Cairo University Faculty of Veterinary Medicine Department of

















- Slides: 59


Cairo University Faculty of Veterinary Medicine Department of Microbiology STUDIES ON MYCOTOXINS-PRODUCING MOULDS IN ANIMALS AND POULTRY FEEDSTUFFS AND EVALUATION OF SOME COMMERCIAL ANTIFUNGALS By Rasha Mahmoud Hamza Sayed El-Ahl Under the Supervision of Prof. Dr. Mohamed Kamal Refai Professor of Microbiology Faculty of Veterinary Medicine Cairo University Prof. Dr. Hosam Ahmed Abdel-Latif Prof. Dr. Atef Abdel Aziz Hassan Prof. of Feeding Faculty of Veterinary Medicine Cairo University ﻣﺮﻛﺰ ﺍﻟﺘﺠﺎﺭﺏ ﺍﻟﺒﻴﻮﻟﻮﺟﻴﺔ ﻭﺍﻟﺠﺮﺍﺣﺔ ﺍﻟﺘﺠﺮﻳﺒﻴﺔ Chief Researcher of Mycology and Mycotoxins – Animal Health Research Institute, Dokki, Giza 2005

INTRODUCTION

Up to date the dramatical increase in population in the world requires an efficient modern animal production industry and the manufacture of good quality feeds and food. This is necessary to give suitable growth performance of broilers, high egg production by layers and good quality meat and milk production from cattle for human consumption. This is in turn will contribute to the production of high quality food material for human consumption and the profitability of agricultural industry. Hence, great attention has been paid to the increased importance of fungi and their mycotoxins, which are serious fungal metabolites. These fungi and mycotoxins have serious effects upon the growth rate and health of human being and animals, as some mycotoxins had been found to be hepatotoxic, carcinogenic, tremorgenic, haemorrhagic and dermatitic.

AIM OF WORK

1. Screening single and compound feeds for fungal contamination. 2. Evaluation of the isolated Aspergillus flavus and Aspergillus ochraceus for production of respective mycotoxins. 3. Testing of some commercial fungal inhibitors and antimycotoxin for prevention and control of fungal growth and mycotoxins degradation.

MATERIALS AND METHODS

I- MATERIALS: 1. Feed samples. A total of 200 samples of feeds and feedstuffs were collected for investigation of fungal contamination and detection of aflatoxin and ochratoxin contamination. These samples included 20 of each of yellow corn, wheat, Soya bean, hay, tiben, layer’s concentrates, bone and blood meals, poultry ration, broiler’s concentrates and processed animal feed. 2. Standards of aflatoxins and ochratoxin A : Standards of aflatoxins B 1, B 2, G 1 and G 2 and ochratoxin A were purchased from Sigma Chemical Company (USA).

3. Media: 1. Sabouraud’s dextrose agar medium (SDA): (Cruickshank et al. , 1975) 2. Czapek- Dox agar medium: (Al Doory, 1980) 3. Malt extract agar medium: (Cruikshank et al. , 1975) 4. Fresh potato dextrose agar medium: (Shotwell et al. , 1966) 5. Yeast extract sucrose liquid medium (Davis et al. , 1969). 4. Chemicals and reagents: 1. Buffered peptone water (BPW): (Refai, 1979) 2. Phenol saline: 3. Other chemicals: 5. Stains: Lactophenol cotton blue stain: (Leanor and Callway, 1978) 6. Equipment, apparatus and glassware: 1. Thermostatic controlled water bath, rotary evaporator. 2. Thin layer chromatographic apparatus

2 - METHODS: 1. Isolation and plate colony count of moulds: (Refai, 1979) 1. 1. Preparation of sample homogenate. 1. 2. Dilution. 1. 3. Plate pouring. 1. 4. Incubation and reporting. 2. Mould identification: - Macroscopic examination: - Microscopic examination: - Direct microscopic examination: - Slide culture technique (Ajello et al. , 1963) 3. 1. Screening of Aspergillus flavus for productions of aflatoxin using liquid medium (Gabal et al. , 1994): - Cultivation and extraction of aflatoxins: 3. 2. Estimation of aflatoxin (Lee, 1975): - Qualitative estimation: - Quantitative estimation of aflatoxin :

4. Production of ochratoxin A by A. ochraceus using liquid medium (Davis et al. , 1969) 1 Extraction of ochratoxin A from liquid medium (Walbeek et al. , 1968; Davis et al. , 1969) 2. Purification of the acidified chloroform from liquid medium: (Vender Merwe et al. , 1965 and Nessheim, 1969) 3. Qualitative determination of the prepared ochratoxin (Vender Merwe et al. , 1965 and Scott and Hand, 1967) 4. Quantitative estimation of the prepared ochratoxin: (Vander Merwe et al. , 1965) 5. Control of A. ochraceus and A. flavus growth by 2 commercial antifungal compounds (muv-anti mould and mycostatine) using plate count assay (Chen and Day, 1974) - Maintaining of culture. - Determination of inhibitory concentration. - Incubation of plates.

- Evaluation of Muv- anti mould in inhibition of fungal growth in poultry feeds: - Colony count of fungi in commercial poultry feeds: - Detection of inhibitory concentration of Muv - anti mould: - Degradation of mycotoxins (aflatoxin B 1 and ochratoxin A) by hydrated sodium calcium aluminosilicate (HSCAS) compound (Harvey et al. (1991).

RESULTS

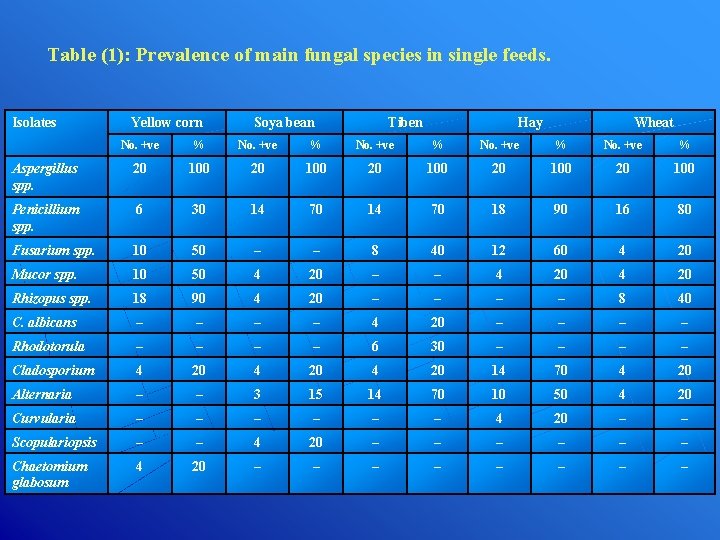
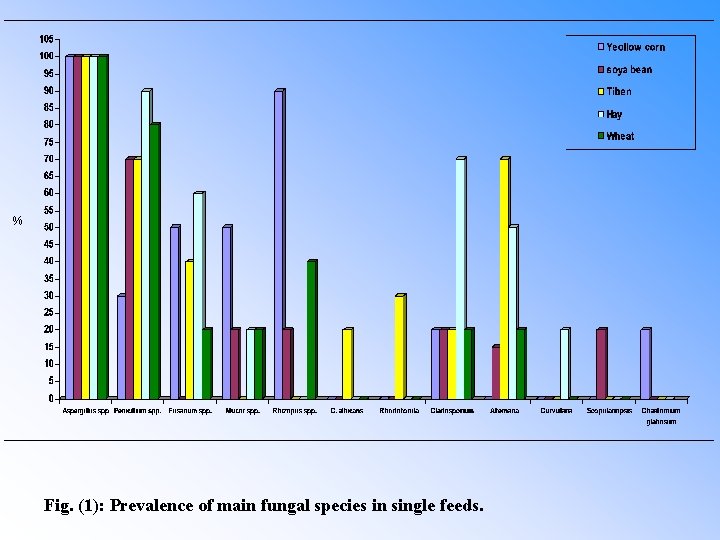
Table (1): Prevalence of main fungal species in single feeds. Isolates Yellow corn Soya bean Tiben Hay Wheat No. +ve % Aspergillus spp. 20 100 20 100 Penicillium spp. 6 30 14 70 18 90 16 80 Fusarium spp. 10 50 – – 8 40 12 60 4 20 Mucor spp. 10 50 4 20 – – 4 20 Rhizopus spp. 18 90 4 20 – – 8 40 C. albicans – – 4 20 – – Rhodotorula – – 6 30 – – Cladosporium 4 20 14 70 4 20 Alternaria – – 3 15 14 70 10 50 4 20 Curvularia – – – 4 20 – – Scopulariopsis – – 4 20 – – – Chaetomium glabosum 4 20 – – – –

% Fig. (1): Prevalence of main fungal species in single feeds.

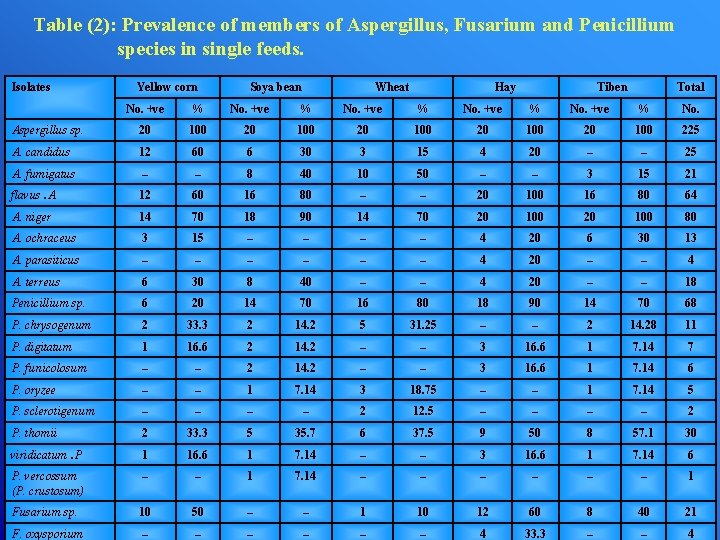
Table (2): Prevalence of members of Aspergillus, Fusarium and Penicillium species in single feeds. Isolates Yellow corn Soya bean Wheat Hay Tiben Total No. +ve % No. Aspergillus sp. 20 100 20 100 225 A. candidus 12 60 6 30 3 15 4 20 – – 25 A. fumigatus – – 8 40 10 50 – – 3 15 21 flavus. A 12 60 16 80 – – 20 100 16 80 64 A. niger 14 70 18 90 14 70 20 100 80 A. ochraceus 3 15 – – 4 20 6 30 13 A. parasiticus – – – 4 20 – – 4 A. terreus 6 30 8 40 – – 4 20 – – 18 Penicillium sp. 6 20 14 70 16 80 18 90 14 70 68 P. chrysogenum 2 33. 3 2 14. 2 5 31. 25 – – 2 14. 28 11 P. digitatum 1 16. 6 2 14. 2 – – 3 16. 6 1 7. 14 7 P. funicolosum – – 2 14. 2 – – 3 16. 6 1 7. 14 6 P. oryzee – – 1 7. 14 3 18. 75 – – 1 7. 14 5 P. sclerotigenum – – 2 12. 5 – – 2 P. thomii 2 33. 3 5 35. 7 6 37. 5 9 50 8 57. 1 30 viridicatum. P 1 16. 6 1 7. 14 – – 3 16. 6 1 7. 14 6 P. vercossum (P. crustosum) – – 1 7. 14 – – – 1 Fusarium sp. 10 50 – – 1 10 12 60 8 40 21 F. oxysporium – – – 4 33. 3 – – 4

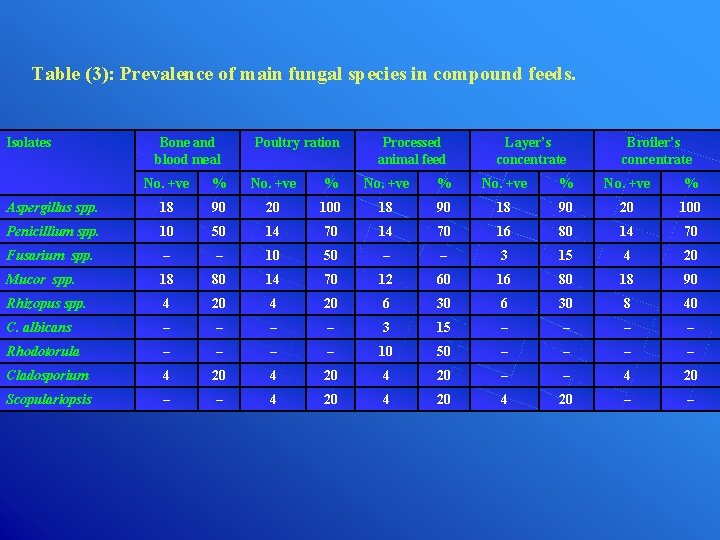
Table (3): Prevalence of main fungal species in compound feeds. Isolates Bone and blood meal Poultry ration Processed animal feed Layer’s concentrate Broiler’s concentrate No. +ve % Aspergillus spp. 18 90 20 100 Penicillium spp. 10 50 14 70 16 80 14 70 Fusarium spp. – – 10 50 – – 3 15 4 20 Mucor spp. 18 80 14 70 12 60 16 80 18 90 Rhizopus spp. 4 20 6 30 8 40 C. albicans – – 3 15 – – Rhodotorula – – 10 50 – – Cladosporium 4 20 – – 4 20 Scopulariopsis – – 4 20 – –

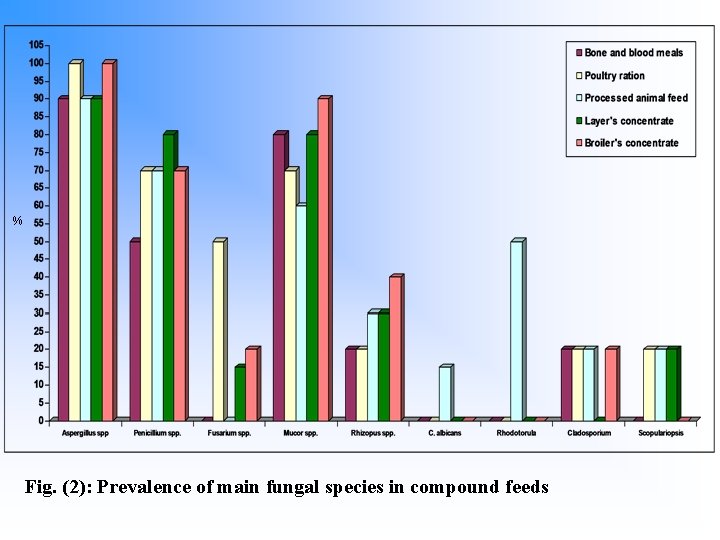
% Fig. (2): Prevalence of main fungal species in compound feeds

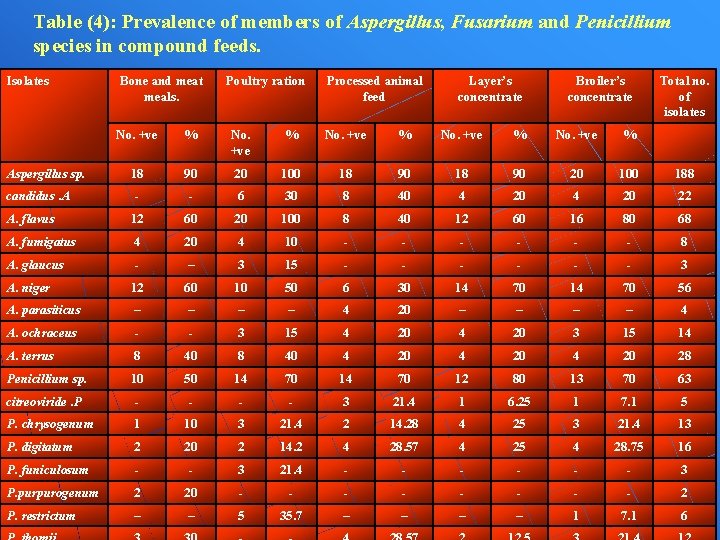
Table (4): Prevalence of members of Aspergillus, Fusarium and Penicillium species in compound feeds. Isolates Bone and meat meals. Poultry ration Processed animal feed Layer’s concentrate Broiler’s concentrate Total no. of isolates No. +ve % 18 90 20 100 188 - - 6 30 8 40 4 20 22 A. flavus 12 60 20 100 8 40 12 60 16 80 68 A. fumigatus 4 20 4 10 - - - 8 A. glaucus - – 3 15 - - - 3 A. niger 12 60 10 50 6 30 14 70 56 A. parasiticus – – 4 20 – – 4 A. ochraceus - - 3 15 4 20 3 15 14 A. terrus 8 40 4 20 28 Penicillium sp. 10 50 14 70 12 80 13 70 63 citreoviride. P - - 3 21. 4 1 6. 25 1 7. 1 5 P. chrysogenum 1 10 3 21. 4 2 14. 28 4 25 3 21. 4 13 P. digitatum 2 20 2 14. 2 4 28. 57 4 25 4 28. 75 16 P. funiculosum - - 3 21. 4 - - - 3 P. purpurogenum 2 20 - - - - 2 P. restrictum – – 5 35. 7 – – 1 7. 1 6 Aspergillus sp. candidus. A

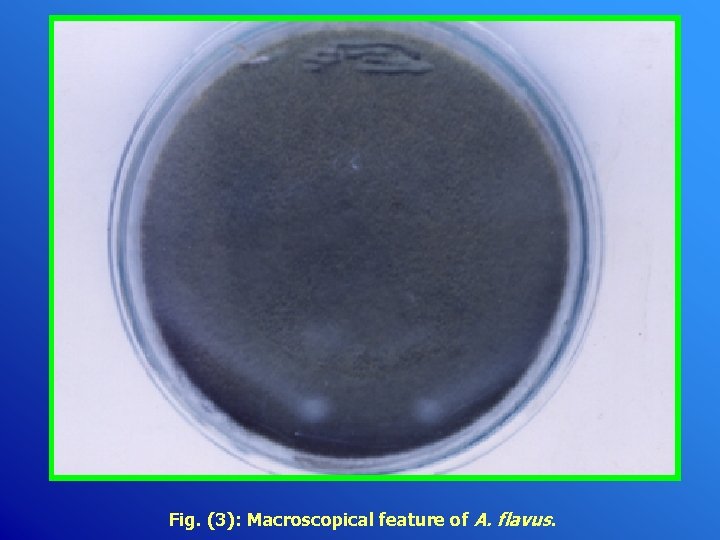
Fig. (3): Macroscopical feature of A. flavus.

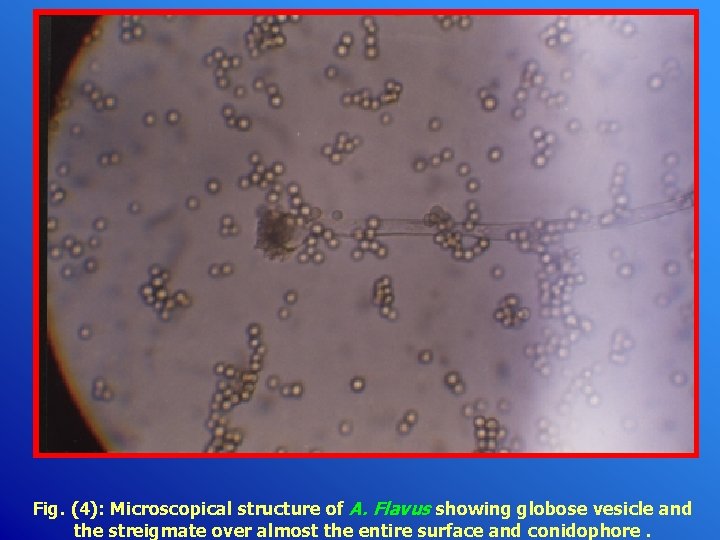
Fig. (4): Microscopical structure of A. Flavus showing globose vesicle and the streigmate over almost the entire surface and conidophore.

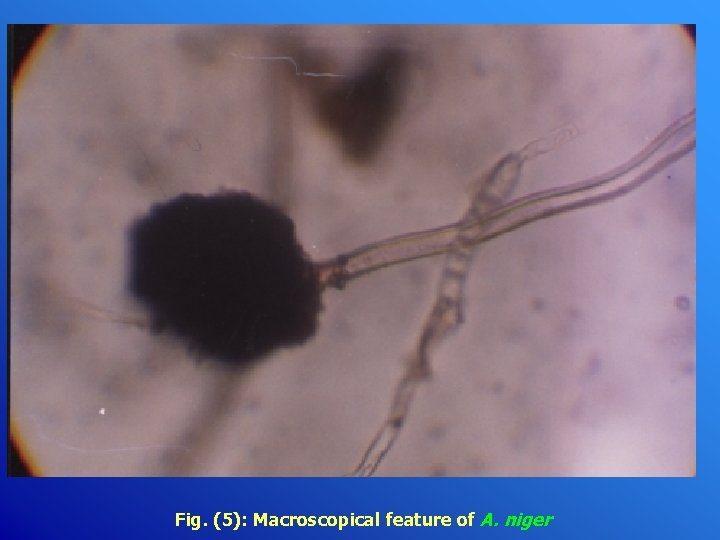
Fig. (5): Macroscopical feature of A. niger

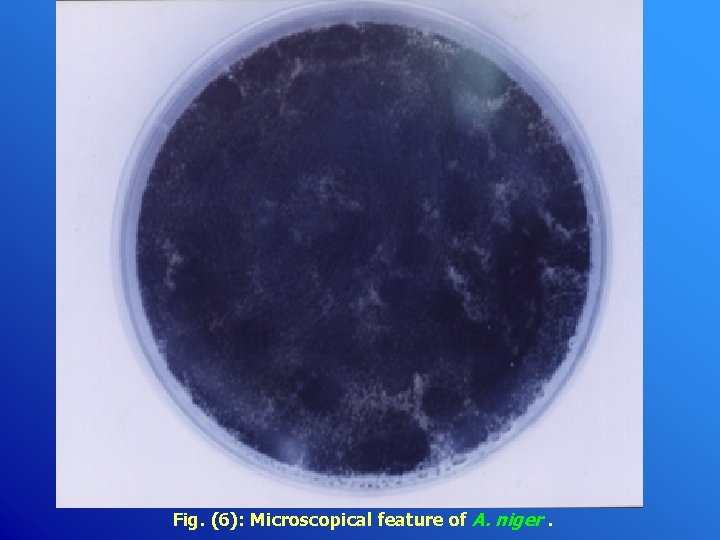
Fig. (6): Microscopical feature of A. niger.

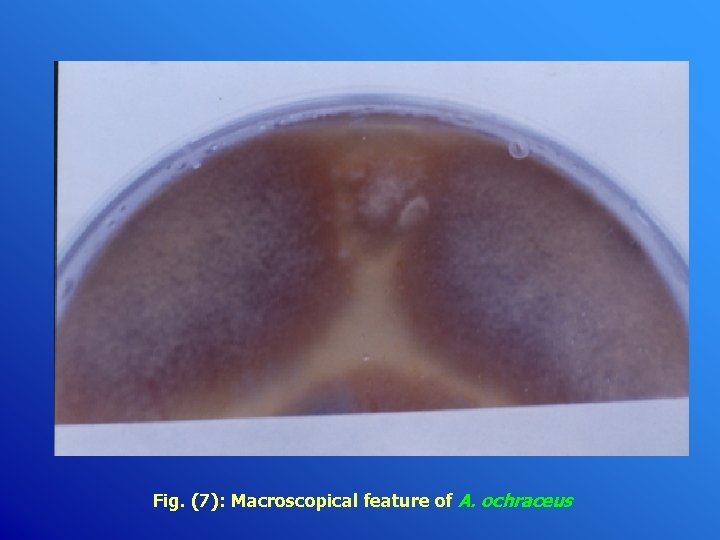
Fig. (7): Macroscopical feature of A. ochraceus

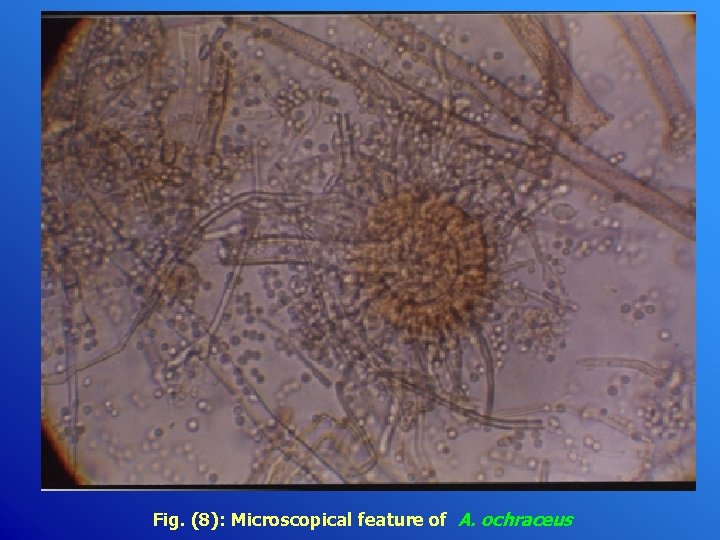
Fig. (8): Microscopical feature of A. ochraceus

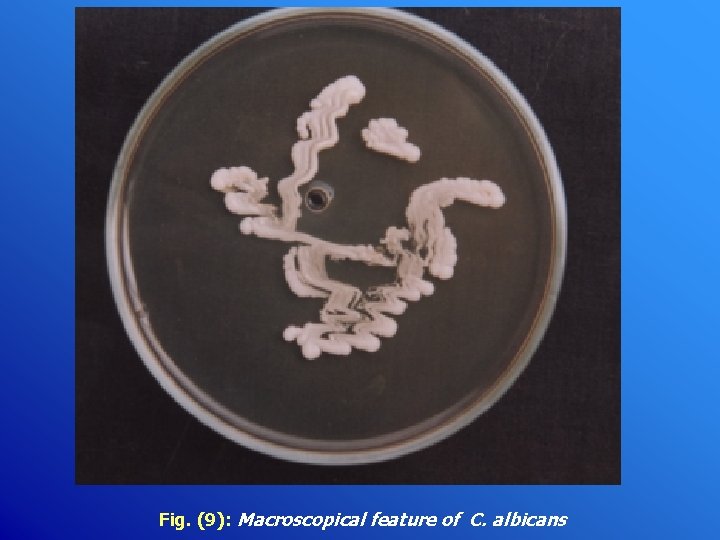
Fig. (9): Macroscopical feature of C. albicans

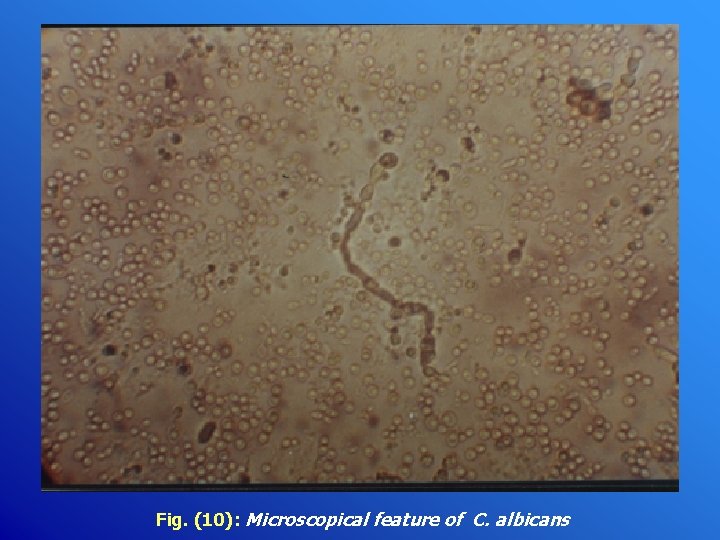
Fig. (10): Microscopical feature of C. albicans

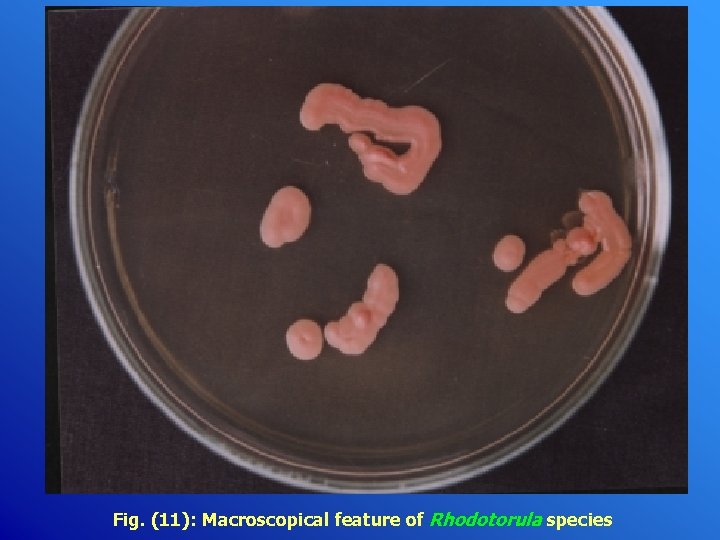
Fig. (11): Macroscopical feature of Rhodotorula species

Fig. (12): Microscopical feature of Rhodotorula species

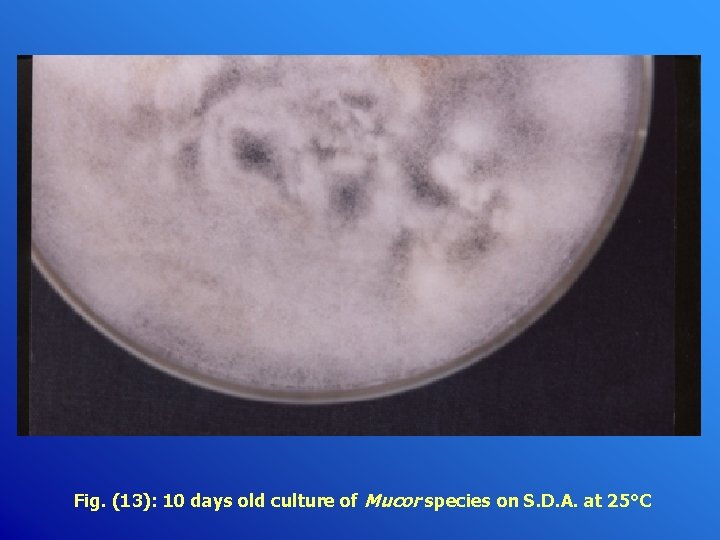
Fig. (13): 10 days old culture of Mucor species on S. D. A. at 25°C

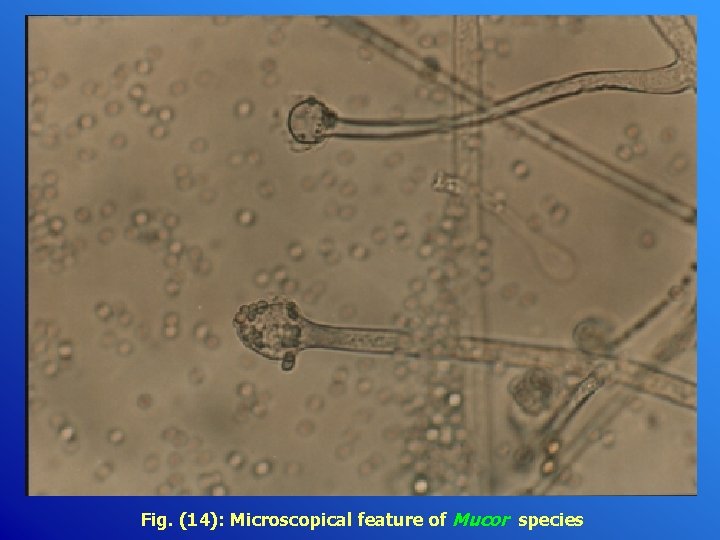
Fig. (14): Microscopical feature of Mucor species

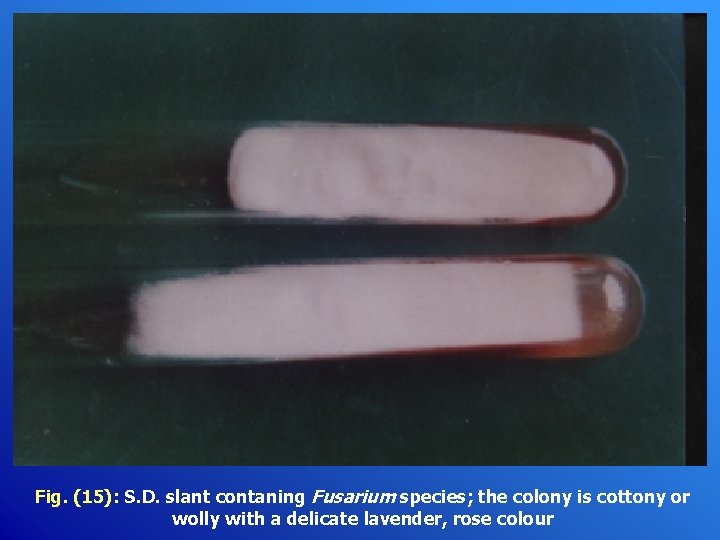
Fig. (15): S. D. slant contaning Fusarium species; the colony is cottony or wolly with a delicate lavender, rose colour

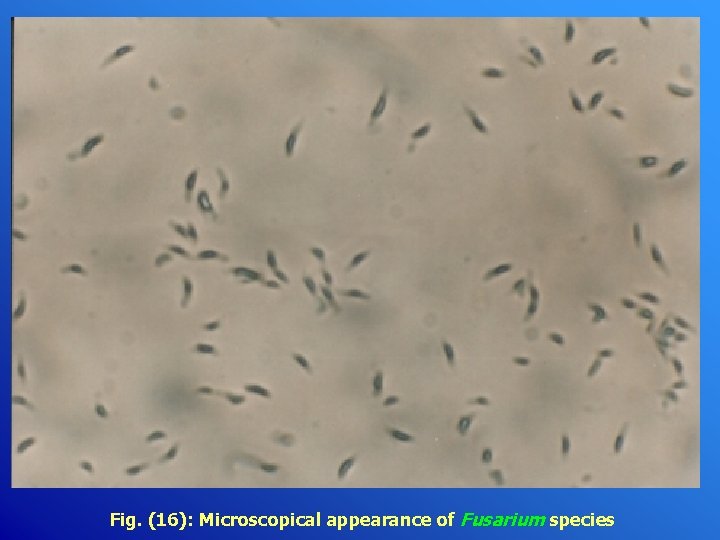
Fig. (16): Microscopical appearance of Fusarium species

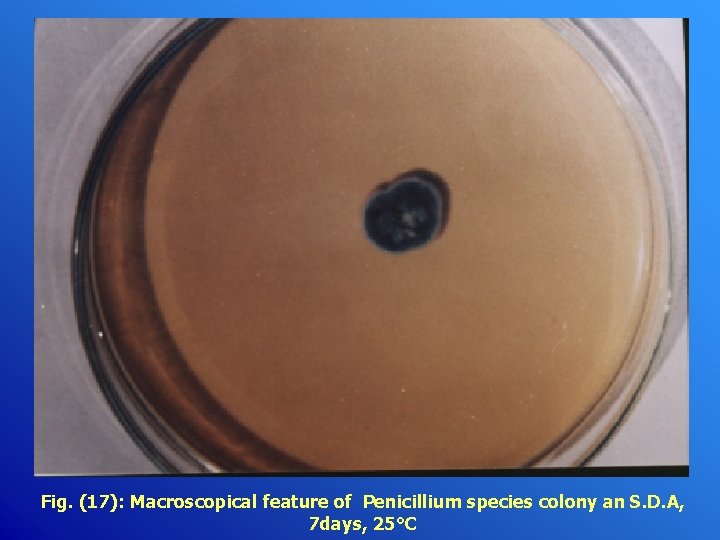
Fig. (17): Macroscopical feature of Penicillium species colony an S. D. A, 7 days, 25°C

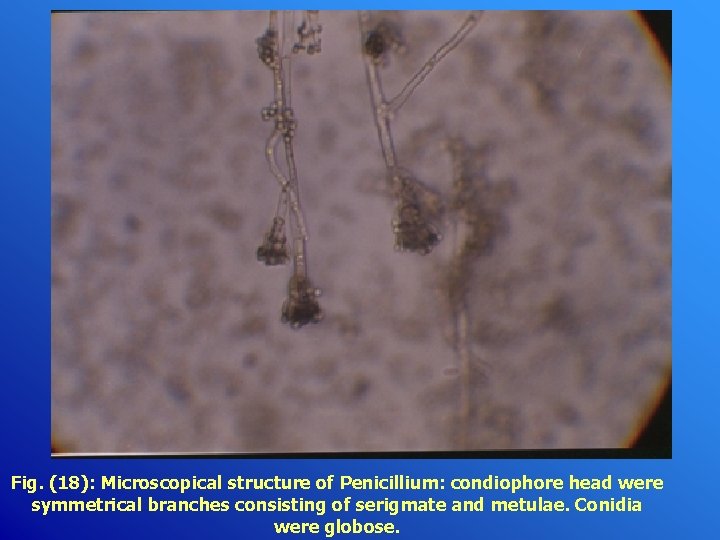
Fig. (18): Microscopical structure of Penicillium: condiophore head were symmetrical branches consisting of serigmate and metulae. Conidia were globose.

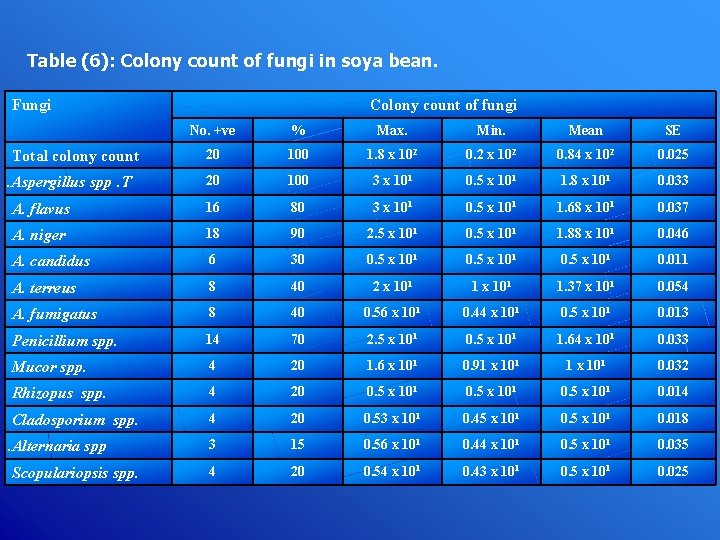
Table (6): Colony count of fungi in soya bean. Fungi Colony count of fungi No. +ve % Max. Min. Mean SE 20 100 1. 8 x 102 0. 2 x 102 0. 84 x 102 0. 025 20 100 3 x 101 0. 5 x 101 1. 8 x 101 0. 033 A. flavus 16 80 3 x 101 0. 5 x 101 1. 68 x 101 0. 037 A. niger 18 90 2. 5 x 101 0. 5 x 101 1. 88 x 101 0. 046 A. candidus 6 30 0. 5 x 101 0. 011 A. terreus 8 40 2 x 101 1. 37 x 101 0. 054 A. fumigatus 8 40 0. 56 x 101 0. 44 x 101 0. 5 x 101 0. 013 Penicillium spp. 14 70 2. 5 x 101 0. 5 x 101 1. 64 x 101 0. 033 Mucor spp. 4 20 1. 6 x 101 0. 91 x 101 0. 032 Rhizopus spp. 4 20 0. 5 x 101 0. 014 Cladosporium spp. 4 20 0. 53 x 101 0. 45 x 101 0. 018 3 15 0. 56 x 101 0. 44 x 101 0. 5 x 101 0. 035 4 20 0. 54 x 101 0. 43 x 101 0. 5 x 101 0. 025 Total colony count. Aspergillus spp. T . Alternaria spp Scopulariopsis spp.

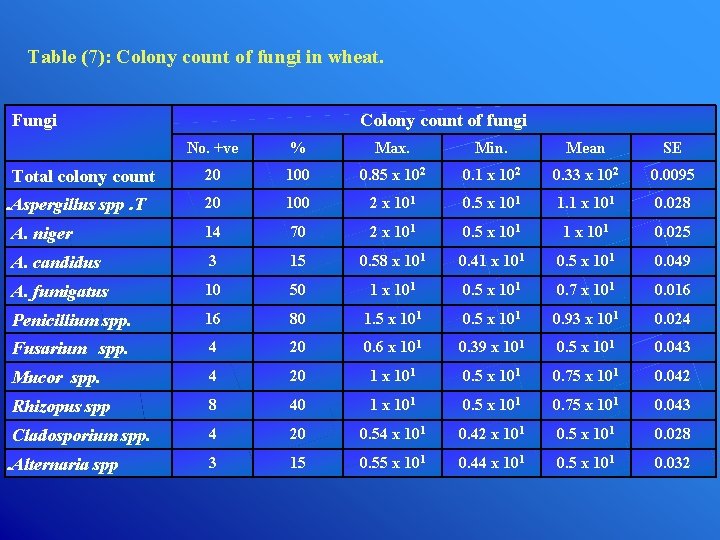
Table (7): Colony count of fungi in wheat. Fungi Colony count of fungi No. +ve % Max. Min. Mean SE 20 100 0. 85 x 102 0. 1 x 102 0. 33 x 102 0. 0095 20 100 2 x 101 0. 5 x 101 1. 1 x 101 0. 028 A. niger 14 70 2 x 101 0. 5 x 101 1 x 101 0. 025 A. candidus 3 15 0. 58 x 101 0. 41 x 101 0. 5 x 101 0. 049 A. fumigatus 10 50 1 x 101 0. 5 x 101 0. 7 x 101 0. 016 Penicillium spp. 16 80 1. 5 x 101 0. 93 x 101 0. 024 Fusarium spp. 4 20 0. 6 x 101 0. 39 x 101 0. 5 x 101 0. 043 Mucor spp. 4 20 1 x 101 0. 5 x 101 0. 75 x 101 0. 042 Rhizopus spp 8 40 1 x 101 0. 5 x 101 0. 75 x 101 0. 043 Cladosporium spp. 4 20 0. 54 x 101 0. 42 x 101 0. 5 x 101 0. 028 3 15 0. 55 x 101 0. 44 x 101 0. 5 x 101 0. 032 Total colony count. Aspergillus spp. T . Alternaria spp

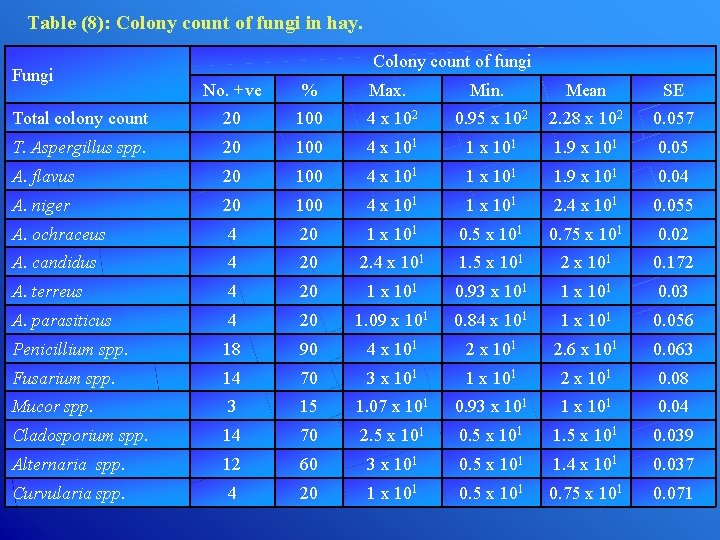
Table (8): Colony count of fungi in hay. Fungi Colony count of fungi No. +ve % Max. Min. Mean SE Total colony count 20 100 4 x 102 0. 95 x 102 2. 28 x 102 0. 057 T. Aspergillus spp. 20 100 4 x 101 1. 9 x 101 0. 05 A. flavus 20 100 4 x 101 1. 9 x 101 0. 04 A. niger 20 100 4 x 101 1 x 101 2. 4 x 101 0. 055 A. ochraceus 4 20 1 x 101 0. 5 x 101 0. 75 x 101 0. 02 A. candidus 4 20 2. 4 x 101 1. 5 x 101 2 x 101 0. 172 A. terreus 4 20 1 x 101 0. 93 x 101 1 x 101 0. 03 A. parasiticus 4 20 1. 09 x 101 0. 84 x 101 1 x 101 0. 056 Penicillium spp. 18 90 4 x 101 2. 6 x 101 0. 063 Fusarium spp. 14 70 3 x 101 1 x 101 2 x 101 0. 08 Mucor spp. 3 15 1. 07 x 101 0. 93 x 101 1 x 101 0. 04 Cladosporium spp. 14 70 2. 5 x 101 0. 5 x 101 1. 5 x 101 0. 039 Alternaria spp. 12 60 3 x 101 0. 5 x 101 1. 4 x 101 0. 037 Curvularia spp. 4 20 1 x 101 0. 5 x 101 0. 75 x 101 0. 071

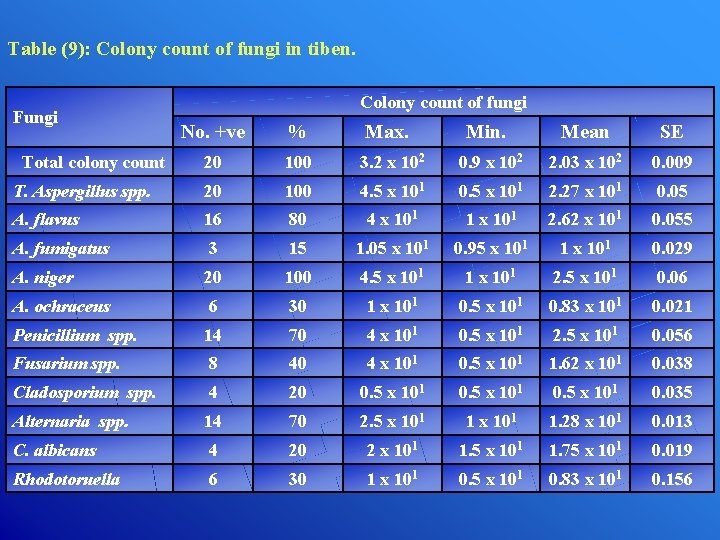
Table (9): Colony count of fungi in tiben. Fungi Colony count of fungi No. +ve % Max. Min. Mean SE 20 100 3. 2 x 102 0. 9 x 102 2. 03 x 102 0. 009 T. Aspergillus spp. 20 100 4. 5 x 101 0. 5 x 101 2. 27 x 101 0. 05 A. flavus 16 80 4 x 101 1 x 101 2. 62 x 101 0. 055 A. fumigatus 3 15 1. 05 x 101 0. 95 x 101 1 x 101 0. 029 A. niger 20 100 4. 5 x 101 1 x 101 2. 5 x 101 0. 06 A. ochraceus 6 30 1 x 101 0. 5 x 101 0. 83 x 101 0. 021 Penicillium spp. 14 70 4 x 101 0. 5 x 101 2. 5 x 101 0. 056 Fusarium spp. 8 40 4 x 101 0. 5 x 101 1. 62 x 101 0. 038 Cladosporium spp. 4 20 0. 5 x 101 0. 035 Alternaria spp. 14 70 2. 5 x 101 1. 28 x 101 0. 013 C. albicans 4 20 2 x 101 1. 5 x 101 1. 75 x 101 0. 019 Rhodotoruella 6 30 1 x 101 0. 5 x 101 0. 83 x 101 0. 156 Total colony count

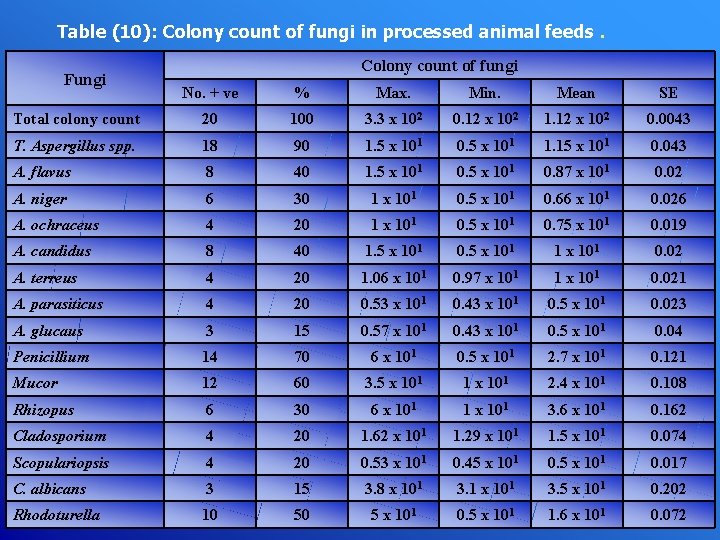
Table (10): Colony count of fungi in processed animal feeds Fungi . Colony count of fungi No. + ve % Max. Min. Mean SE Total colony count 20 100 3. 3 x 102 0. 12 x 102 1. 12 x 102 0. 0043 T. Aspergillus spp. 18 90 1. 5 x 101 0. 5 x 101 1. 15 x 101 0. 043 A. flavus 8 40 1. 5 x 101 0. 87 x 101 0. 02 A. niger 6 30 1 x 101 0. 5 x 101 0. 66 x 101 0. 026 A. ochraceus 4 20 1 x 101 0. 5 x 101 0. 75 x 101 0. 019 A. candidus 8 40 1. 5 x 101 0. 5 x 101 1 x 101 0. 02 A. terreus 4 20 1. 06 x 101 0. 97 x 101 1 x 101 0. 021 A. parasiticus 4 20 0. 53 x 101 0. 43 x 101 0. 5 x 101 0. 023 A. glucaus 3 15 0. 57 x 101 0. 43 x 101 0. 5 x 101 0. 04 Penicillium 14 70 6 x 101 0. 5 x 101 2. 7 x 101 0. 121 Mucor 12 60 3. 5 x 101 1 x 101 2. 4 x 101 0. 108 Rhizopus 6 30 6 x 101 1 x 101 3. 6 x 101 0. 162 Cladosporium 4 20 1. 62 x 101 1. 29 x 101 1. 5 x 101 0. 074 Scopulariopsis 4 20 0. 53 x 101 0. 45 x 101 0. 017 C. albicans 3 15 3. 8 x 101 3. 1 x 101 3. 5 x 101 0. 202 Rhodoturella 10 50 5 x 101 0. 5 x 101 1. 6 x 101 0. 072

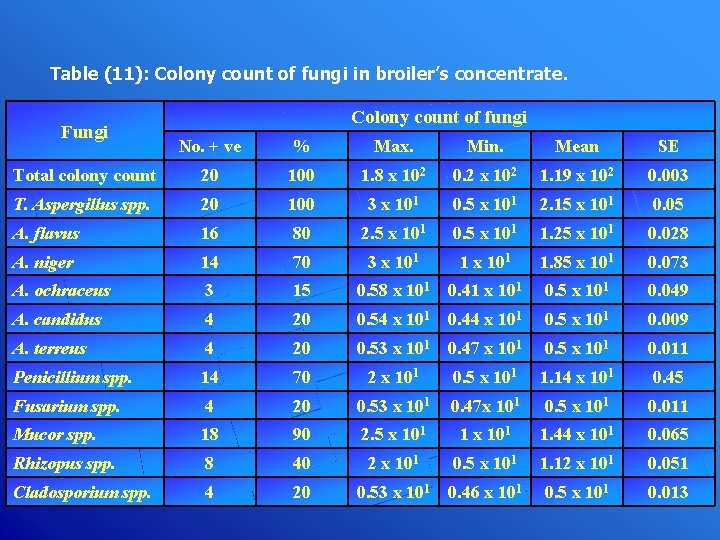
Table (11): Colony count of fungi in broiler’s concentrate. Fungi Colony count of fungi No. + ve % Max. Min. Mean SE Total colony count 20 100 1. 8 x 102 0. 2 x 102 1. 19 x 102 0. 003 T. Aspergillus spp. 20 100 3 x 101 0. 5 x 101 2. 15 x 101 0. 05 A. flavus 16 80 2. 5 x 101 0. 5 x 101 1. 25 x 101 0. 028 A. niger 14 70 3 x 101 1. 85 x 101 0. 073 A. ochraceus 3 15 0. 58 x 101 0. 41 x 101 0. 5 x 101 0. 049 A. candidus 4 20 0. 54 x 101 0. 44 x 101 0. 5 x 101 0. 009 A. terreus 4 20 0. 53 x 101 0. 47 x 101 0. 5 x 101 0. 011 Penicillium spp. 14 70 2 x 101 0. 5 x 101 1. 14 x 101 0. 45 Fusarium spp. 4 20 0. 53 x 101 0. 47 x 101 0. 5 x 101 0. 011 Mucor spp. 18 90 2. 5 x 101 1. 44 x 101 0. 065 Rhizopus spp. 8 40 2 x 101 0. 5 x 101 1. 12 x 101 0. 051 Cladosporium spp. 4 20 0. 53 x 101 0. 46 x 101 0. 5 x 101 0. 013

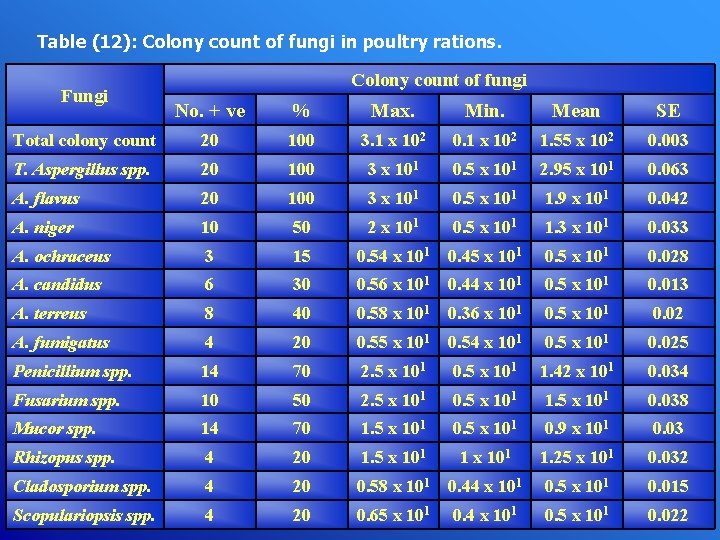
Table (12): Colony count of fungi in poultry rations. Fungi Colony count of fungi No. + ve % Max. Min. Mean SE Total colony count 20 100 3. 1 x 102 0. 1 x 102 1. 55 x 102 0. 003 T. Aspergillus spp. 20 100 3 x 101 0. 5 x 101 2. 95 x 101 0. 063 A. flavus 20 100 3 x 101 0. 5 x 101 1. 9 x 101 0. 042 A. niger 10 50 2 x 101 0. 5 x 101 1. 3 x 101 0. 033 A. ochraceus 3 15 0. 54 x 101 0. 45 x 101 0. 028 A. candidus 6 30 0. 56 x 101 0. 44 x 101 0. 5 x 101 0. 013 A. terreus 8 40 0. 58 x 101 0. 36 x 101 0. 5 x 101 0. 02 A. fumigatus 4 20 0. 55 x 101 0. 54 x 101 0. 5 x 101 0. 025 Penicillium spp. 14 70 2. 5 x 101 0. 5 x 101 1. 42 x 101 0. 034 Fusarium spp. 10 50 2. 5 x 101 0. 5 x 101 1. 5 x 101 0. 038 Mucor spp. 14 70 1. 5 x 101 0. 9 x 101 0. 03 Rhizopus spp. 4 20 1. 5 x 101 1. 25 x 101 0. 032 Cladosporium spp. 4 20 0. 58 x 101 0. 44 x 101 0. 5 x 101 0. 015 Scopulariopsis spp. 4 20 0. 65 x 101 0. 4 x 101 0. 5 x 101 0. 022

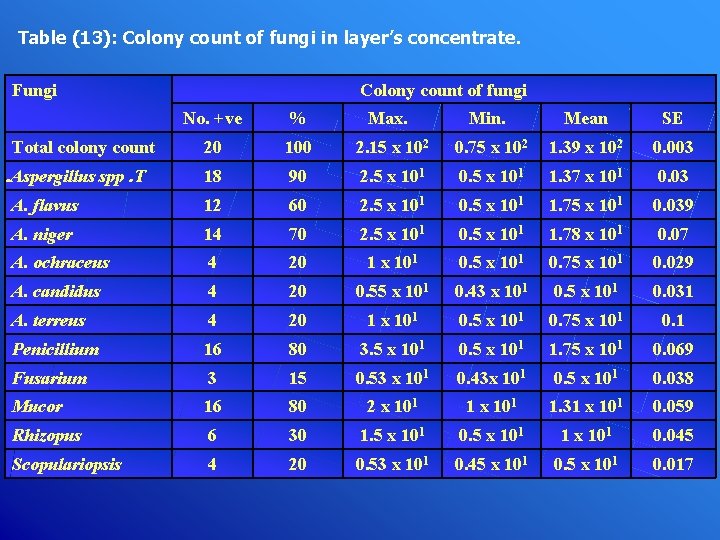
Table (13): Colony count of fungi in layer’s concentrate. Fungi Colony count of fungi No. +ve % Max. Min. Mean SE 20 100 2. 15 x 102 0. 75 x 102 1. 39 x 102 0. 003 18 90 2. 5 x 101 0. 5 x 101 1. 37 x 101 0. 03 A. flavus 12 60 2. 5 x 101 0. 5 x 101 1. 75 x 101 0. 039 A. niger 14 70 2. 5 x 101 0. 5 x 101 1. 78 x 101 0. 07 A. ochraceus 4 20 1 x 101 0. 5 x 101 0. 75 x 101 0. 029 A. candidus 4 20 0. 55 x 101 0. 43 x 101 0. 5 x 101 0. 031 A. terreus 4 20 1 x 101 0. 5 x 101 0. 75 x 101 0. 1 Penicillium 16 80 3. 5 x 101 0. 5 x 101 1. 75 x 101 0. 069 Fusarium 3 15 0. 53 x 101 0. 43 x 101 0. 5 x 101 0. 038 Mucor 16 80 2 x 101 1. 31 x 101 0. 059 Rhizopus 6 30 1. 5 x 101 0. 5 x 101 1 x 101 0. 045 Scopulariopsis 4 20 0. 53 x 101 0. 45 x 101 0. 017 Total colony count. Aspergillus spp. T

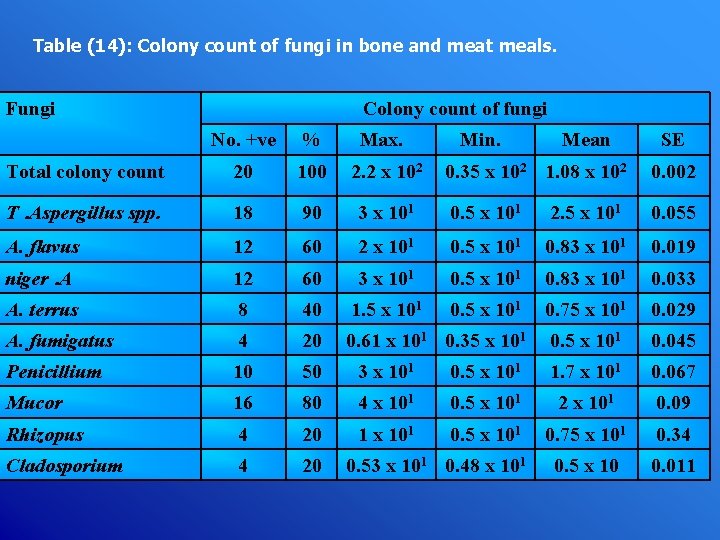
Table (14): Colony count of fungi in bone and meat meals. Fungi Colony count of fungi No. +ve % Max. Total colony count 20 100 2. 2 x 102 T. Aspergillus spp. 18 90 A. flavus 12 niger. A Min. Mean SE 0. 35 x 102 1. 08 x 102 0. 002 3 x 101 0. 5 x 101 2. 5 x 101 0. 055 60 2 x 101 0. 5 x 101 0. 83 x 101 0. 019 12 60 3 x 101 0. 5 x 101 0. 83 x 101 0. 033 A. terrus 8 40 1. 5 x 101 0. 75 x 101 0. 029 A. fumigatus 4 20 0. 61 x 101 0. 35 x 101 0. 045 Penicillium 10 50 3 x 101 0. 5 x 101 1. 7 x 101 0. 067 Mucor 16 80 4 x 101 0. 5 x 101 2 x 101 0. 09 Rhizopus 4 20 1 x 101 0. 5 x 101 0. 75 x 101 0. 34 Cladosporium 4 20 0. 5 x 10 0. 011 0. 53 x 101 0. 48 x 101

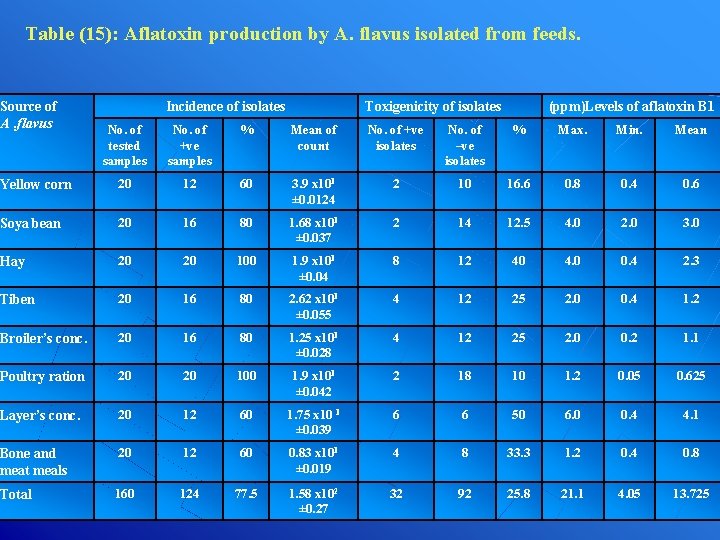
Table (15): Aflatoxin production by A. flavus isolated from feeds. Source of A. flavus Incidence of isolates (ppm)Levels of aflatoxin B 1 Toxigenicity of isolates No. of tested samples No. of +ve samples % Mean of count No. of +ve isolates No. of –ve isolates % Max. Min. Mean Yellow corn 20 12 60 3. 9 x 101 ± 0. 0124 2 10 16. 6 0. 8 0. 4 0. 6 Soya bean 20 16 80 1. 68 x 101 ± 0. 037 2 14 12. 5 4. 0 2. 0 3. 0 Hay 20 20 100 1. 9 x 101 ± 0. 04 8 12 40 4. 0 0. 4 2. 3 Tiben 20 16 80 2. 62 x 101 ± 0. 055 4 12 25 2. 0 0. 4 1. 2 Broiler’s conc. 20 16 80 1. 25 x 101 ± 0. 028 4 12 25 2. 0 0. 2 1. 1 Poultry ration 20 20 100 1. 9 x 101 ± 0. 042 2 18 10 1. 2 0. 05 0. 625 Layer’s conc. 20 12 60 1. 75 x 10 1 ± 0. 039 6 6 50 6. 0 0. 4 4. 1 Bone and meat meals 20 12 60 0. 83 x 101 ± 0. 019 4 8 33. 3 1. 2 0. 4 0. 8 Total 160 124 77. 5 1. 58 x 102 ± 0. 27 32 92 25. 8 21. 1 4. 05 13. 725

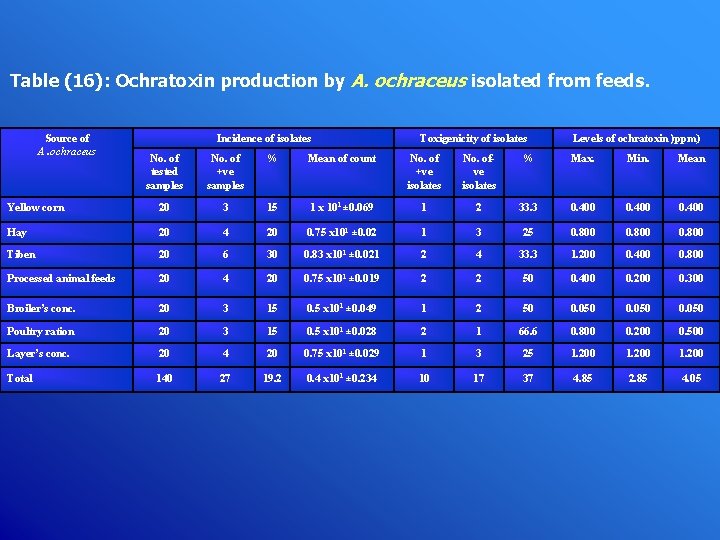
Table (16): Ochratoxin production by A. ochraceus isolated from feeds. Source of A. ochraceus Incidence of isolates Toxigenicity of isolates Levels of ochratoxin )ppm) No. of tested samples No. of +ve samples % Mean of count No. of +ve isolates No. ofve isolates % Max. Min. Mean Yellow corn 20 3 15 1 x 101 ± 0. 069 1 2 33. 3 0. 400 Hay 20 4 20 0. 75 x 101 ± 0. 02 1 3 25 0. 800 Tiben 20 6 30 0. 83 x 101 ± 0. 021 2 4 33. 3 1. 200 0. 400 0. 800 Processed animal feeds 20 4 20 0. 75 x 101 ± 0. 019 2 2 50 0. 400 0. 200 0. 300 Broiler’s conc. 20 3 15 0. 5 x 101 ± 0. 049 1 2 50 0. 050 Poultry ration 20 3 15 0. 5 x 101 ± 0. 028 2 1 66. 6 0. 800 0. 200 0. 500 Layer’s conc. 20 4 20 0. 75 x 101 ± 0. 029 1 3 25 1. 200 Total 140 27 19. 2 0. 4 x 101 ± 0. 234 10 17 37 4. 85 2. 85 4. 05

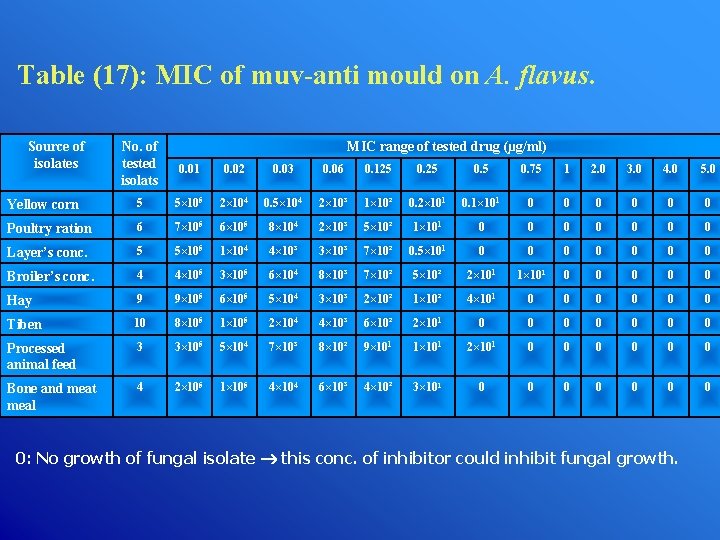
Table (17): MIC of muv-anti mould on A. flavus. Source of isolates No. of tested isolats MIC range of tested drug (µg/ml) 0. 01 0. 02 0. 03 0. 06 0. 125 0. 75 1 2. 0 3. 0 4. 0 5. 0 Yellow corn 5 5× 105 2× 104 0. 5× 104 2× 10³ 1× 10² 0. 2× 101 0. 1× 101 0 0 0 Poultry ration 6 7× 105 6× 105 8× 104 2× 10³ 5× 10² 1× 101 0 0 0 0 Layer’s conc. 5 5× 105 1× 104 4× 10³ 3× 10³ 7× 10² 0. 5× 101 0 0 0 0 Broiler’s conc. 4 4× 105 3× 105 6× 104 8× 10³ 7× 10² 5× 10² 2× 101 1× 101 0 0 0 Hay 9 9× 105 6× 105 5× 104 3× 10³ 2× 10² 1× 10² 4× 101 0 0 0 Tiben 10 8× 105 1× 105 2× 104 4× 10³ 6× 10² 2× 101 0 0 0 0 Processed animal feed 3 3× 105 5× 104 7× 10³ 8× 10² 9× 101 1× 101 2× 101 0 0 0 Bone and meat meal 4 2× 105 1× 105 4× 104 6× 10³ 4× 10² 3× 101 0 0 0 0: No growth of fungal isolate this conc. of inhibitor could inhibit fungal growth.

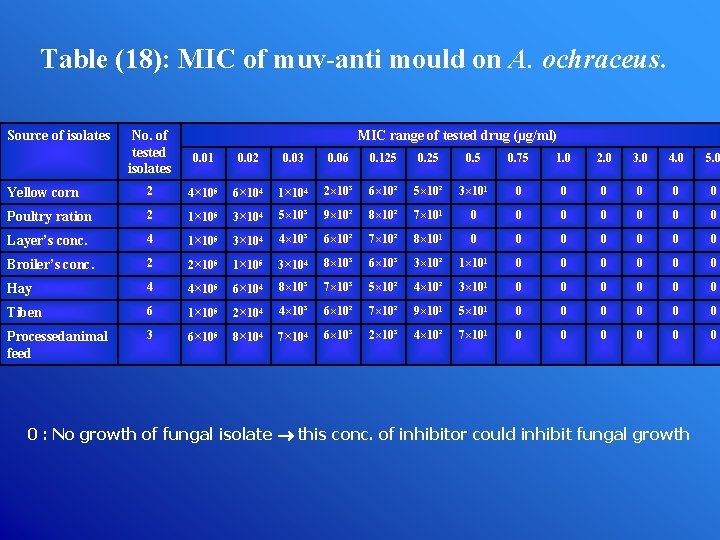
Table (18): MIC of muv-anti mould on A. ochraceus. Source of isolates No. of tested isolates MIC range of tested drug (µg/ml) 0. 01 0. 02 0. 03 0. 06 0. 125 0. 75 1. 0 2. 0 3. 0 4. 0 5. 0 Yellow corn 2 4× 105 6× 104 1× 104 2× 10³ 6× 10² 5× 10² 3× 101 0 0 0 Poultry ration 2 1× 105 3× 104 5× 10³ 9× 10² 8× 10² 7× 101 0 0 0 0 Layer’s conc. 4 1× 105 3× 104 4× 10³ 6× 10² 7× 10² 8× 101 0 0 0 0 Broiler’s conc. 2 2× 105 1× 105 3× 104 8× 10³ 6× 10³ 3× 10² 1× 101 0 0 0 Hay 4 4× 105 6× 104 8× 10³ 7× 10³ 5× 10² 4× 10² 3× 101 0 0 0 Tiben 6 1× 105 2× 104 4× 10³ 6× 10² 7× 10² 9× 101 5× 101 0 0 0 Processedanimal feed 3 6× 105 8× 104 7× 104 6× 10³ 2× 10³ 4× 10² 7× 101 0 0 0 0 : No growth of fungal isolate this conc. of inhibitor could inhibit fungal growth

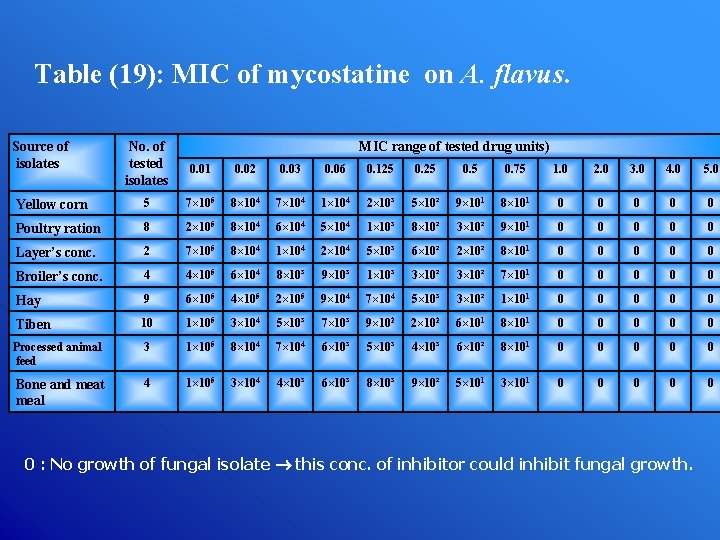
Table (19): MIC of mycostatine on A. flavus. Source of isolates No. of tested isolates MIC range of tested drug units) 0. 01 0. 02 0. 03 0. 06 0. 125 0. 75 1. 0 2. 0 3. 0 4. 0 5. 0 Yellow corn 5 7× 105 8× 104 7× 104 1× 104 2× 10³ 5× 10² 9× 101 8× 101 0 0 0 Poultry ration 8 2× 105 8× 104 6× 104 5× 104 1× 10³ 8× 10² 3× 10² 9× 101 0 0 0 Layer’s conc. 2 7× 105 8× 104 1× 104 2× 104 5× 10³ 6× 10² 2× 10² 8× 101 0 0 0 Broiler’s conc. 4 4× 105 6× 104 8× 10³ 9× 10³ 1× 10³ 3× 10² 7× 101 0 0 0 Hay 9 6× 105 4× 105 2× 105 9× 104 7× 104 5× 10³ 3× 10² 1× 101 0 0 0 Tiben 10 1× 105 3× 104 5× 10³ 7× 10³ 9× 102 2× 102 6× 101 8× 101 0 0 0 Processed animal feed 3 1× 105 8× 104 7× 104 6× 10³ 5× 10³ 4× 10³ 6× 10² 8× 101 0 0 0 Bone and meat meal 4 1× 105 3× 104 4× 10³ 6× 10³ 8× 10³ 9× 10² 5× 101 3× 101 0 0 0 : No growth of fungal isolate this conc. of inhibitor could inhibit fungal growth.

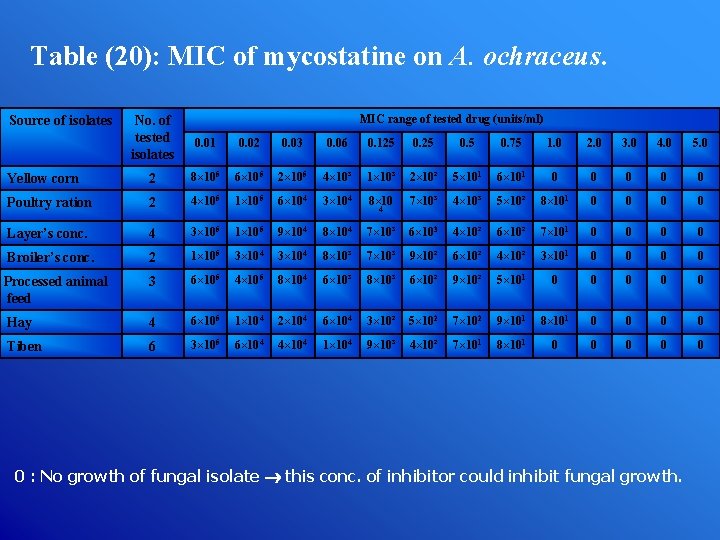
Table (20): MIC of mycostatine on A. ochraceus. Source of isolates MIC range of tested drug (units/ml) No. of tested isolates 0. 01 0. 02 0. 03 0. 06 0. 125 0. 75 1. 0 2. 0 3. 0 4. 0 5. 0 Yellow corn 2 8× 105 6× 105 2× 105 4× 10³ 1× 10³ 2× 10² 5× 101 6× 101 0 0 0 Poultry ration 2 4× 105 1× 105 6× 104 3× 104 8× 10 7× 10³ 4× 10³ 5× 10² 8× 101 0 0 Layer’s conc. 4 3× 105 1× 105 9× 104 8× 104 7× 10³ 6× 103 4× 10² 6× 10² 7× 101 0 0 Broiler’s conc. 2 1× 105 3× 104 8× 10³ 7× 10³ 9× 10² 6× 10² 4× 10² 3× 101 0 0 Processed animal feed 3 6× 105 4× 105 8× 104 6× 10³ 8× 10³ 6× 10² 9× 10² 5× 101 0 0 0 Hay 4 6× 105 1× 104 2× 104 6× 104 3× 10² 5× 102 7× 102 9× 101 8× 101 0 0 Tiben 6 3× 105 6× 104 4× 104 1× 104 9× 10³ 4× 10² 7× 101 8× 101 0 0 0 4 0 : No growth of fungal isolate this conc. of inhibitor could inhibit fungal growth.

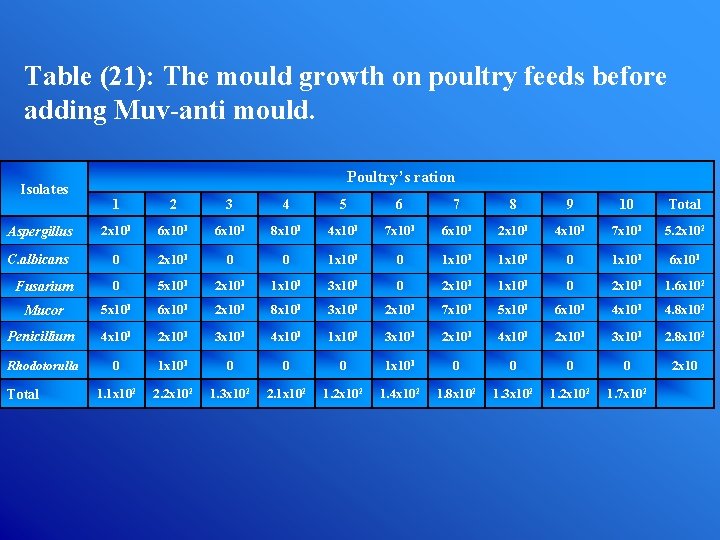
Table (21): The mould growth on poultry feeds before adding Muv-anti mould. Isolates Poultry’s ration 1 2 3 4 5 6 7 8 9 10 Total Aspergillus 2 x 101 6 x 101 8 x 101 4 x 101 7 x 101 6 x 101 2 x 101 4 x 101 7 x 101 5. 2 x 102 C. albicans 0 2 x 101 0 0 1 x 101 0 1 x 101 6 x 101 Fusarium 0 5 x 101 2 x 101 1 x 101 3 x 101 0 2 x 101 1. 6 x 102 Mucor 5 x 101 6 x 101 2 x 101 8 x 101 3 x 101 2 x 101 7 x 101 5 x 101 6 x 101 4. 8 x 102 Penicillium 4 x 101 2 x 101 3 x 101 4 x 101 1 x 101 3 x 101 2 x 101 4 x 101 2 x 101 3 x 101 2. 8 x 102 Rhodotorulla 0 1 x 101 0 0 0 0 2 x 10 1. 1 x 102 2. 2 x 102 1. 3 x 102 2. 1 x 102 1. 2 x 102 1. 4 x 102 1. 8 x 102 1. 3 x 102 1. 2 x 102 1. 7 x 102 Total

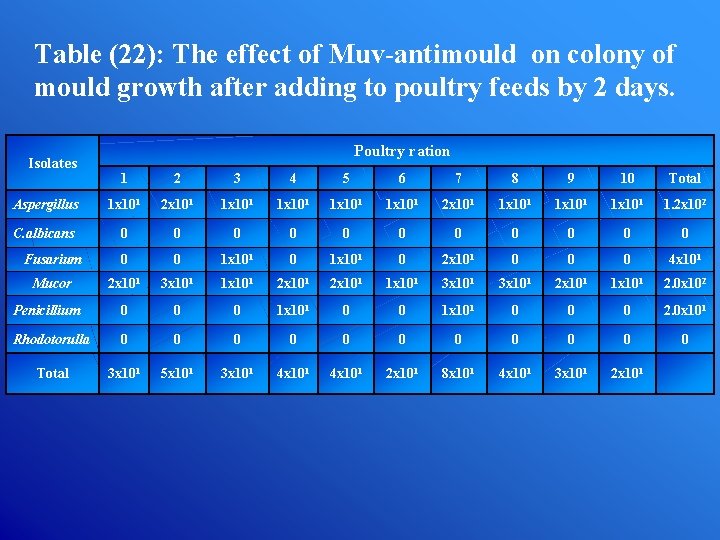
Table (22): The effect of Muv-antimould on colony of mould growth after adding to poultry feeds by 2 days. Isolates Poultry ration 1 2 3 4 5 6 7 8 9 10 Total Aspergillus 1 x 101 2 x 101 1 x 101 1 x 101 1. 2 x 102 C. albicans 0 0 0 Fusarium 0 0 1 x 101 0 2 x 101 0 0 0 4 x 101 Mucor 2 x 101 3 x 101 1 x 101 2 x 101 1 x 101 3 x 101 2 x 101 1 x 101 2. 0 x 102 Penicillium 0 0 0 1 x 101 0 0 0 2. 0 x 101 Rhodotorulla 0 0 0 Total 3 x 101 5 x 101 3 x 101 4 x 101 2 x 101 8 x 101 4 x 101 3 x 101 2 x 101

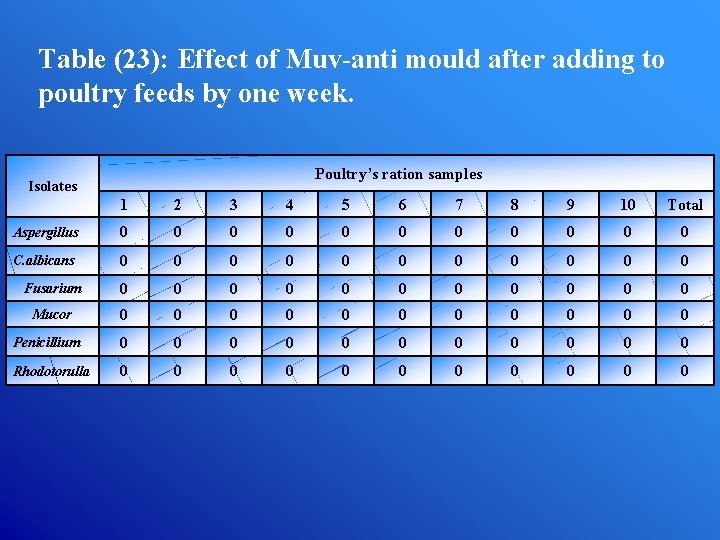
Table (23): Effect of Muv-anti mould after adding to poultry feeds by one week. Poultry’s ration samples Isolates 1 2 3 4 5 6 7 8 9 10 Total Aspergillus 0 0 0 C. albicans 0 0 0 Fusarium 0 0 0 Mucor 0 0 0 Penicillium 0 0 0 Rhodotorulla 0 0 0

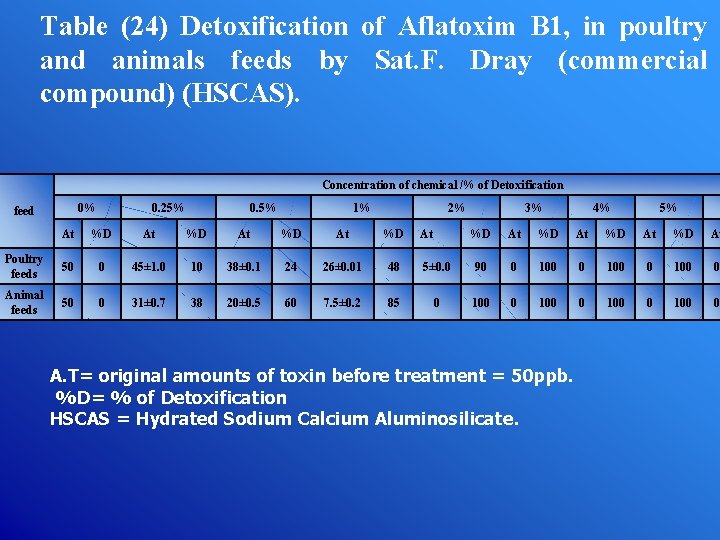
Table (24) Detoxification of Aflatoxim B 1, in poultry and animals feeds by Sat. F. Dray (commercial compound) (HSCAS). Concentration of chemical /% of Detoxification 0% feed 0. 25% 0. 5% 1% 2% At %D Poultry feeds 50 0 45± 1. 0 10 38± 0. 1 24 26± 0. 01 48 Animal feeds 50 0 31± 0. 7 38 20± 0. 5 60 7. 5± 0. 2 85 At 3% 4% 5% %D At 5± 0. 0 90 0 100 0 100 0 A. T= original amounts of toxin before treatment = 50 ppb. %D= % of Detoxification HSCAS = Hydrated Sodium Calcium Aluminosilicate.

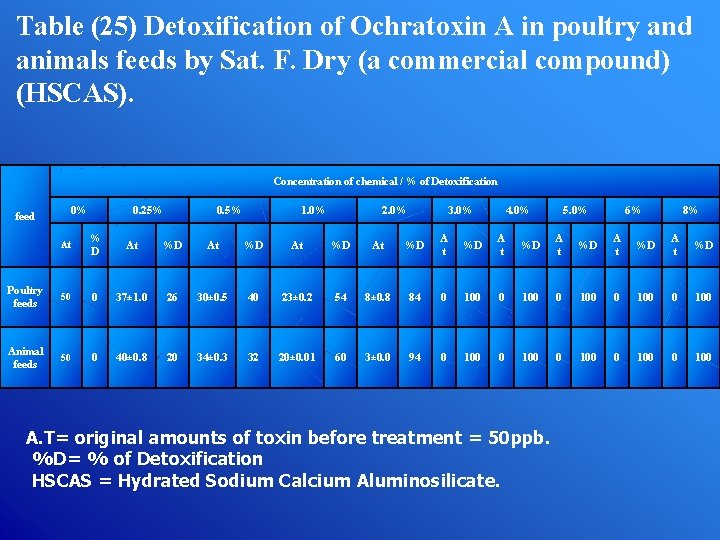
Table (25) Detoxification of Ochratoxin A in poultry and animals feeds by Sat. F. Dry (a commercial compound) (HSCAS). Concentration of chemical / % of Detoxification feed 0% 0. 25% 0. 5% 1. 0% 2. 0% 3. 0% 4. 0% 5. 0% 6% 8% At % D At %D A t %D A t %D Poultry feeds 50 0 37± 1. 0 26 30± 0. 5 40 23± 0. 2 54 8± 0. 8 84 0 100 0 100 Animal feeds 50 0 40± 0. 8 20 34± 0. 3 32 20± 0. 01 60 3± 0. 0 94 0 100 0 100 A. T= original amounts of toxin before treatment = 50 ppb. %D= % of Detoxification HSCAS = Hydrated Sodium Calcium Aluminosilicate.

CONCLUSIONS

From the foregoing results is apparent that 1. The mycoflora of the single feeds is more higher than compound feeds. This may be due to the single feeds were subjected to processes help in their contamination as bad irrigation, cultivation, harvesting, handling and storage condition. While the compound feeds subjected to heat treatment, additives and processing during preparation. These factors may decrease nycoflora of compound feeds. 2. The use of fungal inhibitors and antimycotoxins in addition to proper storage condition provided an effective ways of controlling the growth of fungi and subsequently toxin production.

3. The treated feeds must be remained a time not less than 1 week before used by animal or poultry. Therefore, frequent testing programs of feed and feedstuffs during different steps of production must be monitored before given to animal for consumption. All this way for increasing the quality of human health and animals wealth.

THANK YOU