Faculty of Medicine Introduction to Community Medicine Course






![EPIDEMIOLOGY [DEFINITION OF KEY TERMS] • Distribution : Frequency (including rates & risks) & EPIDEMIOLOGY [DEFINITION OF KEY TERMS] • Distribution : Frequency (including rates & risks) &](https://slidetodoc.com/presentation_image_h2/4ebbee5027d3237756e569ccd1ef0897/image-9.jpg)
![EPIDEMIOLOGY [DEFINITION OF KEY TERMS] • Health related states: infections, chronic diseases & physiological EPIDEMIOLOGY [DEFINITION OF KEY TERMS] • Health related states: infections, chronic diseases & physiological](https://slidetodoc.com/presentation_image_h2/4ebbee5027d3237756e569ccd1ef0897/image-10.jpg)


























- Slides: 68

Faculty of Medicine Introduction to Community Medicine Course (31505201) Unit 4 Epidemiology Introduction to Epidemiology EPIDEMIOLOGICAL STUDY METHODS By Hatim Jaber MD MPH JBCM Ph. D 6+8 12 - 2016 1

Presentation outline Time Introduction and Definitions 12: 00 to 12: 10 CLASSIFICATION 12: 10 to 12: 20 STUDY DESIGNS 12: 20 to 12: 30 VARIOUS DESIGNS 12: 30 to 12: 50 2

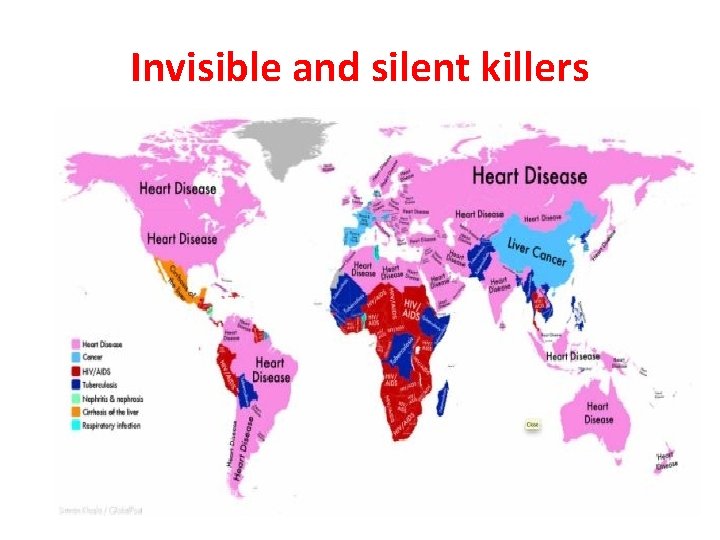
Invisible and silent killers

Invisible killer AIR POLLUTION

Silent killer

Important “What you control!” cant measure you cant epidemiological study methods are used to study your health and my health and its determinants, as we join hands to ensure a healthier us.

INTRODUCTION • The science of epidemiology has matured significantly from the times of Hippocrates and john snow [physician] that the techniques for analyzing data vary depending on the type of disease being monitored but each study will have similarities. environmental factors can influence the occurrence of the diseases. • epidemiology study of what is upon the people • Derived from the greek terms epi=upon, among. demos=people, district. Logos study, word.

DEFINITION OF EPIDEMIOLOGY • Epidemiology is the study of distribution and determinant of health related state or event in a specified human population and the application of this study to the control of health problem.
![EPIDEMIOLOGY DEFINITION OF KEY TERMS Distribution Frequency including rates risks EPIDEMIOLOGY [DEFINITION OF KEY TERMS] • Distribution : Frequency (including rates & risks) &](https://slidetodoc.com/presentation_image_h2/4ebbee5027d3237756e569ccd1ef0897/image-9.jpg)
EPIDEMIOLOGY [DEFINITION OF KEY TERMS] • Distribution : Frequency (including rates & risks) & pattern of health events(person, place, time) • Determinants : factors or events that are capable of bringing about a change in health • Human population : Epidemiology examines health events among population groups rather than individuals.
![EPIDEMIOLOGY DEFINITION OF KEY TERMS Health related states infections chronic diseases physiological EPIDEMIOLOGY [DEFINITION OF KEY TERMS] • Health related states: infections, chronic diseases & physiological](https://slidetodoc.com/presentation_image_h2/4ebbee5027d3237756e569ccd1ef0897/image-10.jpg)
EPIDEMIOLOGY [DEFINITION OF KEY TERMS] • Health related states: infections, chronic diseases & physiological events &various states of health such as disability, injury, mortality • Health related events : immunization, hospital attendance, bed occupancy • Application : basis for directing interventions

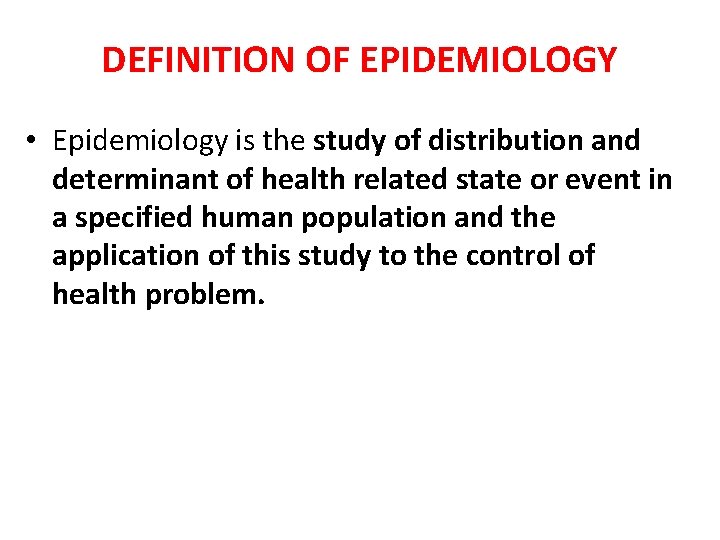
CLASSIFICATION

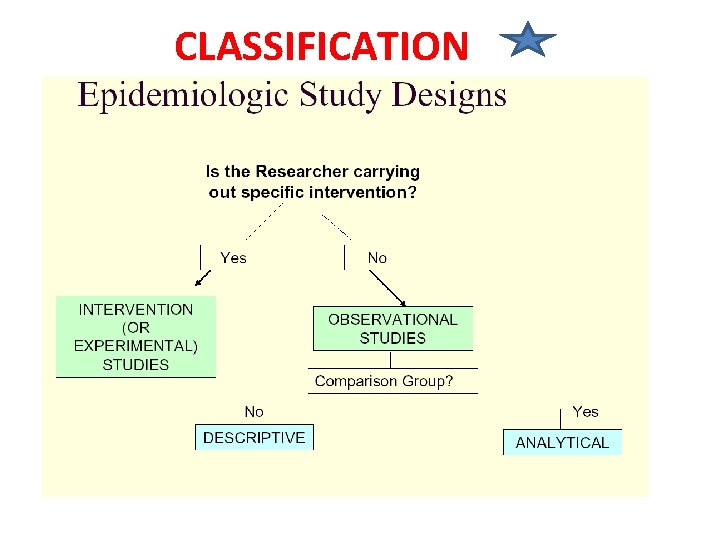
CLASSIFICATION(CONT. ) CORRELATIONAL

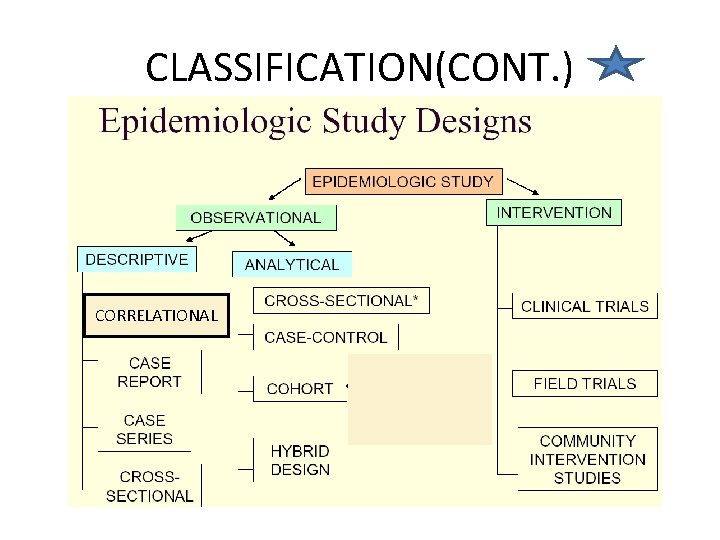
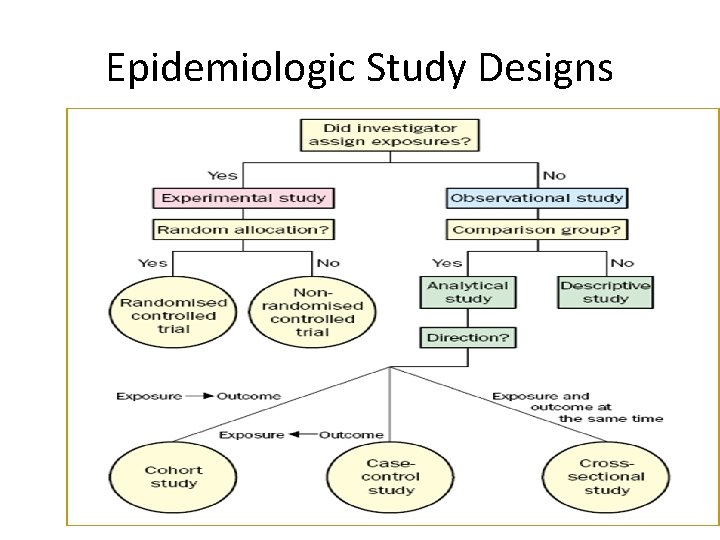
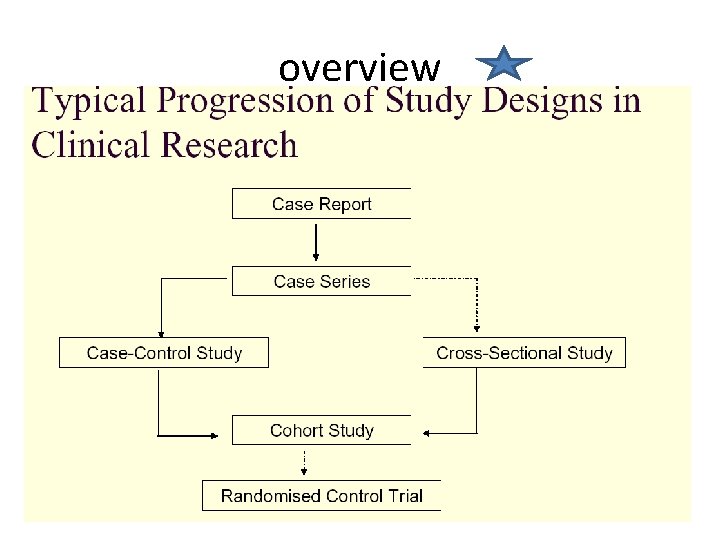
Epidemiologic Study Designs

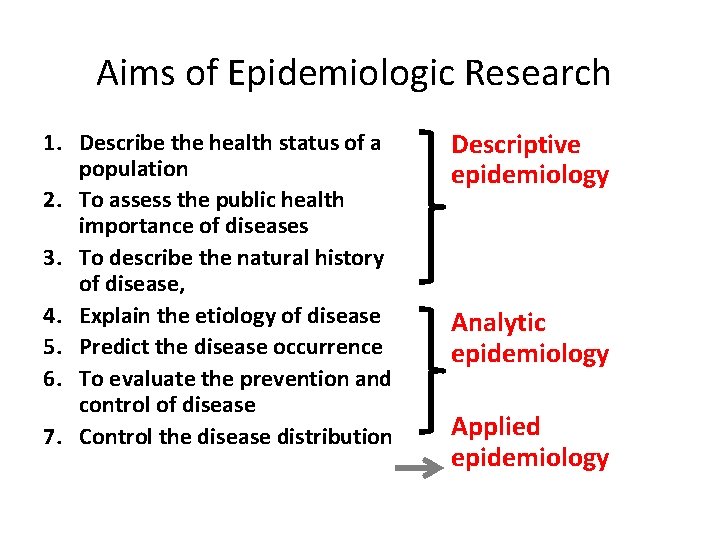
Aims of Epidemiologic Research 1. Describe the health status of a population 2. To assess the public health importance of diseases 3. To describe the natural history of disease, 4. Explain the etiology of disease 5. Predict the disease occurrence 6. To evaluate the prevention and control of disease 7. Control the disease distribution Descriptive epidemiology Analytic epidemiology Applied epidemiology

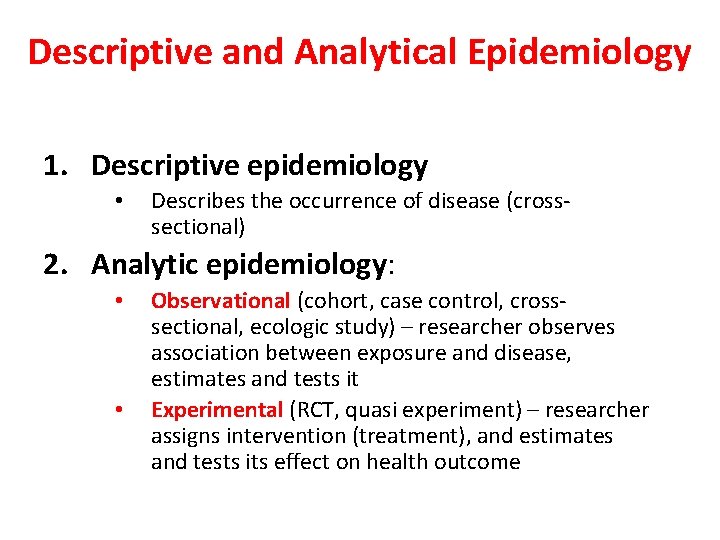
Descriptive and Analytical Epidemiology 1. Descriptive epidemiology • Describes the occurrence of disease (crosssectional) 2. Analytic epidemiology: • • Observational (cohort, case control, crosssectional, ecologic study) – researcher observes association between exposure and disease, estimates and tests it Experimental (RCT, quasi experiment) – researcher assigns intervention (treatment), and estimates and tests its effect on health outcome

OBSERVATIONAL VS EXPERIMENTAL STUDIES • Observational studies Allow nature to take its cause; the investigator measures but does not intervene • Descriptive study: focuses on the description of the occurrence of a disease in a population • Analytical study analyses relationships between health status and other variables

OBSERVATIONAL VS EXPERIMENTAL STUDIES • Experimental or interventional studies: involve an active attempt to change a disease determinant(e. g an exposure or a behaviour) or the progress of a disaese (through treatment) • The studies are based on a group which has had the experience compared with control group which has not had the experience.

PURPOSE OF DESCRIPTIVE EPIDEMIOLOGY • To generate hypothesis • To permit evaluation of trends in health & disease and comparisons among countries and subgroups within countries. • To provide a basis for planning, provision and evaluation of health services • To identify problems to be studied by analytical methods and to suggest areas that may be fruitful for investigation

CASE STUDIES(CASE SERIES) • Case reports: documents unusual medical occurrence and can represent the first clues to the formulation of hypothesis, generally report a new or unique findings and previous undescribed disease. • Case series: collection of individual case reports which may occur within a fairly short time, and experience of a group of patients with similar diagnosis.

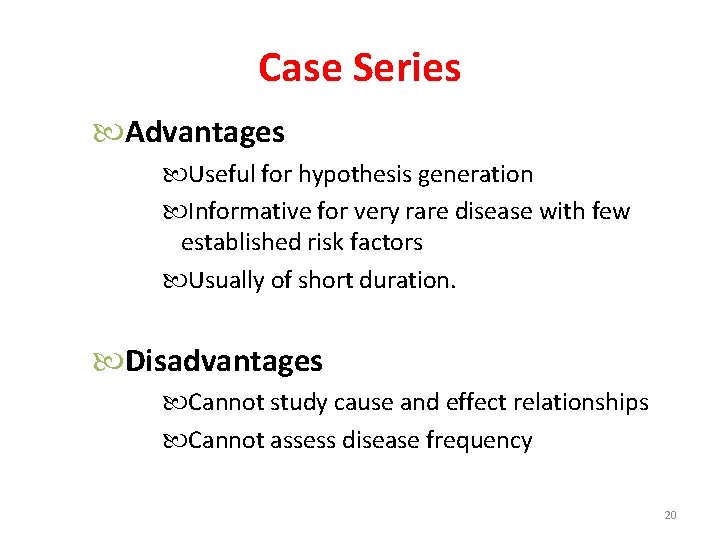
Case Series Advantages Useful for hypothesis generation Informative for very rare disease with few established risk factors Usually of short duration. Disadvantages Cannot study cause and effect relationships Cannot assess disease frequency 20

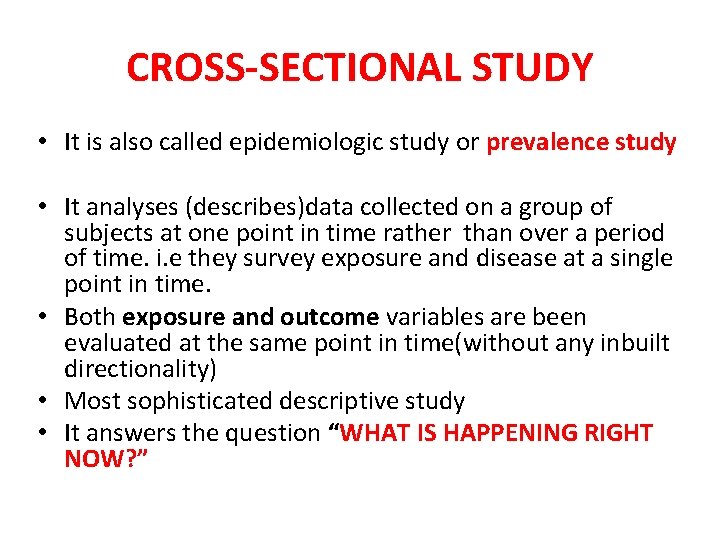
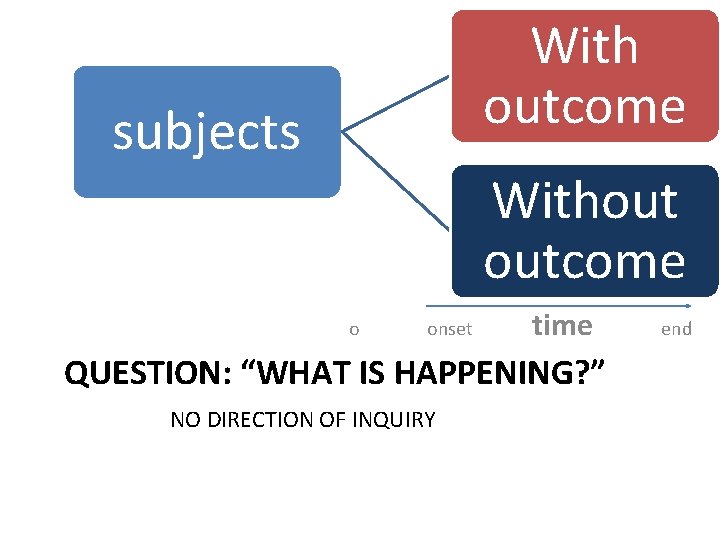
CROSS-SECTIONAL STUDY • It is also called epidemiologic study or prevalence study • It analyses (describes)data collected on a group of subjects at one point in time rather than over a period of time. i. e they survey exposure and disease at a single point in time. • Both exposure and outcome variables are been evaluated at the same point in time(without any inbuilt directionality) • Most sophisticated descriptive study • It answers the question “WHAT IS HAPPENING RIGHT NOW? ”

With outcome subjects Without outcome o onset time QUESTION: “WHAT IS HAPPENING? ” NO DIRECTION OF INQUIRY end

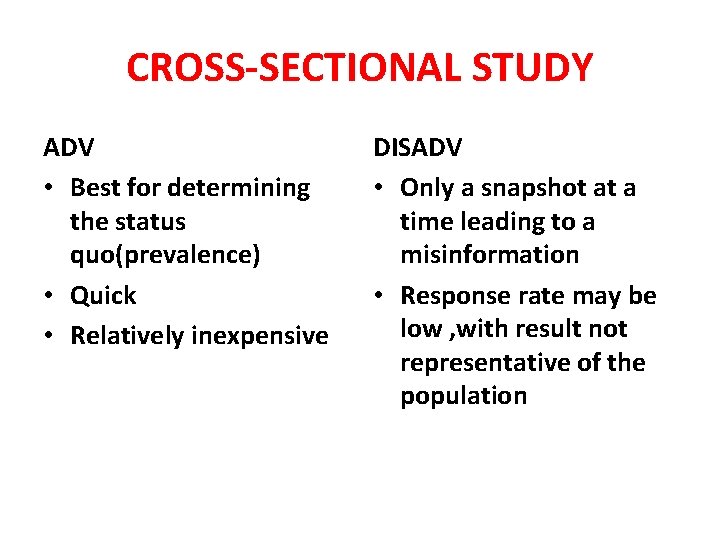
CROSS-SECTIONAL STUDY ADV • Best for determining the status quo(prevalence) • Quick • Relatively inexpensive DISADV • Only a snapshot at a time leading to a misinformation • Response rate may be low , with result not representative of the population

Cross-sectional studies Disadvantages Weakest observational design, (it measures prevalence, not incidence of disease). Prevalent cases are survivors The temporal sequence of exposure and effect may be difficult or impossible to determine Usually don’t know when disease occurred Rare events a problem. Quickly emerging diseases a problem 24

CORRELATIONAL STUDY DESIGN • A study comparing incidence/prevalence of one event against another on a global scale • Measures that represent characteristics of entire populations are used to describe the disease in relation to some factor of interest (such as age, calendar time, food consumption, drug use and utilization of health services)

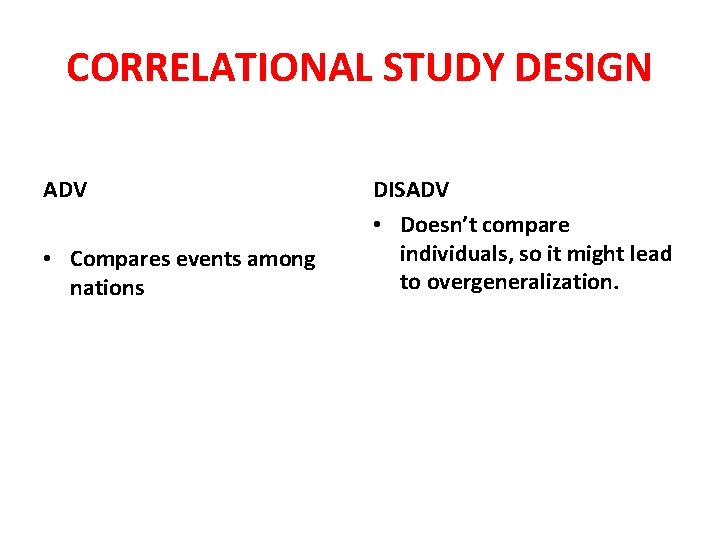
CORRELATIONAL STUDY DESIGN ADV • Compares events among nations DISADV • Doesn’t compare individuals, so it might lead to overgeneralization.

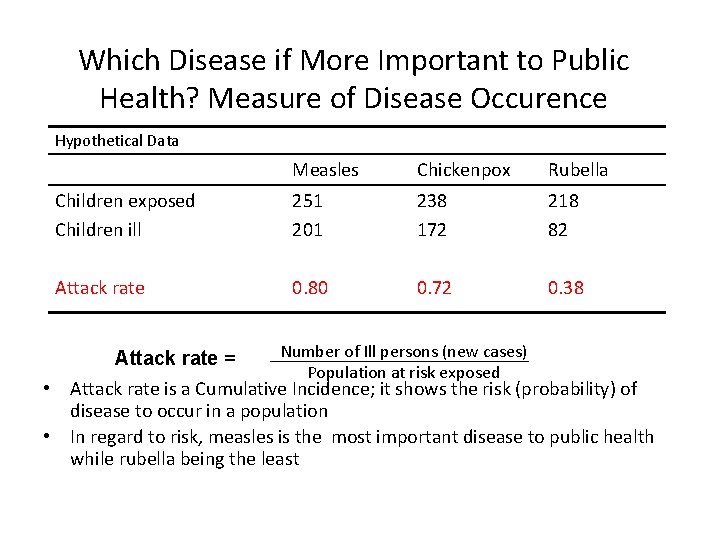
Which Disease if More Important to Public Health? Measure of Disease Occurence Hypothetical Data Measles Chickenpox Rubella Children exposed Children ill 251 201 238 172 218 82 Attack rate 0. 80 0. 72 0. 38 Number of Ill persons (new cases) Attack rate = Population at risk exposed • Attack rate is a Cumulative Incidence; it shows the risk (probability) of disease to occur in a population • In regard to risk, measles is the most important disease to public health while rubella being the least

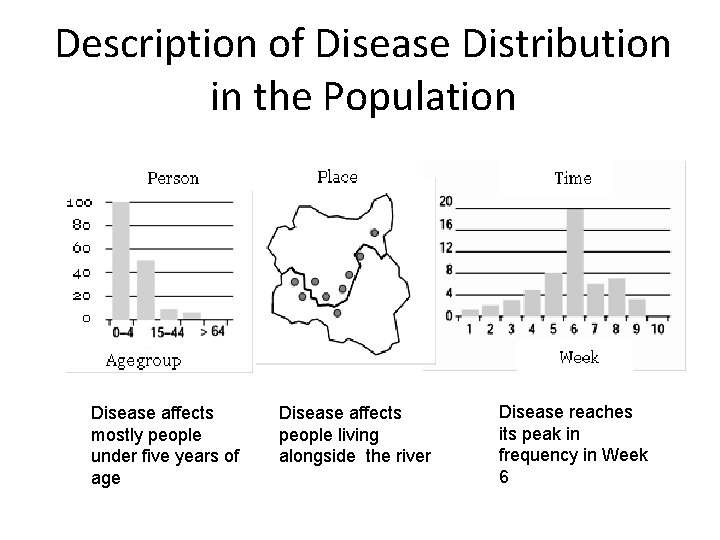
Description of Disease Distribution in the Population Disease affects mostly people under five years of age Disease affects people living alongside the river Disease reaches its peak in frequency in Week 6

ANALYTICAL STUDIES Two basic designs: • Case – control or retrospective study • Cohort or prospective NOTE • There must be a comparison group • No control No conclusion(NCNC)

CASE CONTROL OR CASE HISTORY STUDY • A group of affected people is compared to unaffected people(the control) • It’s a LONGITUDNAL STUDY (like cohort study) because it’s a study over a period of time. • Subjects are selected based on a particular outcome and a study backwards in time to try to detect the causes or risk factors that may have earlier been reported in a descriptive study • Subjects are then matched and assigned into the two groups. Subject selected on the basis of disease[e. g lung cancer]. • Sometimes called a retrospective study because of the direction of study

CASE CONTROL OR CASE HISTORY STUDY

Advantages of case control • It is relatively easy to carry out bcos we go back to existing records in the hospital • It is also rapid and inexpensive • It requires comparatively few subjects • It can assist one in studying different etiological factors • One does not need an ethical clearance • There is no risk to the subject

Disadvantages of case control • It introduces bias • To select an appropriate control could be difficult • It may be difficult to distinguish between the cause of a disease and an associated factor

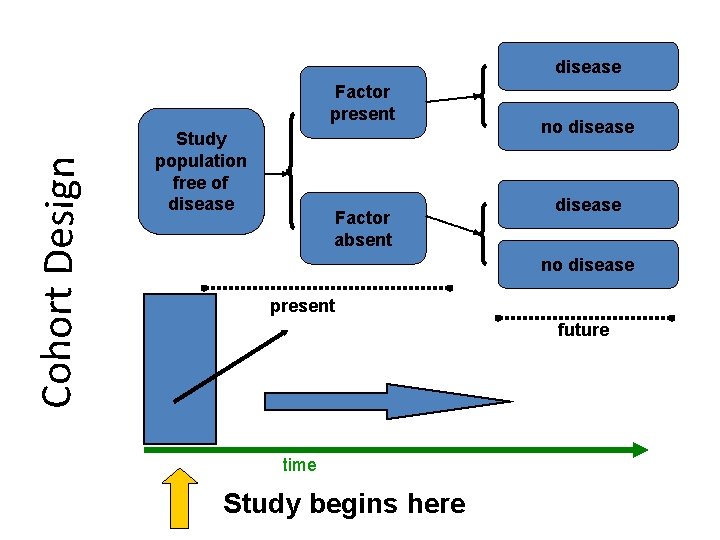
COHORT STUDY • A cohort is a group of people who have something in common and remain part of a group over an extended time • A group of people exposed to a suspected etiological agent are compared with a matched control who have not been similarly exposed. Subject selected on the basis of exposure [etiological factor; cigarette smoking] • Follow-up over a period to compare the outcome • Also a longitudinal study or prospective study

disease Cohort Design Factor present Study population free of disease Factor absent no disease present future time Study begins here


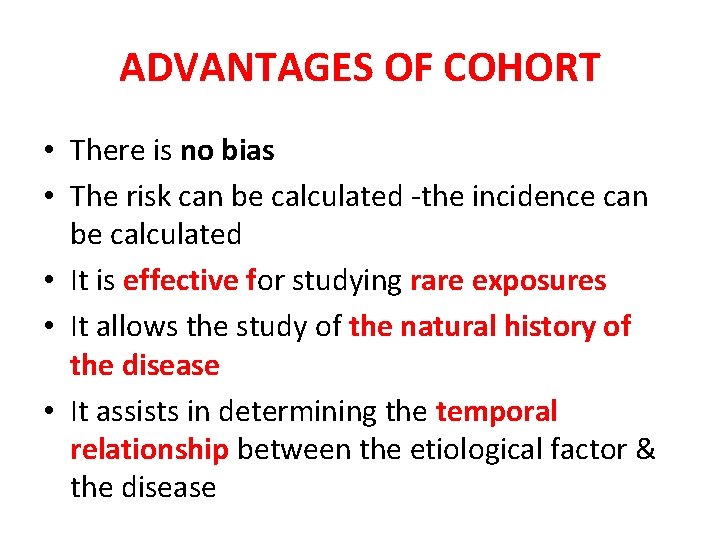
ADVANTAGES OF COHORT • There is no bias • The risk can be calculated -the incidence can be calculated • It is effective for studying rare exposures • It allows the study of the natural history of the disease • It assists in determining the temporal relationship between the etiological factor & the disease

Disadv of cohort study • • It takes a long time It is expensive Large number of subjects are needed There could be changes in the standard methods or diagnostic criteria

EXPERIMENTAL STUDIES • Studies in which 1 group is deliberately subjected to an experience compared with a control group with no similar experience • The gold standard in medicine -it proves causality • Can be controlled or uncontrolled

UNCONTROLLED EXPERIMENTAL STUDIES • Intervention is not compared with a control • The aim is to confirm that the Intervention made a difference

CONTROLLED EXPERIMENTAL STUDIES • In this study, a drug or procedure is compared to: 1. Another drug 2. Procedure 3. Placebo 4. Previously accepted tx • The aim is to proove the difference due to tx

CONTROLLED EXPERIMENTAL STUDIES • Blind trial-single or double • Control could be: A. METHODOLOGY 1. Concurrent or parallel: randomized or nonrandomized(quasi) 2. Sequential control: self controlled or cross over 3. External control

B. STUDY POPULATION 1. Clinical trials 2. Field trials 3. Community trials

EXPERIMENTAL STUDIES ADV DISADV • Best study type • Greatest prove of causality • Gold standard for other design • Least bias • Proves best treatment or procedure efficacy • Greatest expense • Long duration • Unproven facts adopted by community can hinder study acceptance

overview

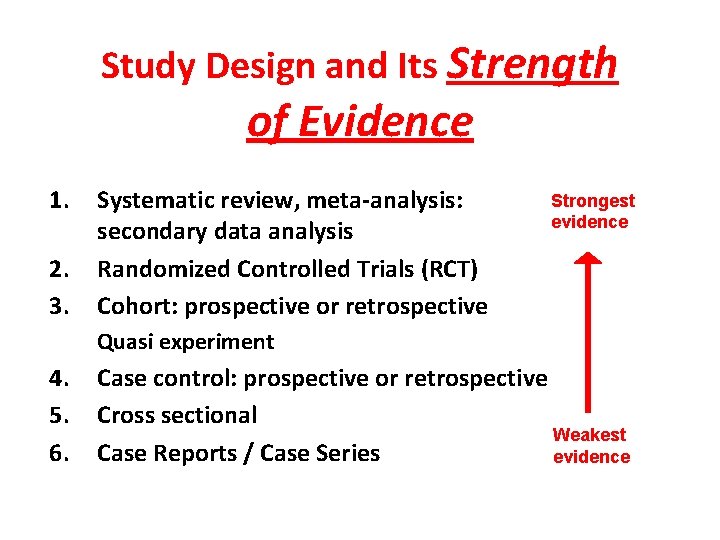
Study Design and Its Strength of Evidence 1. 2. 3. Systematic review, meta-analysis: secondary data analysis Randomized Controlled Trials (RCT) Cohort: prospective or retrospective Strongest evidence Quasi experiment 4. 5. 6. Case control: prospective or retrospective Cross sectional Weakest Case Reports / Case Series evidence



Epidemiologic Measures of Association • Compute & Interpret Relative risk (RR) & Odds ratio (OR) as a measure of association between exposure and Disease • Understand when OR approximates RR 49

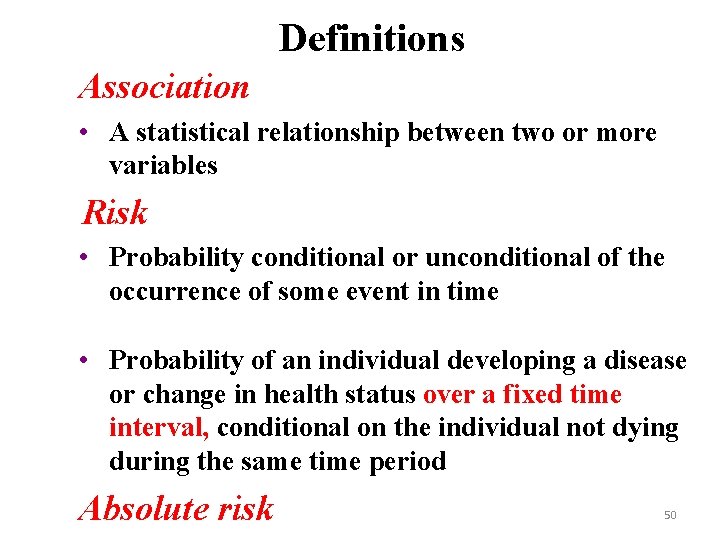
Definitions Association • A statistical relationship between two or more variables Risk • Probability conditional or unconditional of the occurrence of some event in time • Probability of an individual developing a disease or change in health status over a fixed time interval, conditional on the individual not dying during the same time period Absolute risk 50

Association between exposure & Disease • Question: Is there an excess risk associated with a given exposure? • Objective: To determine whether certain exposure is associated with a given disease • Methodology: Use one of the epidemiologic study designs Cohort Case-control 51

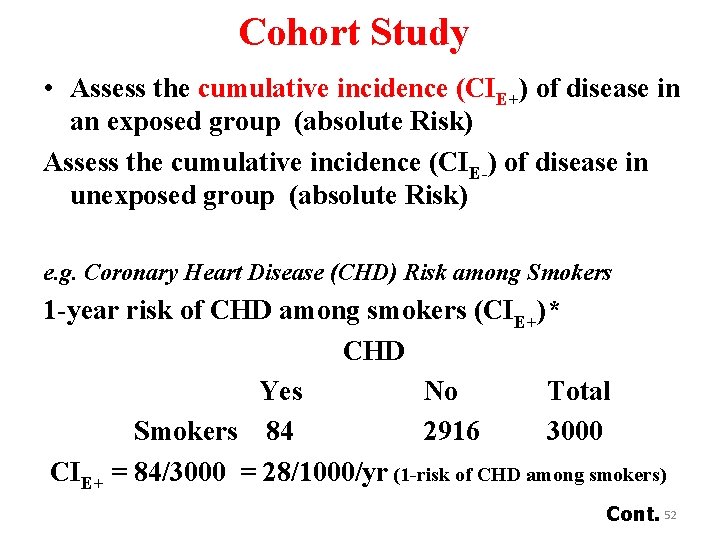
Cohort Study • Assess the cumulative incidence (CIE+) of disease in an exposed group (absolute Risk) Assess the cumulative incidence (CIE-) of disease in unexposed group (absolute Risk) e. g. Coronary Heart Disease (CHD) Risk among Smokers 1 -year risk of CHD among smokers (CIE+)* CHD Yes No Total Smokers 84 2916 3000 CIE+ = 84/3000 = 28/1000/yr (1 -risk of CHD among smokers) Cont. 52

CHD Risk among non-smokers • 1 -year risk of CHD among non-smokers (CIE-) CHD Yes No • Non-smokers 87 4913 5000 CIE-= 87/5000=17. 4/1000/yr (1 -yr risk of CHD among non-smokers) Cont. 53

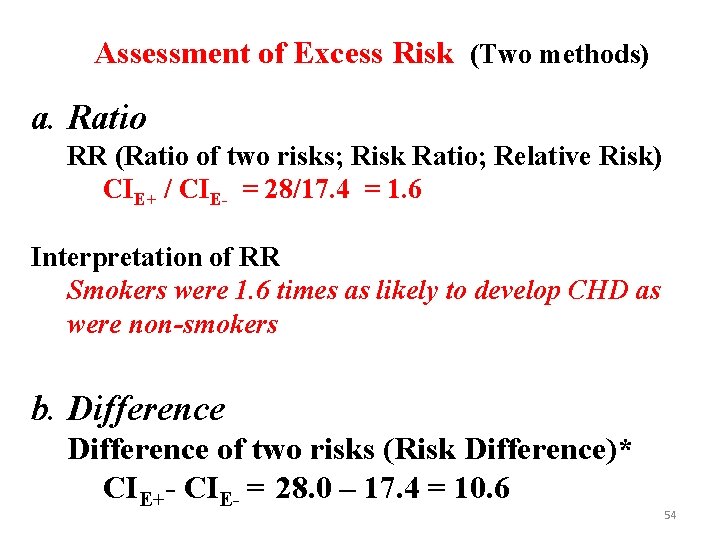
Assessment of Excess Risk (Two methods) a. Ratio RR (Ratio of two risks; Risk Ratio; Relative Risk) CIE+ / CIE- = 28/17. 4 = 1. 6 Interpretation of RR Smokers were 1. 6 times as likely to develop CHD as were non-smokers b. Difference of two risks (Risk Difference)* CIE+- CIE- = 28. 0 – 17. 4 = 10. 6 54

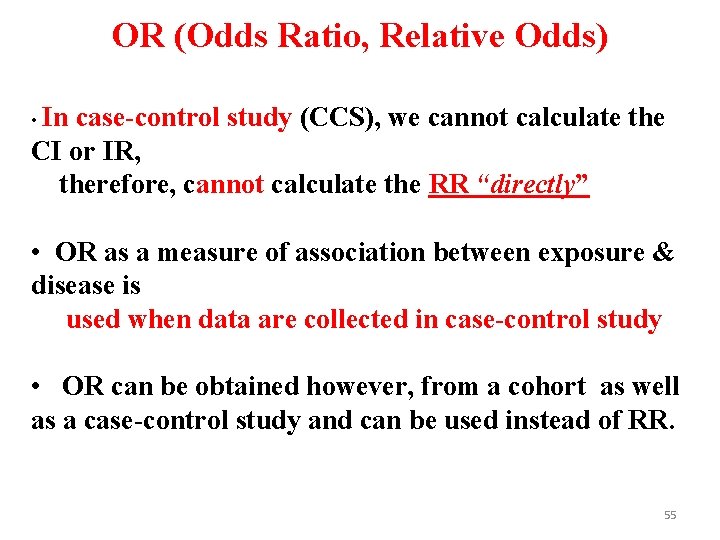
OR (Odds Ratio, Relative Odds) • In case-control study (CCS), we cannot calculate the CI or IR, therefore, cannot calculate the RR “directly” • OR as a measure of association between exposure & disease is used when data are collected in case-control study • OR can be obtained however, from a cohort as well as a case-control study and can be used instead of RR. 55

OR in case-control and cohort studies • Cohort study Ratio of the proportion of exposed subjects who developed the disease to the proportion of non-exposed subjects who developed the disease • Case-control study Ratio of the proportion of cases who were exposed to the proportion of controls who were non-exposed 56

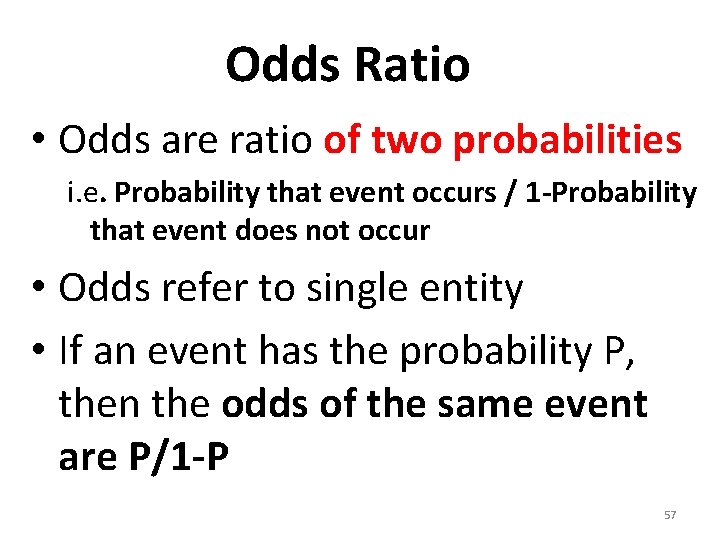
Odds Ratio • Odds are ratio of two probabilities i. e. Probability that event occurs / 1 -Probability that event does not occur • Odds refer to single entity • If an event has the probability P, then the odds of the same event are P/1 -P 57

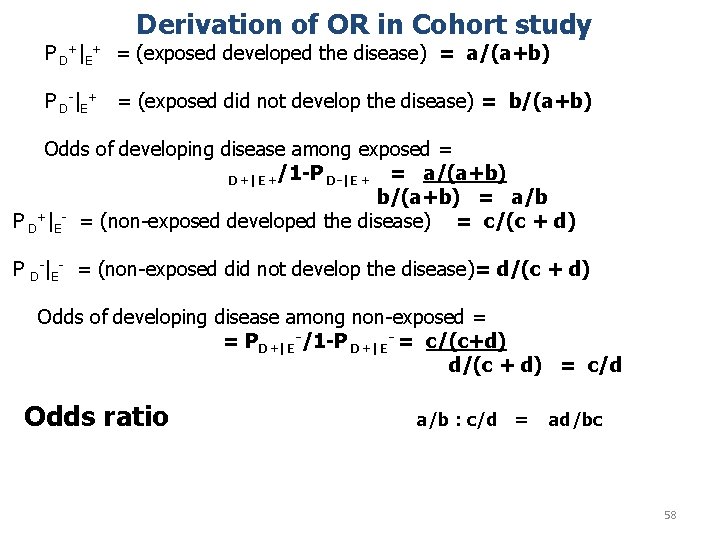
Derivation of OR in Cohort study P D+|E+ = (exposed developed the disease) = a/(a+b) P D-|E+ = (exposed did not develop the disease) = b/(a+b) Odds of developing disease among exposed = D+|E+/1 -P D-|E+ = a/(a+b) b/(a+b) = a/b P D+|E- = (non-exposed developed the disease) = c/(c + d) P - D |E = (non-exposed did not develop the disease)= d/(c + d) Odds of developing disease among non-exposed = = PD+|E-/1 -P D+|E- = c/(c+d) d/(c + d) = c/d Odds ratio a/b : c/d = ad/bc 58

OR in case-control study In case-control study RR cannot be calculated directly to determine the association between exposure and disease. n Don’t know the risk of disease among exposed and un-exposed since we start recruiting cases and controls. n n Can use OR as measure of association between exposure and disease in a case control study. 59

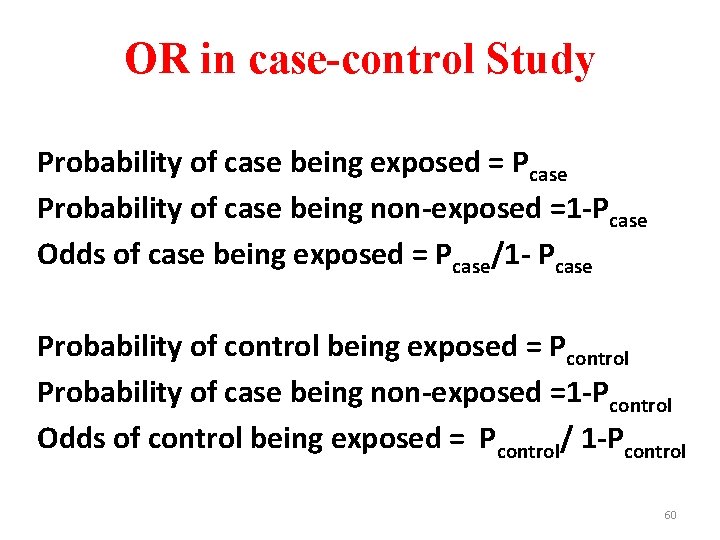
OR in case-control Study Probability of case being exposed = Pcase Probability of case being non-exposed =1 -Pcase Odds of case being exposed = Pcase/1 - Pcase Probability of control being exposed = Pcontrol Probability of case being non-exposed =1 -Pcontrol Odds of control being exposed = Pcontrol/ 1 -Pcontrol 60

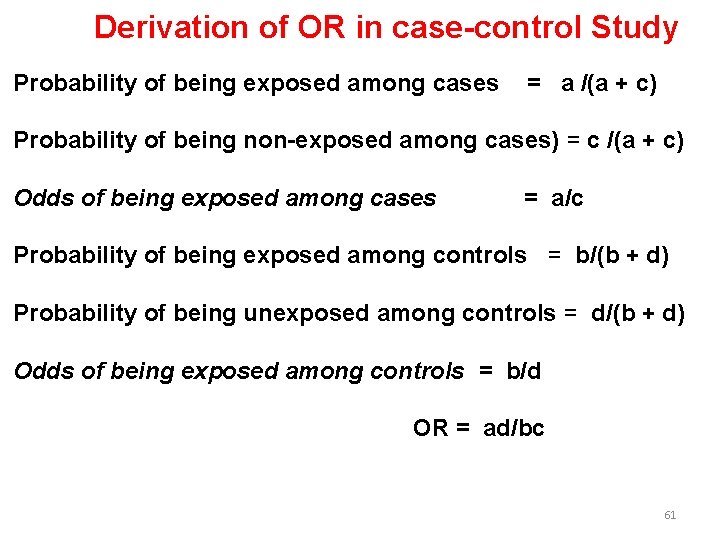
Derivation of OR in case-control Study Probability of being exposed among cases = a /(a + c) Probability of being non-exposed among cases) = c /(a + c) Odds of being exposed among cases = a/c Probability of being exposed among controls = b/(b + d) Probability of being unexposed among controls = d/(b + d) Odds of being exposed among controls = b/d OR = ad/bc 61

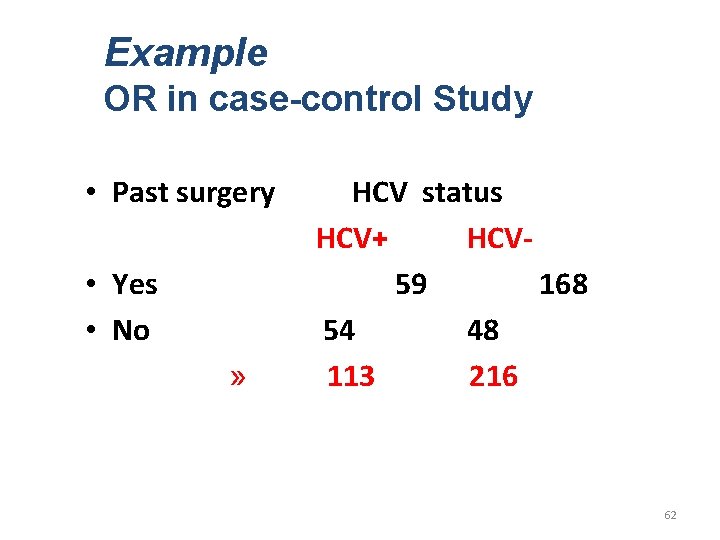
Example OR in case-control Study • Past surgery • Yes • No » HCV status HCV+ HCV 59 168 54 48 113 216 62

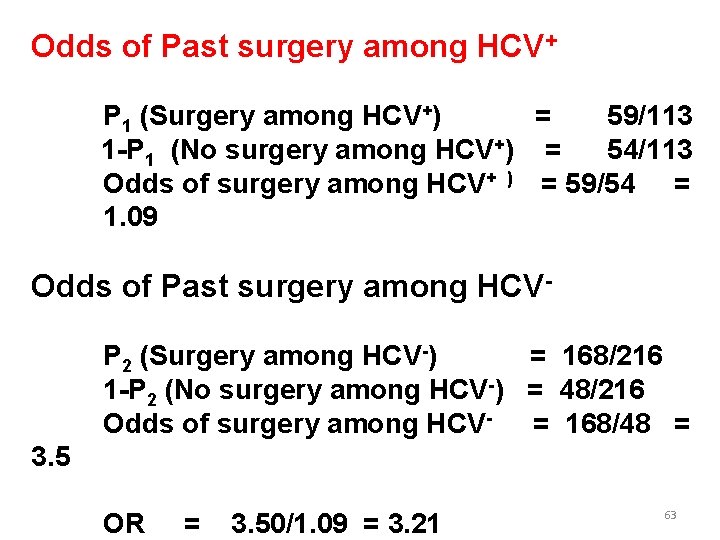
Odds of Past surgery among HCV+ P 1 (Surgery among HCV+) = 59/113 1 -P 1 (No surgery among HCV+) = 54/113 Odds of surgery among HCV+ ) = 59/54 = 1. 09 Odds of Past surgery among HCVP 2 (Surgery among HCV-) = 168/216 1 -P 2 (No surgery among HCV-) = 48/216 Odds of surgery among HCV- = 168/48 = 3. 5 OR = 3. 50/1. 09 = 3. 21 63

When is the OR a good estimate of RR? In CCS, only OR can be calculated as measure of association n In Cohort study, either RR or OR is a valid measure of association n When a RR can be calculated from case control study? n *When exposure prevalence among studied cases in similar and nearly similar to that of disease subjects in the population from which cases are taken. *Prevalence of exposure among studied controls is similar to that of non-diseased population from cases were drawn. 64 *Rare disease (CI < 0. 1) n

Matched case-control study n Matching: In a matched case-control study each case is matched to a control according to variables that are known to be related to disease risk i. e. age, sex, race n Data are analyzed in terms of casecontrol pairs rather than for individual subjects n Four types of case-control combinations are possible in regard to exposure history. 65

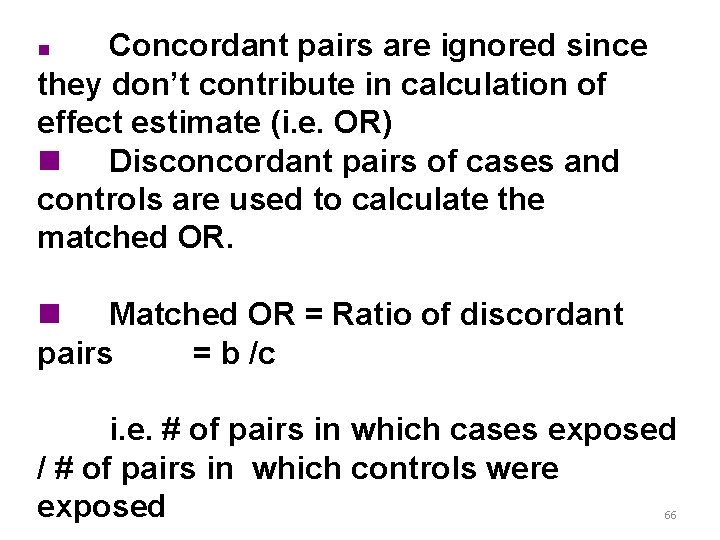
Concordant pairs are ignored since they don’t contribute in calculation of effect estimate (i. e. OR) n Disconcordant pairs of cases and controls are used to calculate the matched OR. n n Matched OR = Ratio of discordant pairs = b /c i. e. # of pairs in which cases exposed / # of pairs in which controls were exposed 66

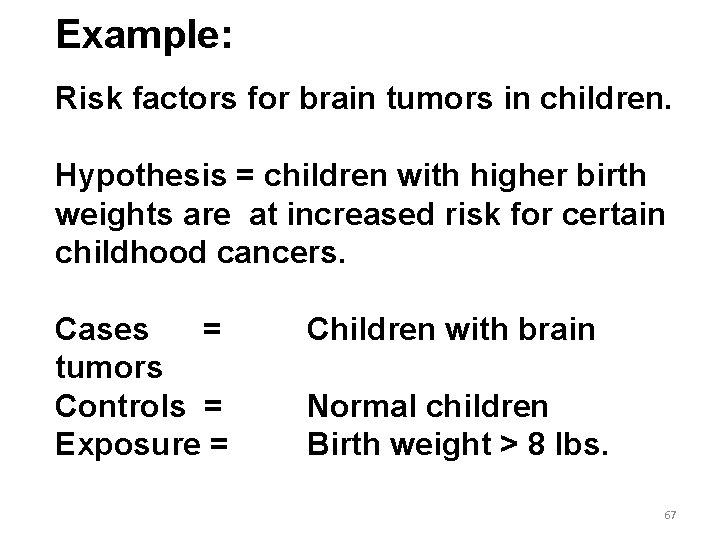
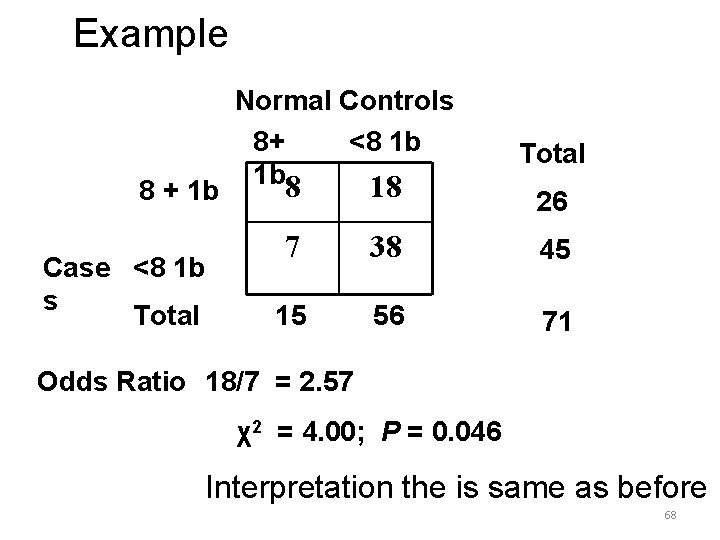
Example: Risk factors for brain tumors in children. Hypothesis = children with higher birth weights are at increased risk for certain childhood cancers. Cases = tumors Controls = Exposure = Children with brain Normal children Birth weight > 8 lbs. 67

Example 8 + 1 b Case <8 1 b s Total Normal Controls 8+ <8 1 b 1 b 8 18 Total 26 7 38 45 15 56 71 Odds Ratio 18/7 = 2. 57 χ2 = 4. 00; P = 0. 046 Interpretation the is same as before 68
 Introduction to community medicine
Introduction to community medicine Concepts of health and disease
Concepts of health and disease Hubert kairuki memorial university faculty of medicine
Hubert kairuki memorial university faculty of medicine Semmelweis university faculty of medicine
Semmelweis university faculty of medicine Faculty of medicine nursing and health sciences
Faculty of medicine nursing and health sciences King abdulaziz university faculty of medicine
King abdulaziz university faculty of medicine Agnes csaki semmelweis
Agnes csaki semmelweis Faculty of veterinary medicine cairo university logo
Faculty of veterinary medicine cairo university logo Hacettepe university faculty of medicine
Hacettepe university faculty of medicine دانشگاه دامپزشکی تهران
دانشگاه دامپزشکی تهران Semmelweis university faculty of medicine
Semmelweis university faculty of medicine Department of medicine mcgill
Department of medicine mcgill Faculty of veterinary medicine cairo university
Faculty of veterinary medicine cairo university Pubh4401
Pubh4401 Emory anticoagulation clinic
Emory anticoagulation clinic Cairo university faculty of veterinary medicine
Cairo university faculty of veterinary medicine Faculty introduction speech
Faculty introduction speech T junction english bond
T junction english bond Course title and course number
Course title and course number Course interne moyenne externe
Course interne moyenne externe Typhoid medicine course
Typhoid medicine course Lt lead time
Lt lead time Types of family in community medicine
Types of family in community medicine Lead time in community medicine
Lead time in community medicine 10 importance of health education
10 importance of health education Basic requirements for sound phc
Basic requirements for sound phc Cohort study community medicine
Cohort study community medicine Duke medicine grand rounds
Duke medicine grand rounds Seqs community medicine
Seqs community medicine Community action cycle
Community action cycle Introduction to banking course
Introduction to banking course Imbe introduction course v2
Imbe introduction course v2 Introduction to software engineering course outline
Introduction to software engineering course outline Pied piping
Pied piping Cleft sentences examples
Cleft sentences examples Ron had a course introduction
Ron had a course introduction Introduction to forensic medicine
Introduction to forensic medicine Introduction of community policing
Introduction of community policing Introduction to community pharmacy
Introduction to community pharmacy Introduction of community health nursing
Introduction of community health nursing An introduction to community asset mapping
An introduction to community asset mapping An introduction to community asset mapping
An introduction to community asset mapping Introduction to community pharmacy
Introduction to community pharmacy Herszon kherson maritime college of merchant marine fleet
Herszon kherson maritime college of merchant marine fleet University of bridgeport computer science faculty
University of bridgeport computer science faculty University of bridgeport computer science faculty
University of bridgeport computer science faculty Alamo colleges salary schedule
Alamo colleges salary schedule Hahnville high school faculty
Hahnville high school faculty Importance of faculty in higher education
Importance of faculty in higher education Http://www-bcf.usc.edu/~gareth/isl/advertising.csv
Http://www-bcf.usc.edu/~gareth/isl/advertising.csv Penn state neurosurgery faculty
Penn state neurosurgery faculty Mercy faculty forward
Mercy faculty forward Lee kong chian faculty of engineering and science
Lee kong chian faculty of engineering and science Carelli
Carelli Florida state university ms in cs
Florida state university ms in cs Mendel university - faculty of business and economics
Mendel university - faculty of business and economics Umd ee
Umd ee Factors influencing faculty staff relationship
Factors influencing faculty staff relationship Nit calicut chemistry department faculty
Nit calicut chemistry department faculty Faculty of civil engineering ctu prague
Faculty of civil engineering ctu prague Faculty 180 ecu
Faculty 180 ecu Faculty of engineering shoubra
Faculty of engineering shoubra Singularity executive program
Singularity executive program Faculty of law maastricht
Faculty of law maastricht Medical faculty in novi sad dean
Medical faculty in novi sad dean Umn faculty dental clinic
Umn faculty dental clinic Sjsu faculty affairs
Sjsu faculty affairs Unlv faculty senate
Unlv faculty senate Ulm nursing faculty
Ulm nursing faculty