Expert guide to universe of choice after metformin















- Slides: 28

Expert guide to universe of choice after metformin – An evidence based approach Dr. A. K. Singh M. D (Medicine), D. M (Endocrinology) Consultant Endocrinologist G D Hospital & Diabetes Institute, Kolkata


Medicine Update Vol. 24; 1449 -1457 2014



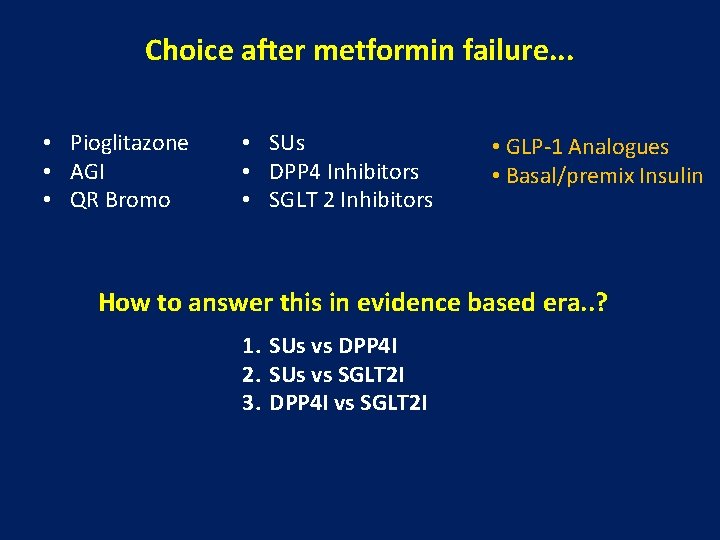
Choice after metformin failure. . . • Pioglitazone • AGI • QR Bromo • SUs • DPP 4 Inhibitors • SGLT 2 Inhibitors • GLP-1 Analogues • Basal/premix Insulin How to answer this in evidence based era. . ? 1. SUs vs DPP 4 I 2. SUs vs SGLT 2 I 3. DPP 4 I vs SGLT 2 I

Sulfonylureas – lessons learnt so far. . . Advantage • Time tested – Since 1950 • Robust glucose reduction in early stage • Inexpensive • RCT did’nt give bad CV signals Disadvantage • “Gluco-centric “ without “Disease-centric” properties • Durability – less (ADOPT) • Hypoglycemia – major issue • Weight gain • Possible beta cell apoptosis • ? Prevents ischemic preconditioning (Glibenclamide) • Observational studies and meta -analysis shows increasingly bad CV signals Singh AK. Indian J Endocr Metab 2014; 18: 617 -23

Gliptins – lessons learnt so far. . . Advantage Disadvantage • A 1 c reduction at par with Met, SUs and SGLT-2 I • Minimal hypoglycaemia with weight neutrality or loss • Possible pleiotropic benefit and beta cell protection • Pancreatitis : not a great concern • Bone friendly • Meta-analysis of pooled data from phase 2/3 showed CV benefit • RCT – TECOS, SAVOR TIMI & EXAMINE suggested CV neutrality • Cost • Possible off-target side effect • Signals of higher CV mortality with VILDA in VIVIDD trial • Increased heart failure hospitalisation with SAXA in SAVOR TIMI - ? Play of chance • Arthritis - FDA Singh AK. Indian J Endocr Metab 2014; 18: 617 -23

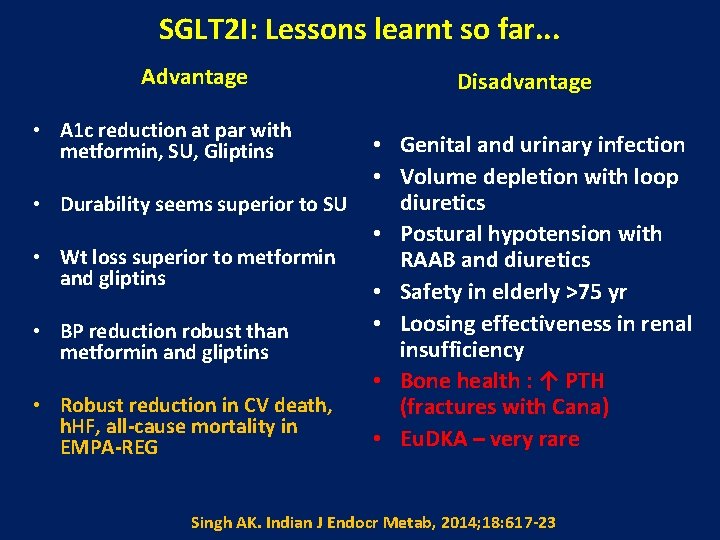
SGLT 2 I: Lessons learnt so far. . . Advantage • A 1 c reduction at par with metformin, SU, Gliptins • Durability seems superior to SU • Wt loss superior to metformin and gliptins • BP reduction robust than metformin and gliptins • Robust reduction in CV death, h. HF, all-cause mortality in EMPA-REG Disadvantage • Genital and urinary infection • Volume depletion with loop diuretics • Postural hypotension with RAAB and diuretics • Safety in elderly >75 yr • Loosing effectiveness in renal insufficiency • Bone health : ↑ PTH (fractures with Cana) • Eu. DKA – very rare Singh AK. Indian J Endocr Metab, 2014; 18: 617 -23

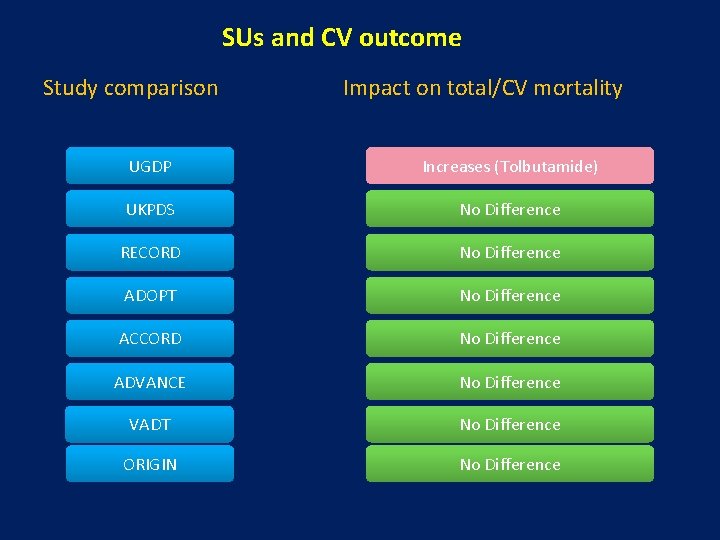
SUs and CV outcome Study comparison Impact on total/CV mortality UGDP Increases (Tolbutamide) UKPDS No Difference RECORD No Difference ADOPT No Difference ACCORD No Difference ADVANCE No Difference VADT No Difference ORIGIN No Difference

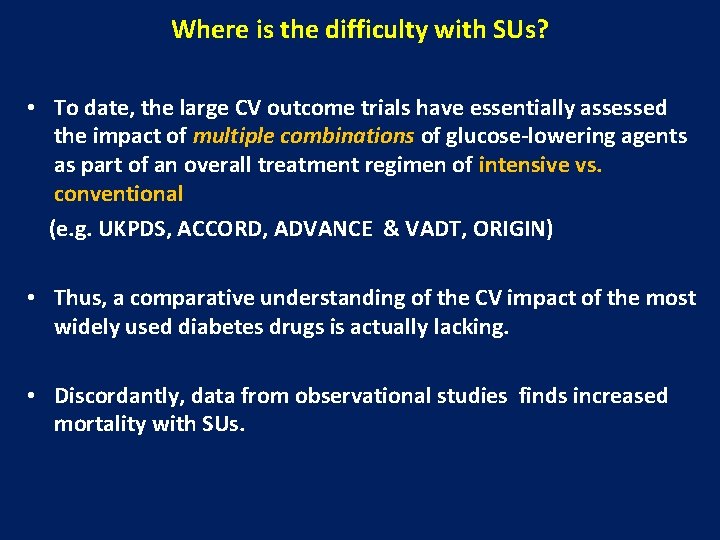
Where is the difficulty with SUs? • To date, the large CV outcome trials have essentially assessed the impact of multiple combinations of glucose-lowering agents as part of an overall treatment regimen of intensive vs. conventional (e. g. UKPDS, ACCORD, ADVANCE & VADT, ORIGIN) • Thus, a comparative understanding of the CV impact of the most widely used diabetes drugs is actually lacking. • Discordantly, data from observational studies finds increased mortality with SUs.

SUs and CV outcome • Most neutral CV outcome data lies with Gliclazide followed by Glimepiride • Glipizide had intermediate risk • Glibenclamide has worst outcome Singh AK. Expert Review of Clinical Pharmacology; 2016

Head-to-head study 1. SUs vs DPP 4 I 2. SUs vs SGLT 2 I 3. DPP 4 I vs SGLT 2 I


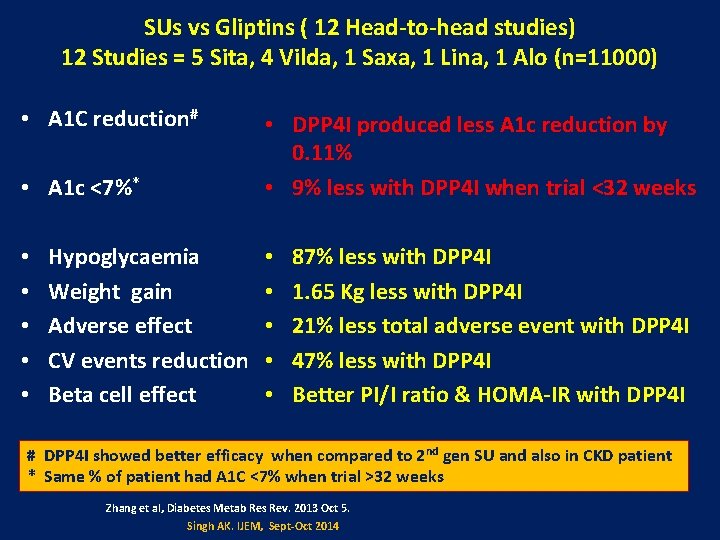
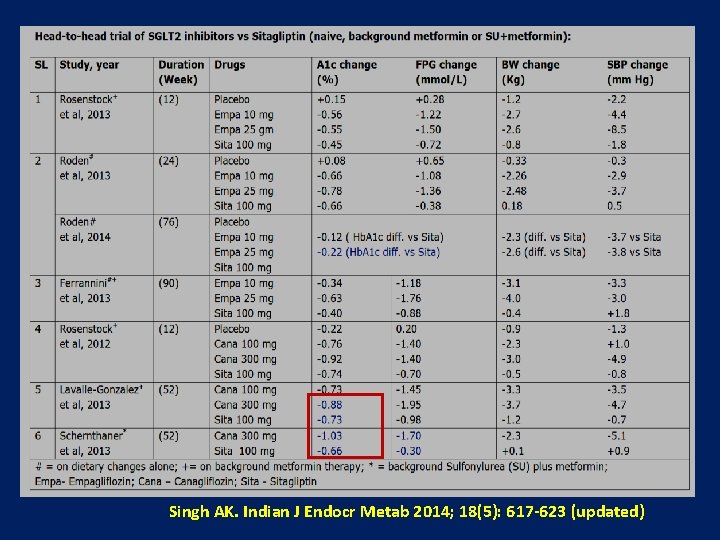
SUs vs Gliptins ( 12 Head-to-head studies) 12 Studies = 5 Sita, 4 Vilda, 1 Saxa, 1 Lina, 1 Alo (n=11000) • A 1 C reduction# • A 1 c <7%* • • • Hypoglycaemia Weight gain Adverse effect CV events reduction Beta cell effect • DPP 4 I produced less A 1 c reduction by 0. 11% • 9% less with DPP 4 I when trial <32 weeks • • • 87% less with DPP 4 I 1. 65 Kg less with DPP 4 I 21% less total adverse event with DPP 4 I 47% less with DPP 4 I Better PI/I ratio & HOMA-IR with DPP 4 I # DPP 4 I showed better efficacy when compared to 2 nd gen SU and also in CKD patient * Same % of patient had A 1 C <7% when trial >32 weeks Zhang et al, Diabetes Metab Res Rev. 2013 Oct 5. Singh AK. IJEM, Sept-Oct 2014

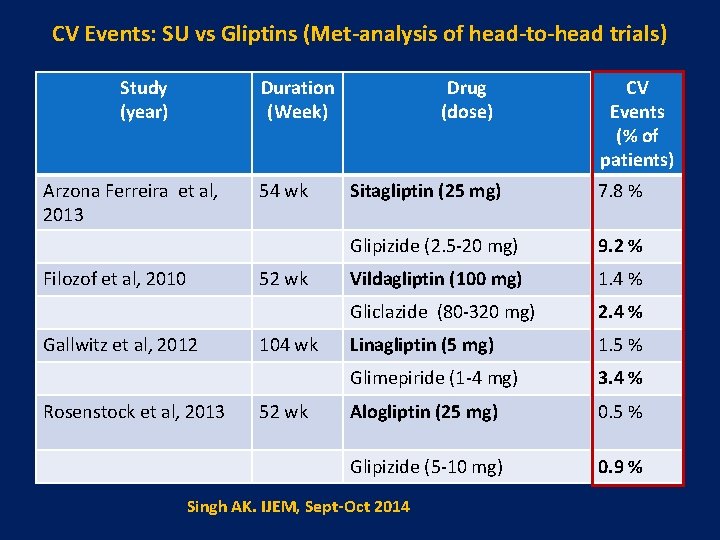
CV Events: SU vs Gliptins (Met-analysis of head-to-head trials) Study (year) Duration (Week) Arzona Ferreira et al, 2013 Filozof et al, 2010 54 wk 52 wk Gallwitz et al, 2012 Rosenstock et al, 2013 104 wk 52 wk Drug (dose) CV Events (% of patients) Sitagliptin (25 mg) 7. 8 % Glipizide (2. 5 -20 mg) 9. 2 % Vildagliptin (100 mg) 1. 4 % Gliclazide (80 -320 mg) 2. 4 % Linagliptin (5 mg) 1. 5 % Glimepiride (1 -4 mg) 3. 4 % Alogliptin (25 mg) 0. 5 % Glipizide (5 -10 mg) 0. 9 % Singh AK. IJEM, Sept-Oct 2014 Zhang et al, Diabetes Metab Res Rev. 2013 Oct 5. doi: 10. 1002/dmrr. 2482. [Epub ahead of print]

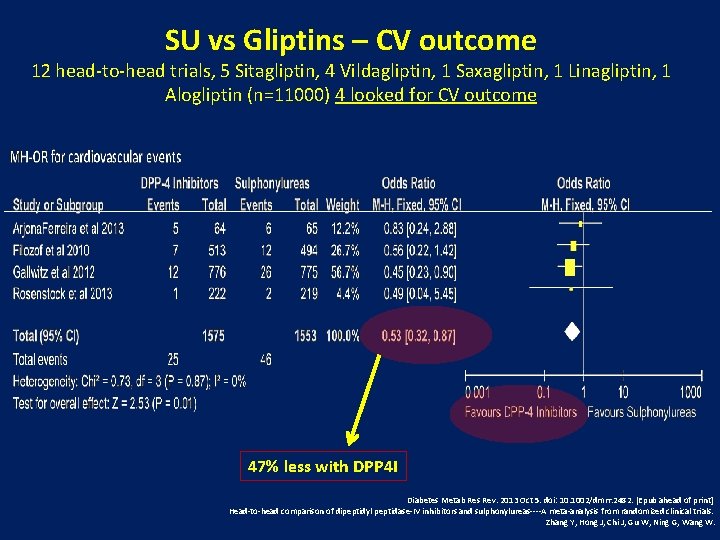
SU vs Gliptins – CV outcome 12 head-to-head trials, 5 Sitagliptin, 4 Vildagliptin, 1 Saxagliptin, 1 Linagliptin, 1 Alogliptin (n=11000) 4 looked for CV outcome 47% less with DPP 4 I Diabetes Metab Res Rev. 2013 Oct 5. doi: 10. 1002/dmrr. 2482. [Epub ahead of print] Head-to-head comparison of dipeptidyl peptidase-IV inhibitors and sulphonylureas----A meta-analysis from randomized clinical trials. Zhang Y, Hong J, Chi J, Gu W, Ning G, Wang W.

Summary: SUs or DPP-4 I? • SUs and DPP-4 I are both insulinotropic, but with different mechanisms. SUs may cause (severe) hypoglycamia, whereas DPP-4 I don‘t. • Whether SUs elicit cardio-vascular problems is still not known. By direct (head-to-head) comparison, DPP-4 I are associated with less cardio-vascular events than SUs. Whether this indicates a harm of SUs or a benefit of DPP-4 inhibitors needs to be clarified from further CV outcome studies. • CAROLINA may answer or at least enlighten further.


Head-to-head study 1. SUs vs DPP 4 I 2. SUs vs SGLT 2 I 3. DPP 4 I vs SGLT 2 I

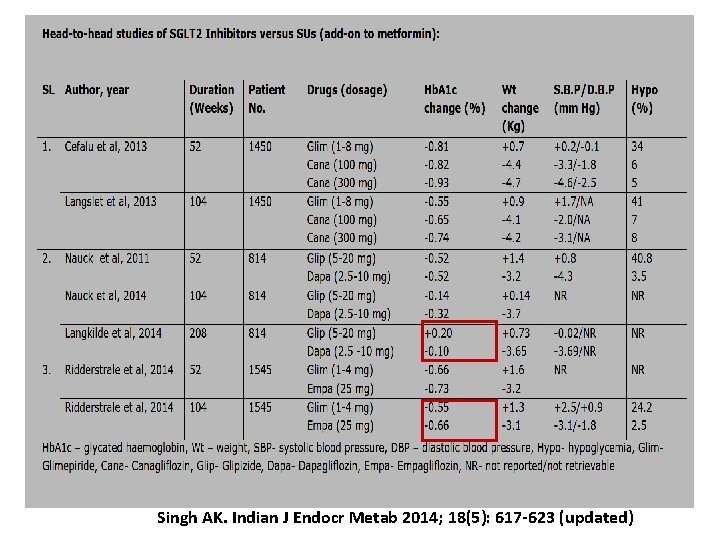
Singh AK. Indian J Endocr Metab 2014; 18(5): 617 -623 (updated)

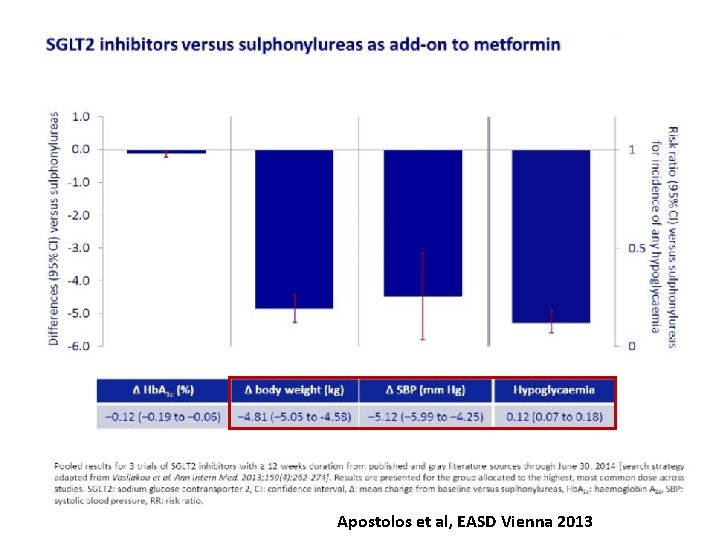
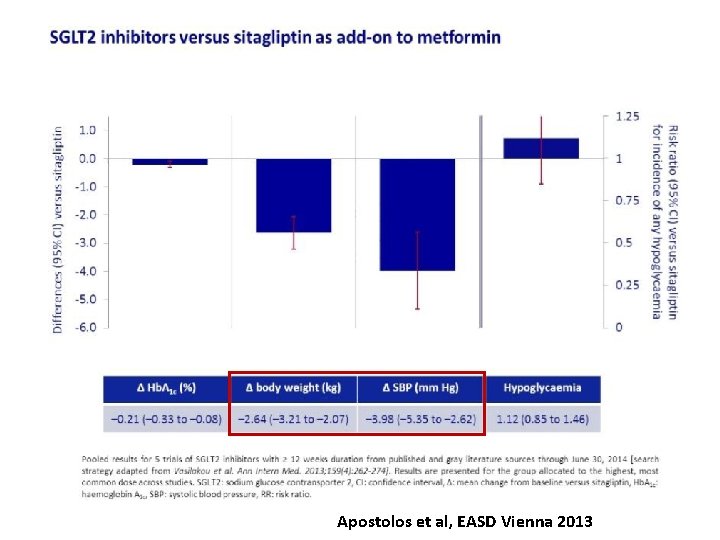
Apostolos et al, EASD Vienna 2013

Head-to-head study 1. SUs vs DPP 4 I 2. SUs vs SGLT 2 I 3. DPP 4 I vs SGLT 2 I

Singh AK. Indian J Endocr Metab 2014; 18(5): 617 -623 (updated)

Apostolos et al, EASD Vienna 2013

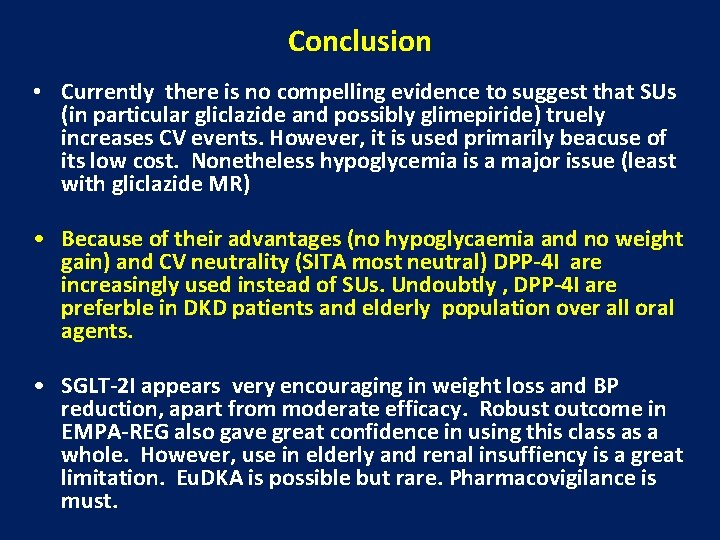
Conclusion • Currently there is no compelling evidence to suggest that SUs (in particular gliclazide and possibly glimepiride) truely increases CV events. However, it is used primarily beacuse of its low cost. Nonetheless hypoglycemia is a major issue (least with gliclazide MR) • Because of their advantages (no hypoglycaemia and no weight gain) and CV neutrality (SITA most neutral) DPP-4 I are increasingly used instead of SUs. Undoubtly , DPP-4 I are preferble in DKD patients and elderly population over all oral agents. • SGLT-2 I appears very encouraging in weight loss and BP reduction, apart from moderate efficacy. Robust outcome in EMPA-REG also gave great confidence in using this class as a whole. However, use in elderly and renal insuffiency is a great limitation. Eu. DKA is possible but rare. Pharmacovigilance is must.

Criteria for ideal oral drug after metformin. . . • Efficacy • Safety – ü short term – hypoglycemia ü long term – CV, All-cause, Cancer mortality • Durability • Good partner to insulin (eventually) • Corrects the pathogenesis of T 2 DM – desirable • Safe in co-morbid condition – DKD, CLD, IHD, CCF

THANKS
 Rythme prandial
Rythme prandial After me after me after me
After me after me after me If any man come after me
If any man come after me Expert choice 2000
Expert choice 2000 Hướng dẫn sử dụng expert choice
Hướng dẫn sử dụng expert choice Metformin triglycerides
Metformin triglycerides Metformin etki mekanizması
Metformin etki mekanizması Metformin lactic acidosis symptoms
Metformin lactic acidosis symptoms Metformin lactic acidosis symptoms
Metformin lactic acidosis symptoms Propiltiourasil paten
Propiltiourasil paten Metformin etki mekanizması
Metformin etki mekanizması Metformin triglycerides
Metformin triglycerides Metformin njurar
Metformin njurar Cholinomimetic
Cholinomimetic σδιι
σδιι Introduction of metformin
Introduction of metformin Metiglinide
Metiglinide Metformin side effects
Metformin side effects Metformin and constipation
Metformin and constipation Pco metformin
Pco metformin Sekretagog nedir
Sekretagog nedir Good choice or bad choice
Good choice or bad choice Dollar universe tutorial
Dollar universe tutorial Ordonnanceur dollar universe
Ordonnanceur dollar universe Coquille tribe
Coquille tribe The scale of the universe 2
The scale of the universe 2 There is no neutral ground in the universe
There is no neutral ground in the universe The last book in the universe
The last book in the universe ❄️ heat death of the universe
❄️ heat death of the universe