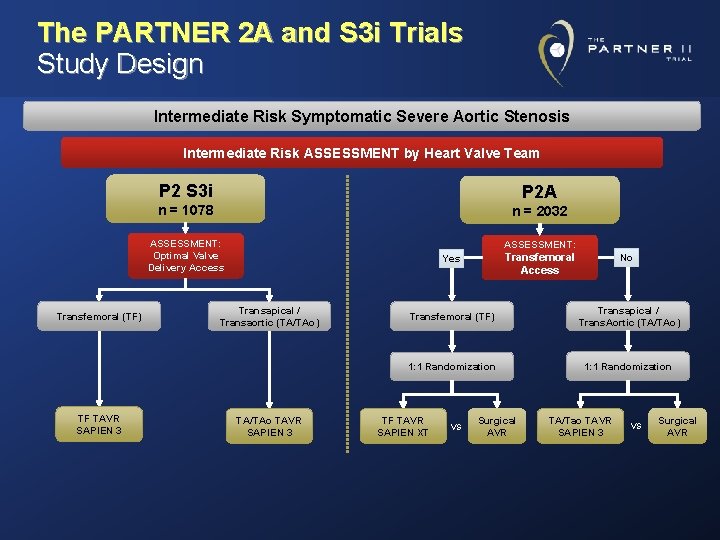
The PARTNER 2 A and S 3 i Trials Study Design Intermediate Risk Symptomatic Severe Aortic Stenosis Intermediate Risk ASSESSMENT by Heart Valve Team P 2 S 3 i P 2 A n = 1078 n = 2032 ASSESSMENT: Optimal Valve Delivery Access Transfemoral (TF) TF TAVR SAPIEN 3 ASSESSMENT: Transfemoral Access Yes Transapical / Transaortic (TA/TAo) TA/TAo TAVR SAPIEN 3 No Transfemoral (TF) Transapical / Trans. Aortic (TA/TAo) 1: 1 Randomization TF TAVR SAPIEN XT VS Surgical AVR TA/Tao TAVR SAPIEN 3 VS Surgical AVR

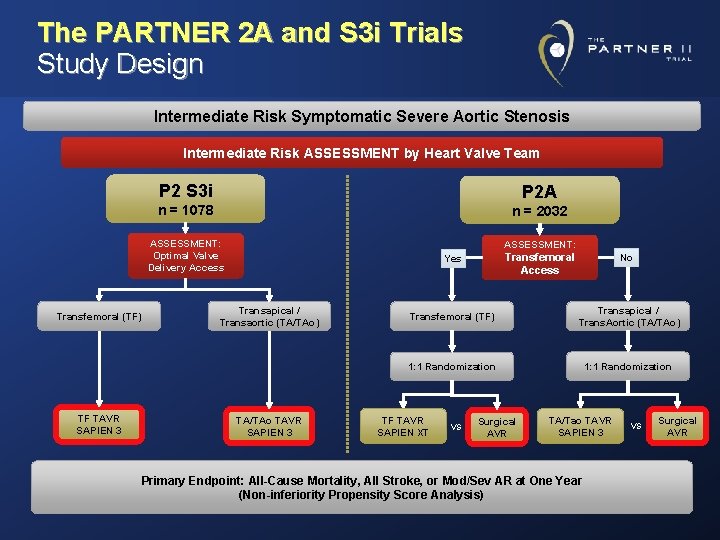
The PARTNER 2 A and S 3 i Trials Study Design Intermediate Risk Symptomatic Severe Aortic Stenosis Intermediate Risk ASSESSMENT by Heart Valve Team P 2 S 3 i P 2 A n = 1078 n = 2032 ASSESSMENT: Optimal Valve Delivery Access Transfemoral (TF) TF TAVR SAPIEN 3 ASSESSMENT: Transfemoral Access Yes Transapical / Transaortic (TA/TAo) TA/TAo TAVR SAPIEN 3 No Transfemoral (TF) Transapical / Trans. Aortic (TA/TAo) 1: 1 Randomization TF TAVR SAPIEN XT VS Surgical AVR TA/Tao TAVR SAPIEN 3 Primary Endpoint: All-Cause Mortality, All Stroke, or Mod/Sev AR at One Year (Non-inferiority Propensity Score Analysis) VS Surgical AVR

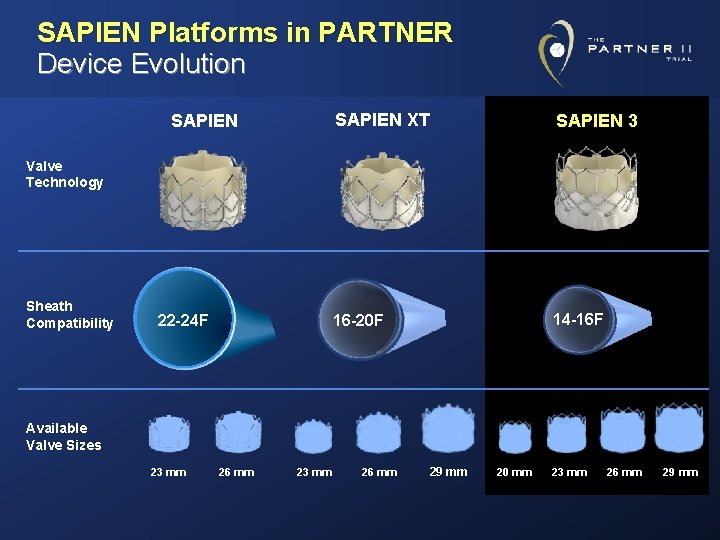
SAPIEN Platforms in PARTNER Device Evolution SAPIEN XT SAPIEN 3 16 -20 F 14 -16 F Valve Technology Sheath Compatibility 22 -24 F Available Valve Sizes 23 mm 26 mm 29 mm 20 mm 23 mm 26 mm 29 mm

The PARTNER 2 A and S 3 i Trials Inclusion Criteria • Severe AS: Echo-derived AVA ≤ 0. 8 cm 2 (or AVA index < 0. 5 cm 2/m 2) and mean AVG > 40 mm. Hg or peak jet velocity > 4. 0 m/s • Cardiac Symptoms: NYHA Functional Class ≥ II • Intermediate Risk: 1. Determined by a multi-disciplinary Heart Team 2. Using a guideline STS between 4 -8%*, and 3. Adjudicated by case review committee * PARTNER 2 A used guideline STS ≥ 4%

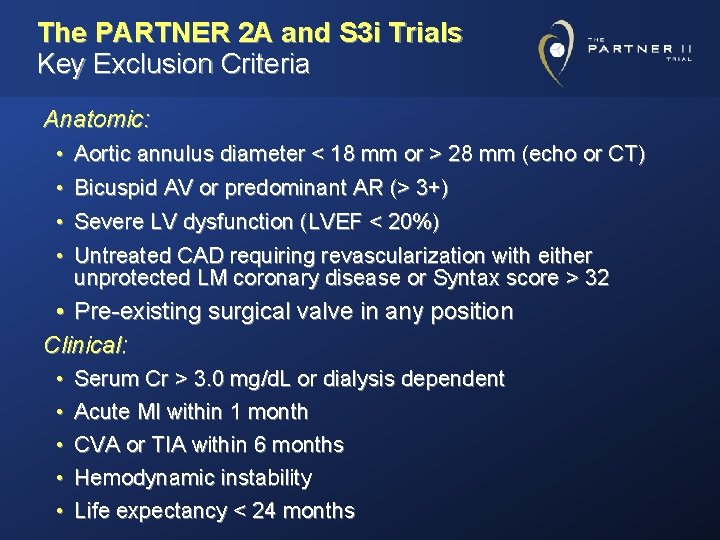
The PARTNER 2 A and S 3 i Trials Key Exclusion Criteria Anatomic: • Aortic annulus diameter < 18 mm or > 28 mm (echo or CT) • Bicuspid AV or predominant AR (> 3+) • Severe LV dysfunction (LVEF < 20%) • Untreated CAD requiring revascularization with either unprotected LM coronary disease or Syntax score > 32 • Pre-existing surgical valve in any position Clinical: • • • Serum Cr > 3. 0 mg/d. L or dialysis dependent Acute MI within 1 month CVA or TIA within 6 months Hemodynamic instability Life expectancy < 24 months

The PARTNER 2 A and S 3 i Trials Primary Endpoint • Non-hierarchical composite of all-cause mortality, all stroke, or ≥ moderate aortic regurgitation at one year • Propensity score analysis of the valve implant (VI) populations from S 3 i compared to the surgical arm of the PARTNER 2 A trial • All patients followed for at least 1 year • Event rates by Kaplan-Meier estimates • Non-inferiority trial design followed by superiority testing for the primary endpoint and components

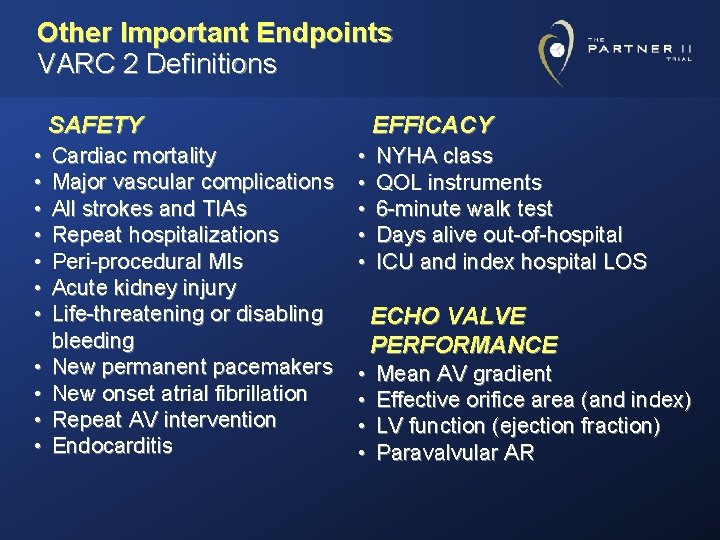
Other Important Endpoints VARC 2 Definitions SAFETY • • • Cardiac mortality Major vascular complications All strokes and TIAs Repeat hospitalizations Peri-procedural MIs Acute kidney injury Life-threatening or disabling bleeding New permanent pacemakers New onset atrial fibrillation Repeat AV intervention Endocarditis EFFICACY • • • NYHA class QOL instruments 6 -minute walk test Days alive out-of-hospital ICU and index hospital LOS ECHO VALVE PERFORMANCE • • Mean AV gradient Effective orifice area (and index) LV function (ejection fraction) Paravalvular AR

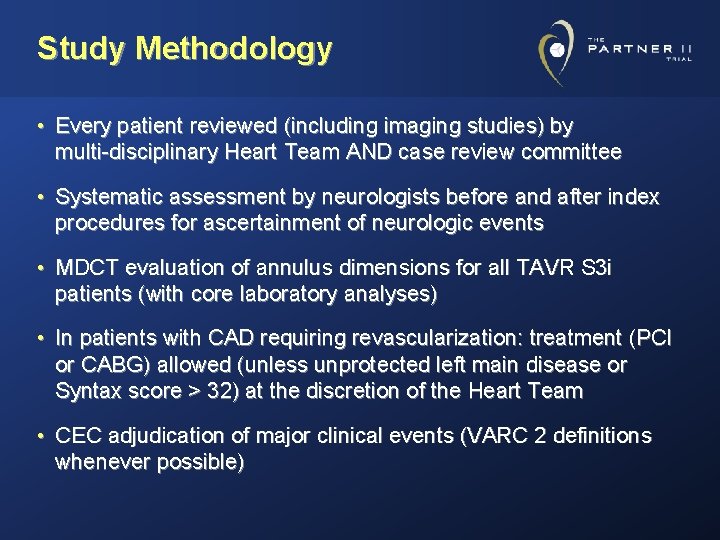
Study Methodology • Every patient reviewed (including imaging studies) by multi-disciplinary Heart Team AND case review committee • Systematic assessment by neurologists before and after index procedures for ascertainment of neurologic events • MDCT evaluation of annulus dimensions for all TAVR S 3 i patients (with core laboratory analyses) • In patients with CAD requiring revascularization: treatment (PCI or CABG) allowed (unless unprotected left main disease or Syntax score > 32) at the discretion of the Heart Team • CEC adjudication of major clinical events (VARC 2 definitions whenever possible)

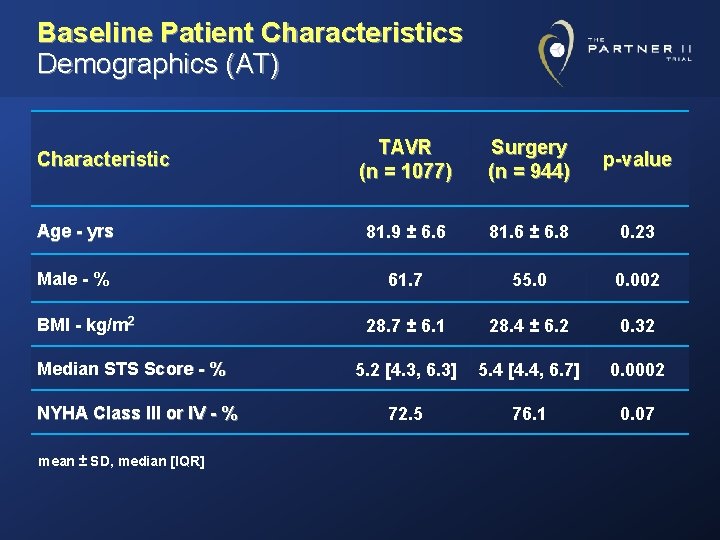
Baseline Patient Characteristics Demographics (AT) TAVR (n = 1077) Surgery (n = 944) p-value Age - yrs 81. 9 ± 6. 6 81. 6 ± 6. 8 0. 23 Male - % 61. 7 55. 0 0. 002 28. 7 ± 6. 1 28. 4 ± 6. 2 0. 32 5. 2 [4. 3, 6. 3] 5. 4 [4. 4, 6. 7] 0. 0002 72. 5 76. 1 0. 07 Characteristic BMI - kg/m 2 Median STS Score - % NYHA Class III or IV - % mean ± SD, median [IQR]

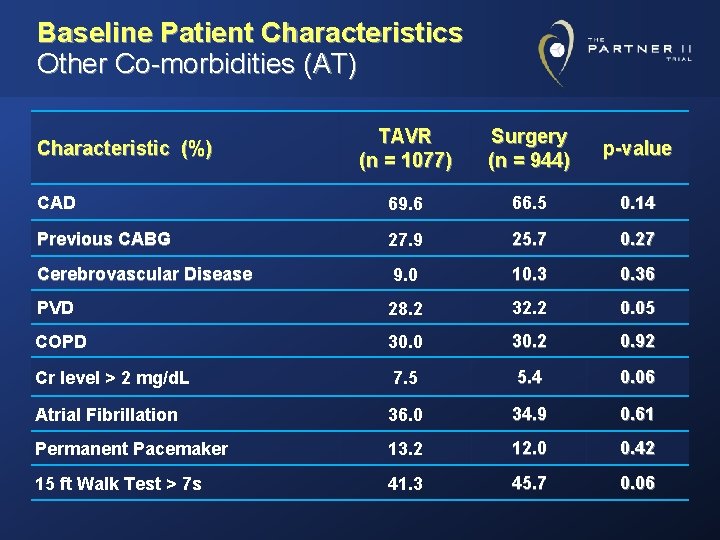
Baseline Patient Characteristics Other Co-morbidities (AT) TAVR (n = 1077) Surgery (n = 944) p-value CAD 69. 6 66. 5 0. 14 Previous CABG 27. 9 25. 7 0. 27 Cerebrovascular Disease 9. 0 10. 36 PVD 28. 2 32. 2 0. 05 COPD 30. 0 30. 2 0. 92 Cr level > 2 mg/d. L 7. 5 5. 4 0. 06 Atrial Fibrillation 36. 0 34. 9 0. 61 Permanent Pacemaker 13. 2 12. 0 0. 42 15 ft Walk Test > 7 s 41. 3 45. 7 0. 06 Characteristic (%)

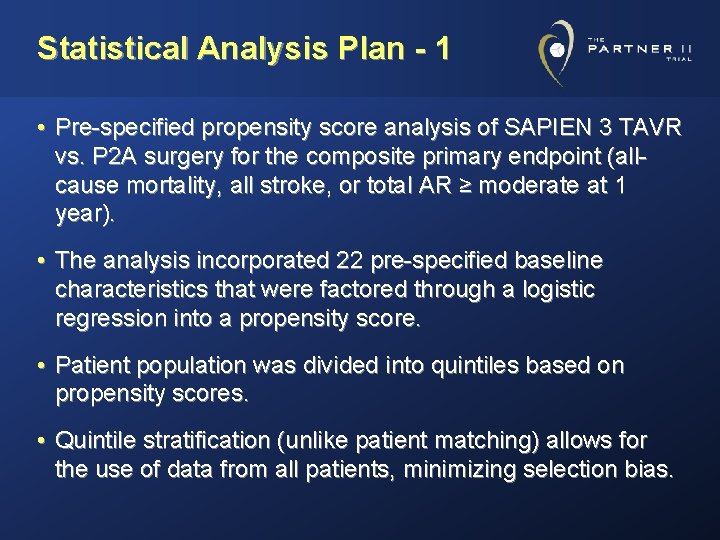
Statistical Analysis Plan - 1 • Pre-specified propensity score analysis of SAPIEN 3 TAVR vs. P 2 A surgery for the composite primary endpoint (allcause mortality, all stroke, or total AR ≥ moderate at 1 year). • The analysis incorporated 22 pre-specified baseline characteristics that were factored through a logistic regression into a propensity score. • Patient population was divided into quintiles based on propensity scores. • Quintile stratification (unlike patient matching) allows for the use of data from all patients, minimizing selection bias.

Statistical Analysis Plan - 2 • The primary hypothesis was non-inferiority of SAPIEN 3 vs. surgery from P 2 A for the primary endpoint at 1 year. • Non-inferiority: – One-sided alpha: 0. 05 – Hypothesis testing: upper bound of the one-sided 95% CI of bound the primary weighted difference of proportions endpoint (weighted avg test - control) < 7. 5% • Superiority: – Two-sided alpha: 0. 05 – Hypothesis testing: upper or lower bound of the two-sided 95% CI of the primary weighted difference of proportions endpoint (weighted avg test - control) < 0 for TAVR and > 0 for surgery

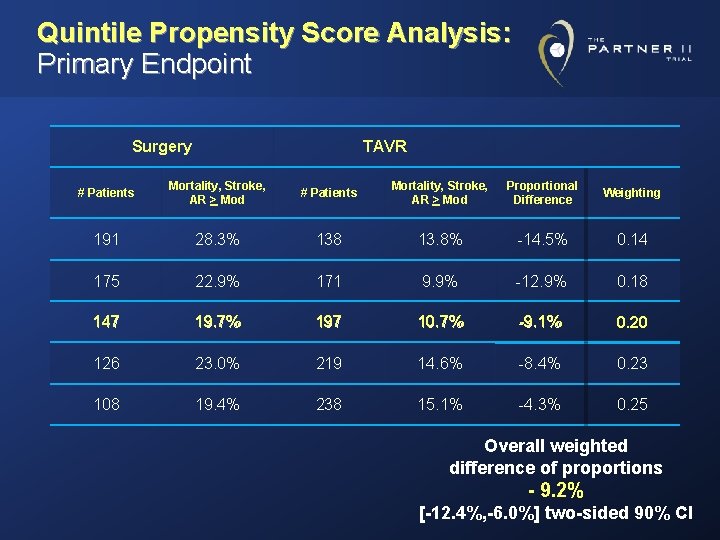
Quintile Propensity Score Analysis: Primary Endpoint Surgery TAVR # Patients Mortality, Stroke, AR > Mod Proportional Difference Weighting 191 28. 3% 138 13. 8% -14. 5% 0. 14 175 22. 9% 171 9. 9% -12. 9% 0. 18 147 19. 7% 197 10. 7% -9. 1% 0. 20 126 23. 0% 219 14. 6% -8. 4% 0. 23 108 19. 4% 238 15. 1% -4. 3% 0. 25 Overall weighted difference of proportions - 9. 2% [-12. 4%, -6. 0%] two-sided 90% CI

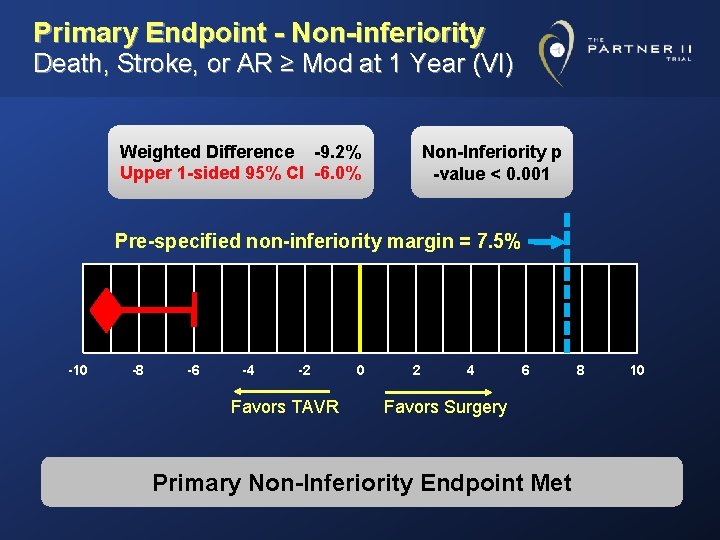
Primary Endpoint - Non-inferiority Death, Stroke, or AR ≥ Mod at 1 Year (VI) Weighted Difference -9. 2% Upper 1 -sided 95% CI -6. 0% Non-Inferiority p -value < 0. 001 Pre-specified non-inferiority margin = 7. 5% -10 -8 -6 -4 -2 Favors TAVR 0 2 4 6 Favors Surgery Primary Non-Inferiority Endpoint Met 8 10

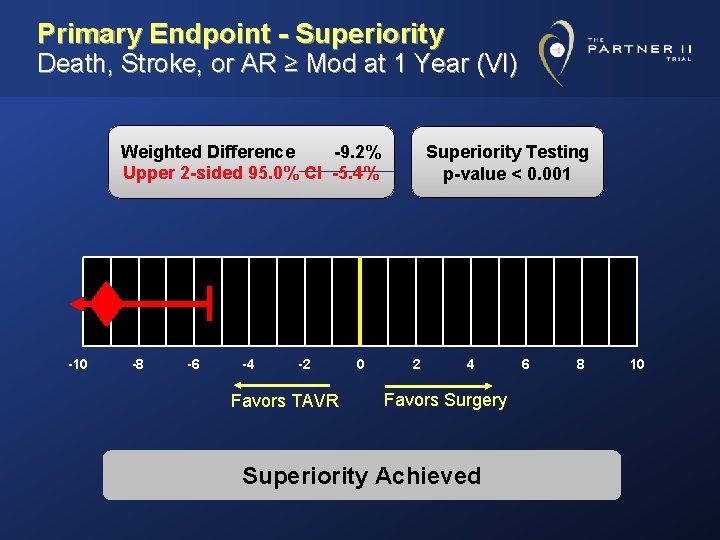
Primary Endpoint - Superiority Death, Stroke, or AR ≥ Mod at 1 Year (VI) Weighted Difference -9. 2% Upper 2 -sided 95. 0% CI -5. 4% -10 -8 -6 -4 -2 Favors TAVR 0 Superiority Testing p-value < 0. 001 2 4 Favors Surgery Superiority Achieved 6 8 10

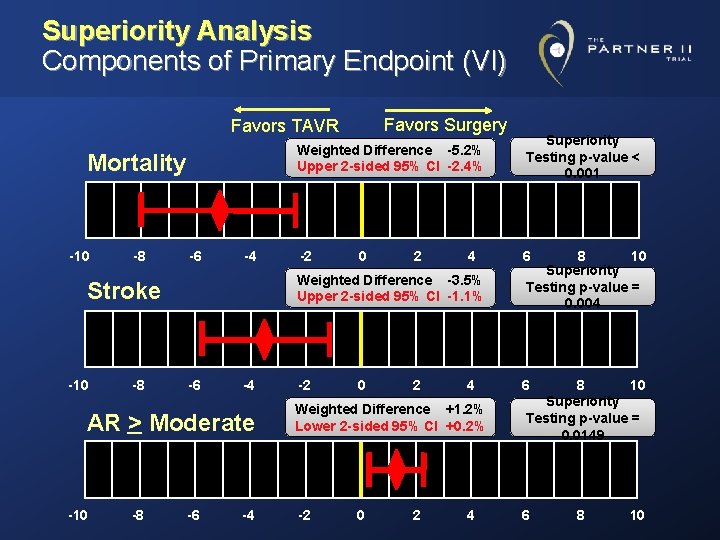
Superiority Analysis Components of Primary Endpoint (VI) Favors Surgery Favors TAVR Mortality -10 -8 -6 -4 AR > Moderate -10 -8 Superiority Testing p-value < 0. 001 -2 6 0 2 4 Weighted Difference -3. 5% Upper 2 -sided 95% CI -1. 1% Stroke -10 Weighted Difference -5. 2% Upper 2 -sided 95% CI -2. 4% -6 -4 -2 0 2 4 Weighted Difference +1. 2% Lower 2 -sided 95% CI +0. 2% -2 0 2 4 8 10 Superiority Testing p-value = 0. 004 6 8 10 Superiority Testing p-value = 0. 0149 6 8 10

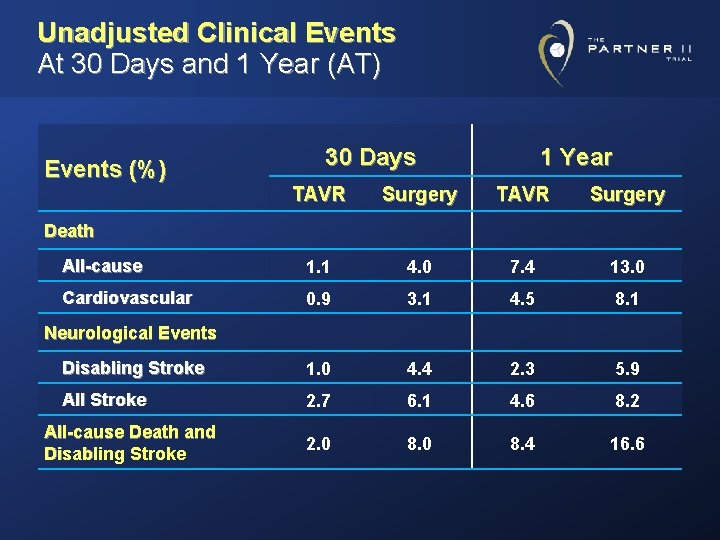
Unadjusted Clinical Events At 30 Days and 1 Year (AT) Events (%) 30 Days 1 Year TAVR Surgery All-cause 1. 1 4. 0 7. 4 13. 0 Cardiovascular 0. 9 3. 1 4. 5 8. 1 Disabling Stroke 1. 0 4. 4 2. 3 5. 9 All Stroke 2. 7 6. 1 4. 6 8. 2 2. 0 8. 4 16. 6 Death Neurological Events All-cause Death and Disabling Stroke

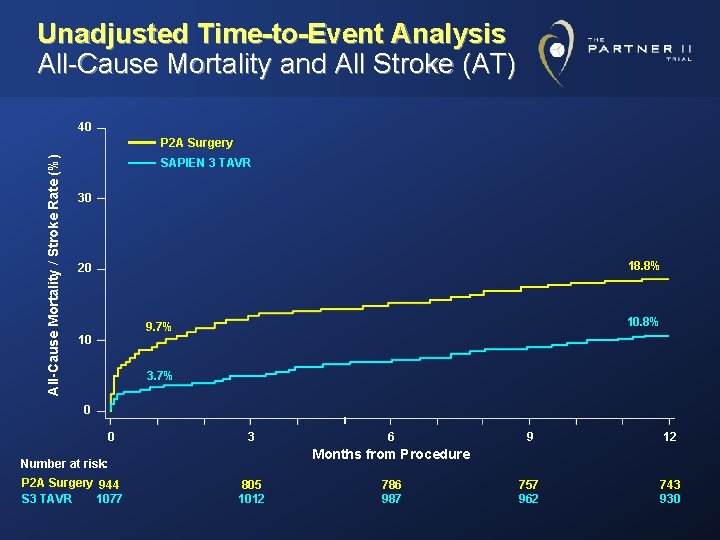
Unadjusted Time-to-Event Analysis All-Cause Mortality and All Stroke (AT) 40 All-Cause Mortality / Stroke Rate (%) P 2 A Surgery SAPIEN 3 TAVR 30 18. 8% 20 10. 8% 9. 7% 10 3. 7% 0 0 3 9 12 757 962 743 930 Months from Procedure Number at risk: P 2 A Surgery 944 S 3 TAVR 1077 6 805 1012 786 987

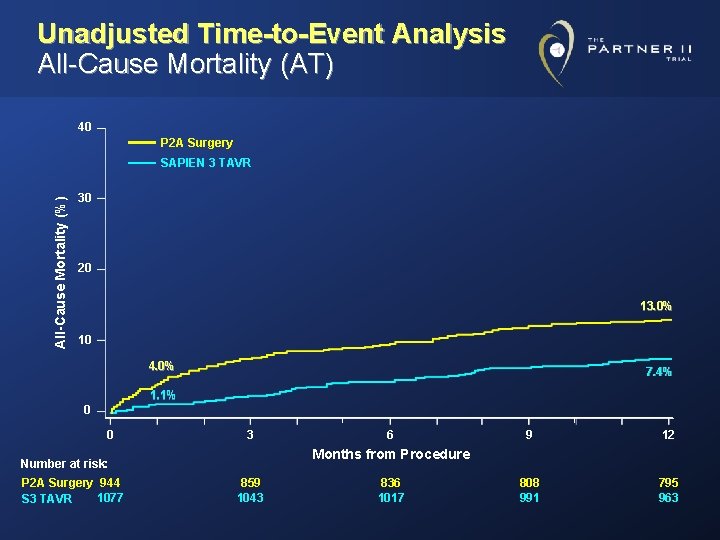
Unadjusted Time-to-Event Analysis All-Cause Mortality (AT) 40 P 2 A Surgery All-Cause Mortality (%) SAPIEN 3 TAVR 30 20 13. 0% 10 4. 0% 7. 4% 1. 1% 0 0 3 9 12 808 991 795 963 Months from Procedure Number at risk: P 2 A Surgery 944 1077 S 3 TAVR 6 859 1043 836 1017

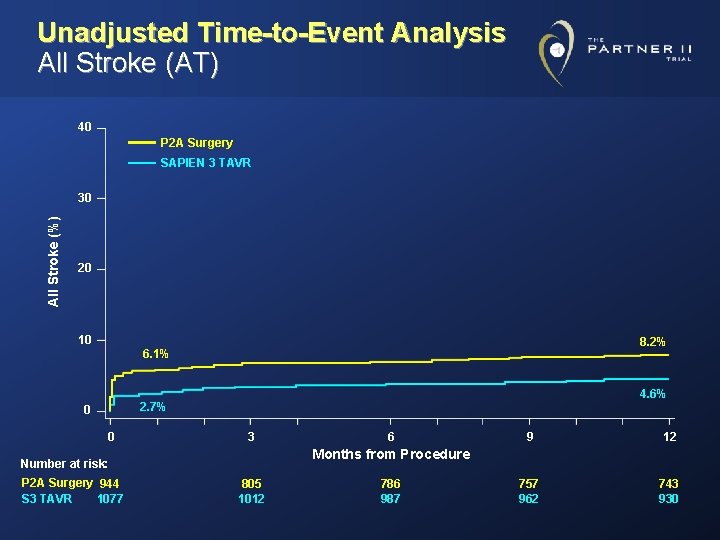
Unadjusted Time-to-Event Analysis All Stroke (AT) 40 P 2 A Surgery SAPIEN 3 TAVR All Stroke (%) 30 20 10 8. 2% 6. 1% 4. 6% 2. 7% 0 0 3 9 12 757 962 743 930 Months from Procedure Number at risk: P 2 A Surgery 944 1077 S 3 TAVR 6 805 1012 786 987

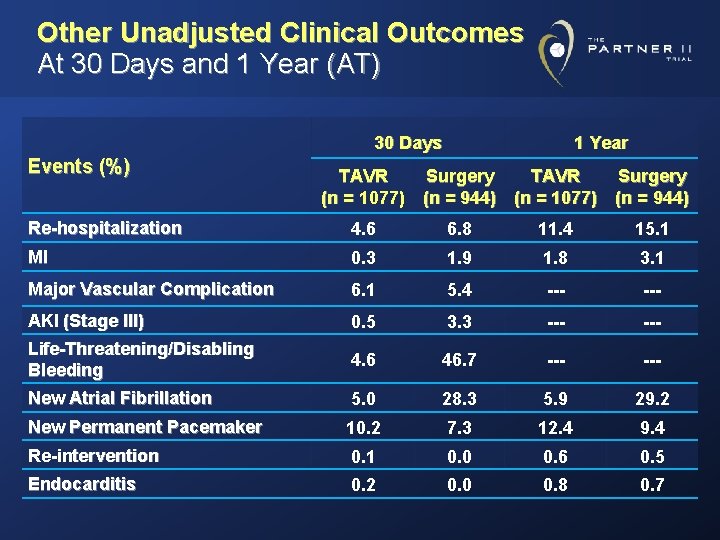
Other Unadjusted Clinical Outcomes At 30 Days and 1 Year (AT) 30 Days Events (%) 1 Year TAVR Surgery (n = 1077) (n = 944) Re-hospitalization 4. 6 6. 8 11. 4 15. 1 MI 0. 3 1. 9 1. 8 3. 1 Major Vascular Complication 6. 1 5. 4 --- AKI (Stage III) 0. 5 3. 3 --- Life-Threatening/Disabling Bleeding 4. 6 46. 7 --- New Atrial Fibrillation 5. 0 28. 3 5. 9 29. 2 New Permanent Pacemaker 10. 2 7. 3 12. 4 9. 4 Re-intervention 0. 1 0. 0 0. 6 0. 5 Endocarditis 0. 2 0. 0 0. 8 0. 7

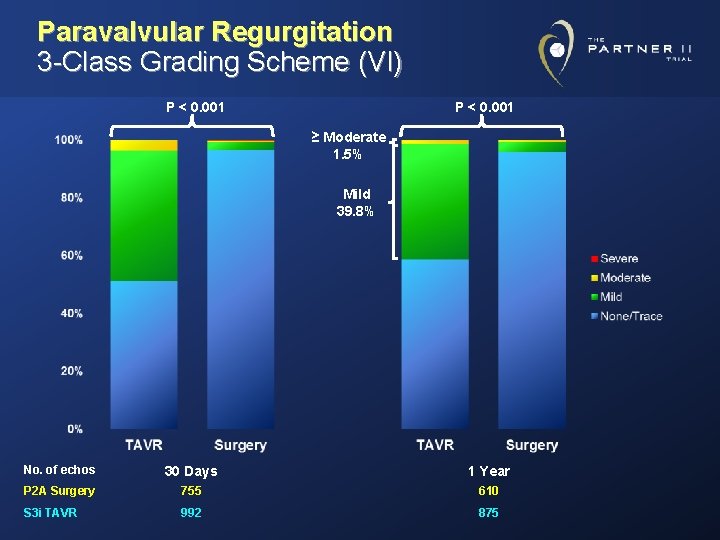
Paravalvular Regurgitation 3 -Class Grading Scheme (VI) P < 0. 001 ≥ Moderate 1. 5% Mild 39. 8% No. of echos 30 Days 1 Year P 2 A Surgery 755 610 S 3 i TAVR 992 875

The PARTNER 2 A and S 3 i Trials Conclusions - 1 • In intermediate-risk patients, SAPIEN 3 TAVR resulted in low 1 -year rates of all-cause mortality (7. 4%), all stroke (4. 6%), and moderate or severe aortic regurgitation (1. 5%)

The PARTNER 2 A and S 3 i Trials Conclusions - 2 • A rigorous propensity score analysis comparing SAPIEN 3 TAVR with surgery from PARTNER 2 A in intermediate-risk patients at 1 year demonstrated: – Non-inferiority for the primary endpoint (composite of all-cause mortality, all stroke, or AR ≥ moderate) – Superiority of SAPIEN 3 TAVR for the primary endpoint, all-cause mortality, and all stroke – Superiority of surgery for AR ≥ moderate • Time-to-event analyses indicated that the benefits of SAPIEN 3 TAVR occurred in the first few months, suggesting procedure-related effects

The PARTNER 2 A and S 3 i Trial Clinical Implications • The conclusions from the PARTNER 2 A randomized trial and this propensity score analysis provide strong evidence that in intermediate-risk patients with severe aortic stenosis, SAPIEN 3 TAVR compared with surgery improves clinical outcomes and is the preferred therapy.

The PARTNER 2 A and S 3 i Trial Lancet On-line Special thanks to the PARTNER sites and patients, the clinical research teams, and the writing group!

The PARTNER 2 A and S 3 i Trial Lancet On-line

The PARTNER 2 A and S 3 i Trial Lancet On-line