EDUCATIONAL SLIDE MODULES Module C Evidence for effects




- Slides: 36

EDUCATIONAL SLIDE MODULES Module C: Evidence for effects of older glucose-lowering agents on CV risk

ACROSS T 2 D educational slide modules Module A • CV disease and T 2 D Module B • Approaches to managing CV risk in patients with T 2 D Module C • Evidence for effects of older glucose-lowering agents on CV risk Module D • Evaluating CV safety and potential for CV risk reduction with newer T 2 D agents Module E • EMPA-REG OUTCOME® results. 2

Metformin CVOT interpretation FDA mandate 3 SU CV safety TZD

Metformi n CVOT interpretation FDA mandate 4 SU CV safety TZD

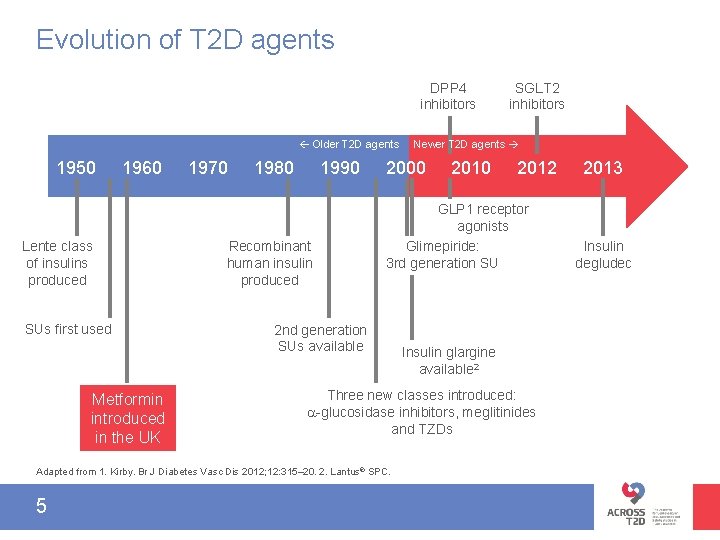
Evolution of T 2 D agents DPP 4 inhibitors Older T 2 D agents 1950 1960 Lente class of insulins produced SUs first used Metformin introduced in the UK 1970 1980 1990 Recombinant human insulin produced Newer T 2 D agents 2000 2012 GLP 1 receptor agonists Glimepiride: 3 rd generation SU 2 nd generation SUs available Insulin glargine available 2 Three new classes introduced: -glucosidase inhibitors, meglitinides and TZDs Adapted from 1. Kirby. Br J Diabetes Vasc Dis 2012; 12: 315– 20. 2. Lantus® SPC. 5 SGLT 2 inhibitors 2013 Insulin degludec

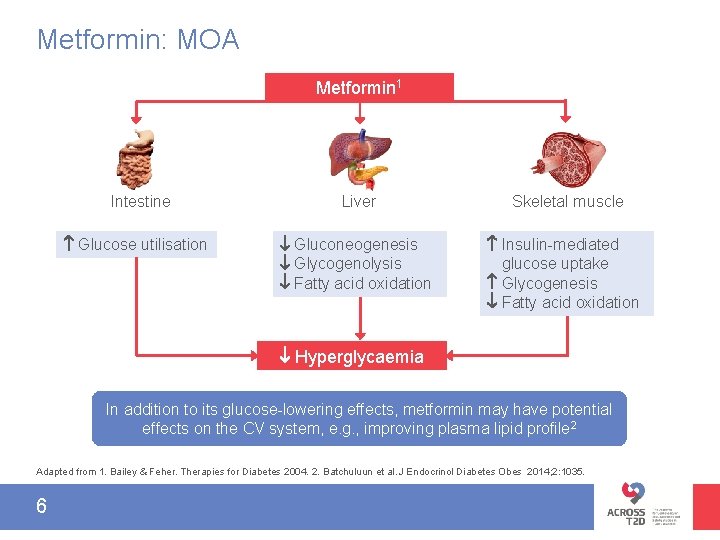
Metformin: MOA Metformin 1 Intestine Glucose utilisation Liver Skeletal muscle Gluconeogenesis Glycogenolysis Fatty acid oxidation Insulin-mediated glucose uptake Glycogenesis Fatty acid oxidation Hyperglycaemia In addition to its glucose-lowering effects, metformin may have potential effects on the CV system, e. g. , improving plasma lipid profile 2 Adapted from 1. Bailey & Feher. Therapies for Diabetes 2004. 2. Batchuluun et al. J Endocrinol Diabetes Obes 2014; 2: 1035. 6

Metformin is recommended as first-line therapy in T 2 D • Metformin is indicated for the treatment of T 2 D, and generally recommended as first-line therapy 1, 2 • Evidence for effect on CV risk cited in international prescribing information differs for US vs EU – US prescribing information 3 • States that there are no clinical studies establishing conclusive evidence of macrovascular risk reduction with metformin (or any other anti-diabetes drug) – EU prescribing information 1 • Cites UKPDS analysis from 342 overweight patients treated with metformin after failure of diet alone 1, 4 – Metformin significantly reduced any diabetes-related complication, diabetes-related and overall mortality, and absolute risk of MI vs diet alone after 10. 7 years 1. http: //www. medicines. org. uk/emc/medicine/23244/SPC. 2. American Diabetes Association. Diabetes Care 2015; 38(suppl. 1): S 1–S 94. 3. http: //www. drugs. com/pro/metformin. html 4. UKPDS 34. Lancet 1998; 352: 854– 65. 7

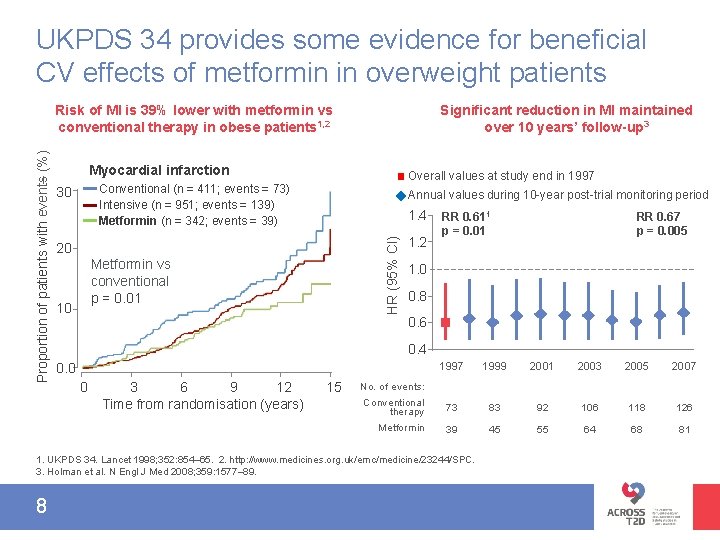
UKPDS 34 provides some evidence for beneficial CV effects of metformin in overweight patients Significant reduction in MI maintained over 10 years’ follow-up 3 Myocardial infarction Overall values at study end in 1997 Conventional (n = 411; events = 73) Intensive (n = 951; events = 139) Metformin (n = 342; events = 39) 30 Annual values during 10 -year post-trial monitoring period 1. 4 HR (95% CI) Proportion of patients with events (%) Risk of MI is 39% lower with metformin vs conventional therapy in obese patients 1, 2 20 Metformin vs conventional p = 0. 01 10 1. 2 RR 0. 611 p = 0. 01 1. 0 0. 8 0. 6 0. 4 0. 0 0 3 6 9 12 Time from randomisation (years) 15 1997 1999 2001 2003 2005 2007 Conventional therapy 73 83 92 106 118 126 Metformin 39 45 55 64 68 81 No. of events: 1. UKPDS 34. Lancet 1998; 352: 854– 65. 2. http: //www. medicines. org. uk/emc/medicine/23244/SPC. 3. Holman et al. N Engl J Med 2008; 359: 1577– 89. 8 RR 0. 67 p = 0. 005

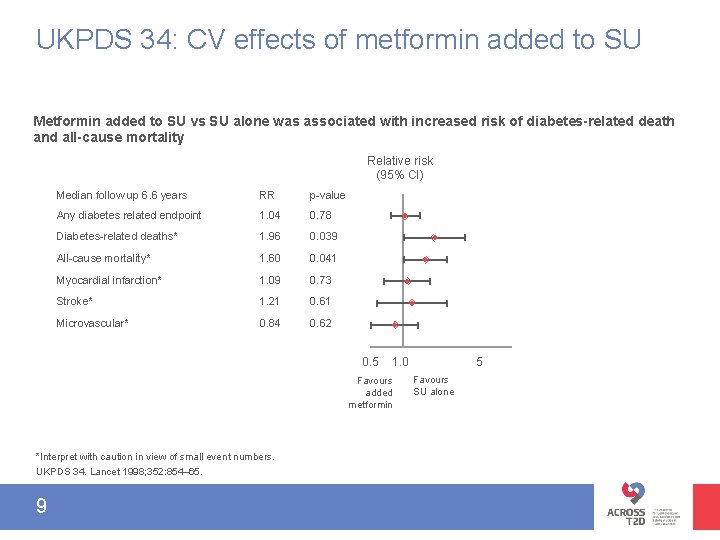
UKPDS 34: CV effects of metformin added to SU Metformin added to SU vs SU alone was associated with increased risk of diabetes-related death and all-cause mortality Relative risk (95% CI) Median follow up 6. 6 years RR p-value Any diabetes related endpoint 1. 04 0. 78 Diabetes-related deaths* 1. 96 0. 039 All-cause mortality* 1. 60 0. 041 Myocardial infarction* 1. 09 0. 73 Stroke* 1. 21 0. 61 Microvascular* 0. 84 0. 62 0. 5 Favours added metformin *Interpret with caution in view of small event numbers. UKPDS 34. Lancet 1998; 352: 854– 65. 9 5 1. 0 Favours SU alone

Section recap CV safety of metformin • Metformin is generally recommended as first-line therapy 1, 2 • Some evidence to suggest a CV benefit in overweight patients 1 • There remains a paucity of evidence from large, long-term, placebo-controlled CV outcome trials 3 1. http: //www. medicines. org. uk/emc/medicine/23244/SPC. 2. American Diabetes Association. Diabetes Care 2015; 38(suppl. 1): S 1–S 94. 3. Boussageaon et al. PLo. S Med. 2012; 9: e 1001204. 10

Metformi n CVOT interpretation FDA mandate 11 SU CV safety TZD

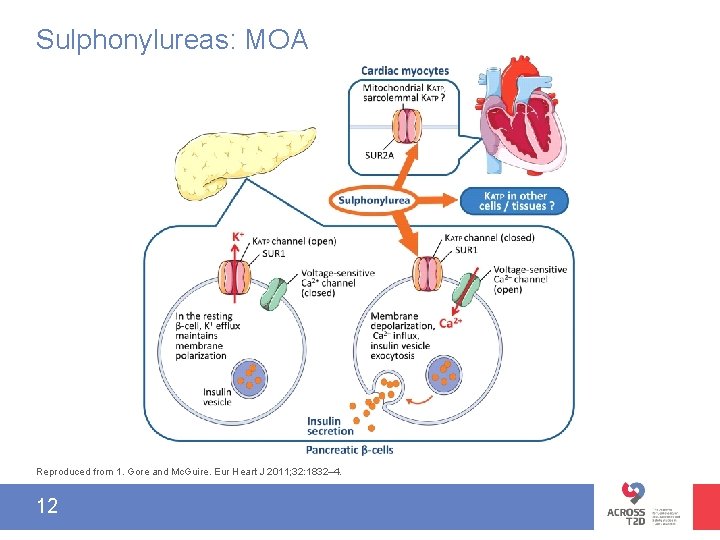
Sulphonylureas: MOA Reproduced from 1. Gore and Mc. Guire. Eur Heart J 2011; 32: 1832– 4. 12

Sulphonylureas and CV safety • In the US, SUs carry a special warning around increased risk of CV mortality 1– 3 – The warning is based on findings from the UGDP trial that reported an excess of cardiac deaths in patients receiving tolbutamide versus placebo 4 • In the EU, the same SUs do not carry safety warnings around increased CV mortality with SUs 5– 7 1. Glimepiride PI at http: //www. accessdata. fda. gov/drugsatfda_docs/label/2009/020496 s 021 lbl. pdf. 2. Tolbutamide PI at http: //www. drugs. com/pro/tolbutamide. html. 3. Glipizide PI at http: //www. drugs. com/pro/glipizide. html. 4. Meinert et al. Diabetes 1970; 19 (suppl): 789– 830. 5. Glimepiride EU Sm. PC at http: //www. medicines. org. uk/emc/medicine/27033. 6. Tolbutamide EU Sm. PC at http: //www. medicines. org. uk/emc/medicine/26366. 7. Glipizide EU Sm. PC at http: //www. medicines. org. uk/emc/medicine/9851. 13

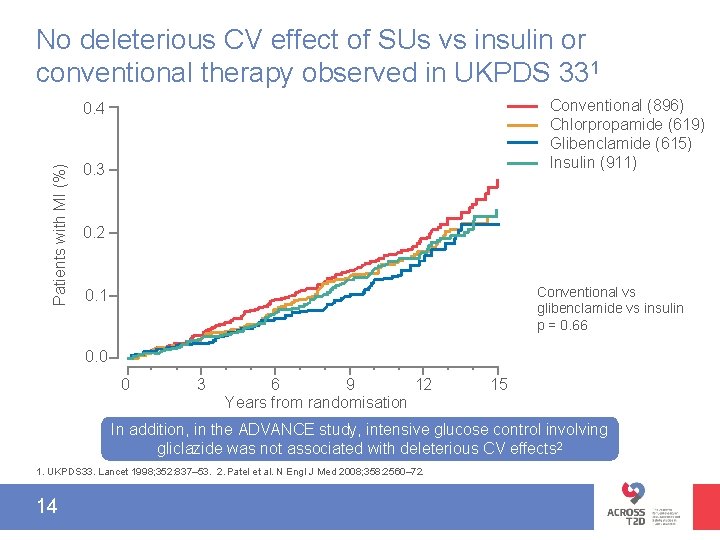
No deleterious CV effect of SUs vs insulin or conventional therapy observed in UKPDS 331 Conventional (896) Chlorpropamide (619) Glibenclamide (615) Insulin (911) Patients with MI (%) 0. 4 0. 3 0. 2 Conventional vs glibenclamide vs insulin p = 0. 66 0. 1 0. 0 0 3 6 9 12 Years from randomisation 15 In addition, in the ADVANCE study, intensive glucose control involving gliclazide was not associated with deleterious CV effects 2 1. UKPDS 33. Lancet 1998; 352: 837– 53. 2. Patel et al. N Engl J Med 2008; 358: 2560– 72. 14

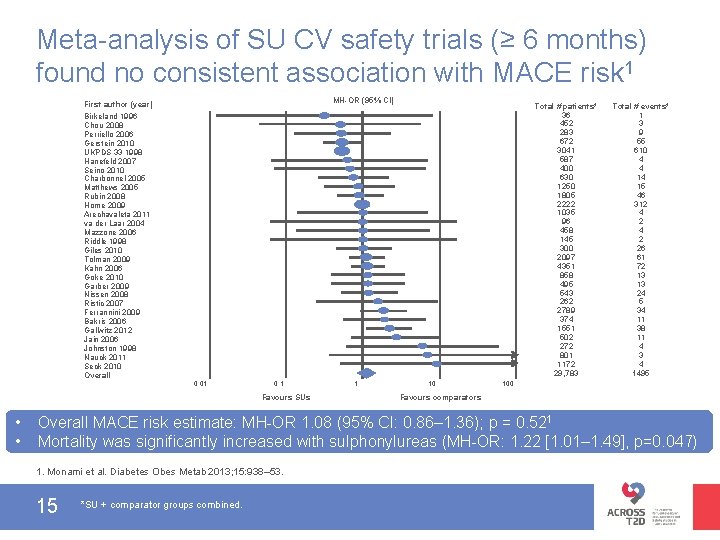
Meta-analysis of SU CV safety trials (≥ 6 months) found no consistent association with MACE risk 1 MH-OR (95% CI) First author (year) Total # patients* 36 452 283 672 3041 587 400 630 1250 1805 2222 1035 96 458 145 300 2097 4351 858 495 543 262 2789 374 1551 502 272 801 1172 29, 783 Birkeland 1996 Chou 2008 Perriello 2006 Gerstein 2010 UKPDS 33 1998 Hanefeld 2007 Seino 2010 Charbonnel 2005 Matthews 2005 Rubin 2008 Home 2009 Arechavaleta 2011 va der Laar 2004 Mazzone 2006 Riddle 1998 Giles 2010 Tolman 2009 Kahn 2006 Goke 2010 Garber 2009 Nissen 2008 Ristic 2007 Ferrannini 2009 Bakris 2006 Gallwitz 2012 Jain 2006 Johnston 1998 Nauck 2011 Seck 2010 Overall 0. 01 0. 1 Favours SUs • • 1 10 Total # events* 1 3 9 55 610 4 4 14 15 46 312 4 2 26 61 72 13 13 24 5 34 11 38 11 4 3 4 1495 100 Favours comparators Overall MACE risk estimate: MH-OR 1. 08 (95% CI: 0. 86– 1. 36); p = 0. 521 Mortality was significantly increased with sulphonylureas (MH-OR: 1. 22 [1. 01– 1. 49], p=0. 047) 1. Monami et al. Diabetes Obes Metab 2013; 15: 938– 53. 15 *SU + comparator groups combined.

Section recap CV safety of SUs • The UGDP study raised safety concerns with tolbutamide (excess of cardiac deaths vs placebo)1 • UKPDS 33 demonstrated no deleterious effect of SUs on CV safety compared with insulin or conventional management 2 • In ADVANCE, intensive glucose-lowering including gliclazide was not associated with negative CV outcomes vs standard treatment 3 • In a meta-analysis of 115 RCTs, overall MACE risk estimate for SUs vs comparators was not statistically increased (OR 1. 08, p = 0. 52)4 ‘CV safety of SUs cannot be considered established unless evaluated in long-term CVOTs’ 4 1. Meinert et al. Diabetes 1970; 19(suppl): 789– 830. 2. UKPDS Group. Lancet 1998; 352: 837– 53. 3. Patel et al. N Engl J Med 2008; 358: 2560– 72. 4. 5. Monami et al. Diabetes Obes Metab 2013; 15: 938– 53. 16

Metformi n CVOT interpretation FDA mandate 17 SU CV safety TZD

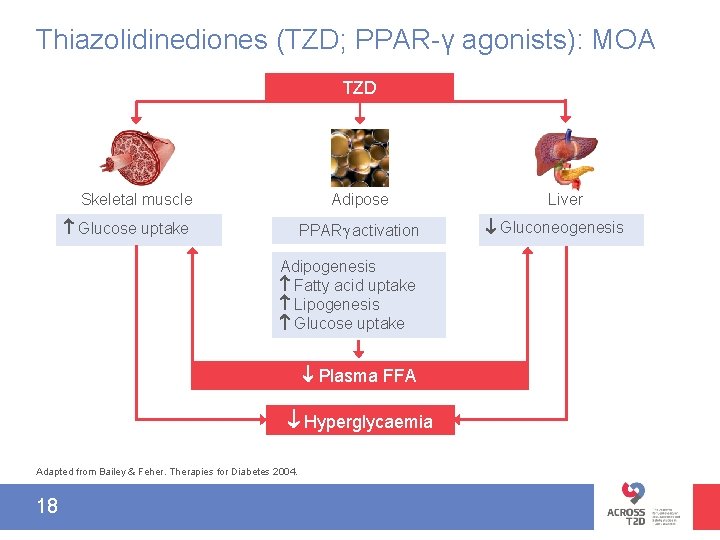
Thiazolidinediones (TZD; PPAR-γ agonists): MOA TZD Skeletal muscle Glucose uptake Adipose PPAR activation Adipogenesis Fatty acid uptake Lipogenesis Glucose uptake Plasma FFA Hyperglycaemia Adapted from Bailey & Feher. Therapies for Diabetes 2004. 18 Liver Gluconeogenesis

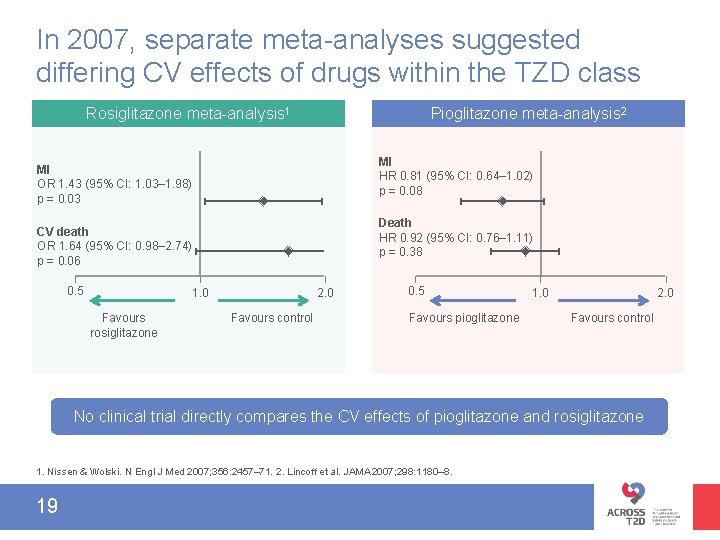
In 2007, separate meta-analyses suggested differing CV effects of drugs within the TZD class Rosiglitazone meta-analysis 1 Pioglitazone meta-analysis 2 MI HR 0. 81 (95% CI: 0. 64‒ 1. 02) p = 0. 08 MI OR 1. 43 (95% CI: 1. 03‒ 1. 98) p = 0. 03 Death HR 0. 92 (95% CI: 0. 76‒ 1. 11) p = 0. 38 CV death OR 1. 64 (95% CI: 0. 98‒ 2. 74) p = 0. 06 0. 5 1. 0 Favours rosiglitazone 2. 0 Favours control 0. 5 Favours pioglitazone 1. 0 2. 0 Favours control No clinical trial directly compares the CV effects of pioglitazone and rosiglitazone 1. Nissen & Wolski. N Engl J Med 2007; 356: 2457– 71. 2. Lincoff et al. JAMA 2007; 298: 1180– 8. 19

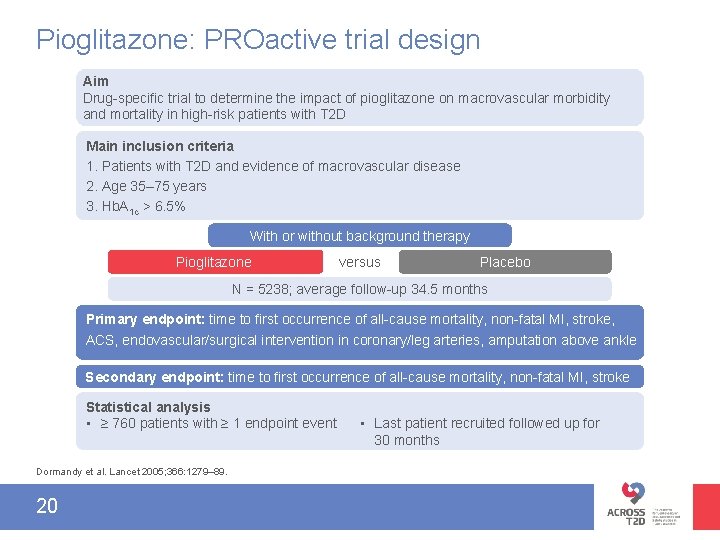
Pioglitazone: PROactive trial design Aim Drug-specific trial to determine the impact of pioglitazone on macrovascular morbidity and mortality in high-risk patients with T 2 D Main inclusion criteria 1. Patients with T 2 D and evidence of macrovascular disease 2. Age 35– 75 years 3. Hb. A 1 c > 6. 5% With or without background therapy Pioglitazone versus Placebo N = 5238; average follow-up 34. 5 months Primary endpoint: time to first occurrence of all-cause mortality, non-fatal MI, stroke, ACS, endovascular/surgical intervention in coronary/leg arteries, amputation above ankle Secondary endpoint: time to first occurrence of all-cause mortality, non-fatal MI, stroke Statistical analysis • ≥ 760 patients with ≥ 1 endpoint event Dormandy et al. Lancet 2005; 366: 1279– 89. 20 • Last patient recruited followed up for 30 months

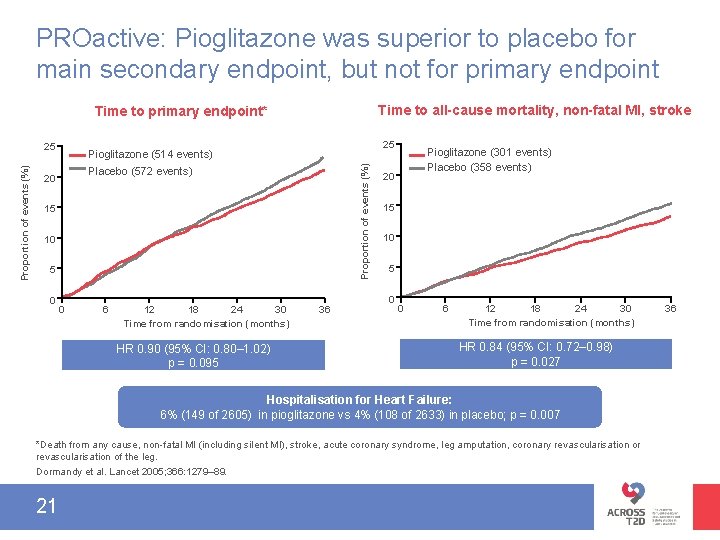
PROactive: Pioglitazone was superior to placebo for main secondary endpoint, but not for primary endpoint Time to all-cause mortality, non-fatal MI, stroke Time to primary endpoint* 25 Pioglitazone (514 events) Proportion of events (%) 25 Placebo (572 events) 20 15 10 5 0 0 6 12 18 24 30 Time from randomisation (months) HR 0. 90 (95% CI: 0. 80– 1. 02) p = 0. 095 36 Pioglitazone (301 events) Placebo (358 events) 20 15 10 5 0 0 6 12 18 24 30 Time from randomisation (months) HR 0. 84 (95% CI: 0. 72– 0. 98) p = 0. 027 Hospitalisation for Heart Failure: 6% (149 of 2605) in pioglitazone vs 4% (108 of 2633) in placebo; p = 0. 007 *Death from any cause, non-fatal MI (including silent MI), stroke, acute coronary syndrome, leg amputation, coronary revascularisation or revascularisation of the leg. Dormandy et al. Lancet 2005; 366: 1279– 89. 21 36

Rosiglitazone: RECORD trial design Aim Drug-specific trial to compare macrovascular morbidity and mortality in patients with T 2 D treated with rosiglitazone + metformin / SU Main inclusion criteria 1. Patients with T 2 D on maximum tolerated doses of metformin or SU monotherapy 2. Age 40– 75 years 3. BMI ≥ 25. 0 kg/m 2 OPEN-LABEL Rosiglitazone + metformin or Rosiglitazone + SU versus OPEN-LABEL Metformin + SU N = 4447; follow-up 5– 7 years Primary endpoint: Time to first occurrence of cardiovascular hospitalisation or cardiovascular death Time to first occurrence of CV hospitalisation or CV death Statistical analysis • Non-inferiority margin of 1. 20 for HR Home et al. Lancet 2009; 373: 2125– 35. 22 • 4000 participants followed for a median of 6 years to give 99% power

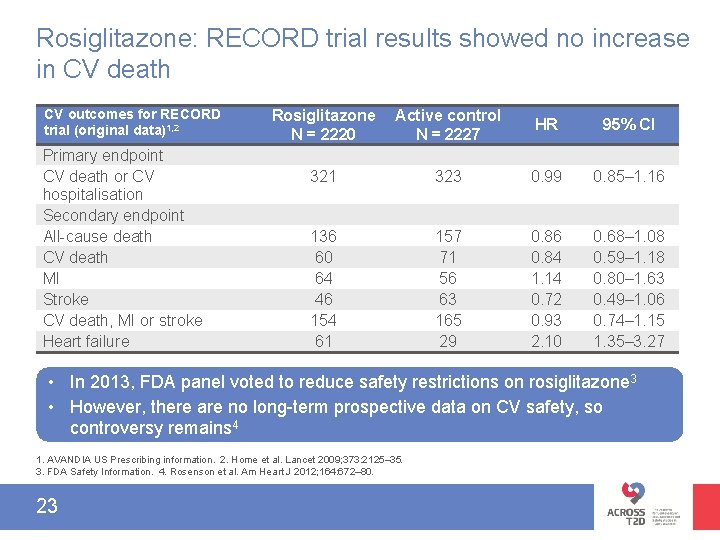
Rosiglitazone: RECORD trial results showed no increase in CV death CV outcomes for RECORD trial (original data)1, 2 Primary endpoint CV death or CV hospitalisation Secondary endpoint All-cause death CV death MI Stroke CV death, MI or stroke Heart failure Rosiglitazone N = 2220 Active control N = 2227 HR 95% CI 321 323 0. 99 0. 85– 1. 16 136 60 64 46 154 61 157 71 56 63 165 29 0. 86 0. 84 1. 14 0. 72 0. 93 2. 10 0. 68– 1. 08 0. 59– 1. 18 0. 80– 1. 63 0. 49– 1. 06 0. 74– 1. 15 1. 35– 3. 27 • In 2013, FDA panel voted to reduce safety restrictions on rosiglitazone 3 • However, there are no long-term prospective data on CV safety, so controversy remains 4 1. AVANDIA US Prescribing information. 2. Home et al. Lancet 2009; 373: 2125– 35. 3. FDA Safety Information. 4. Rosenson et al. Am Heart J 2012; 164: 672– 80. 23

Section recap CV safety of TZDs • TZDs cause or exacerbate heart failure in some patients 1 • CV meta-analyses in 2007 suggested differing effects on CV outcomes • Pioglitazone was associated with a significant 16% reduction in 3 P-MACE (as a secondary endpoint) vs placebo in PROactive 2 • Rosiglitazone open-label RECORD data showed no increase in CV death 1 • FDA reduced the safety restrictions on rosiglitazone imposed following 2007 meta-analysis 3 but controversy over CV safety remains ‘Within the PPAR family, there is no “class effect” and each agent must be considered unique. The FDA has mandated that each agent within this class be evaluated individually in a variety of ways including clinical outcome studies’ 4 1. AVANDIA US Prescribing information. 2. Dormandy et al. Lancet 2005; 366: 1279– 89. 3. FDA Safety Information. 4. Rosenson et al. Am Heart J. 2012; 164: 672– 80. 24

Metformi n CVOT interpretation FDA mandate 25 SU CV safety TZD

Adverse CV events led the FDA to require demonstration of CV safety for new glucose-lowering drugs 1961 UGDP trial: tolbutamide discontinued due to increased CV mortality vs other treatment groups 1 2005 Muraglitazar found to potentially increase CV risk during FDA assessment 2 • Sponsor withdrew application 1 2007 Rosiglitazone associated with increased risk for MI and CV-related death 3 • Withdrawn in the EU 1 • Use restricted in US 1* 2008 ACCORD trial: intensive glucose lowering was associated with increased all-cause mortality 4 *In 2013, FDA panel voted to reduce safety restrictions on rosiglitazone 7 HR 1. 22 (95% CI 1. 01‒ 1. 46); p = 0. 04 2008 2012 New FDA requirements 5 New EMA requirements 6 New diabetes drugs should demonstrate CV safety with meta-analysis and a CV outcome trial (CVOT) 1. Nissen. Ann Intern Med 2012; 157: 671– 2. 2. Nissen et al. JAMA 2005; 294: 2581– 6. 3. Nissen et al. N Engl J Med 2007; 356: 2457– 71. 4. ACCORD Study Group. N Engl J Med 2008; 358: 2545– 59. 5. http: //www. fda. gov/downloads/drugs/guidancecomplianceregulatoryinformation/% 20 guidances/ucm 071627. pdf 6. http: //www. ema. europa. eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC 500129256. pdf 7. http: //www. fda. gov/Safety/Med. Watch/Safety. Information/Safety. Alertsfor. Human. Medical. Products/ucm 376683. htm? source=govdelivery&utm_medium=email&utm_source=govdelivery 26

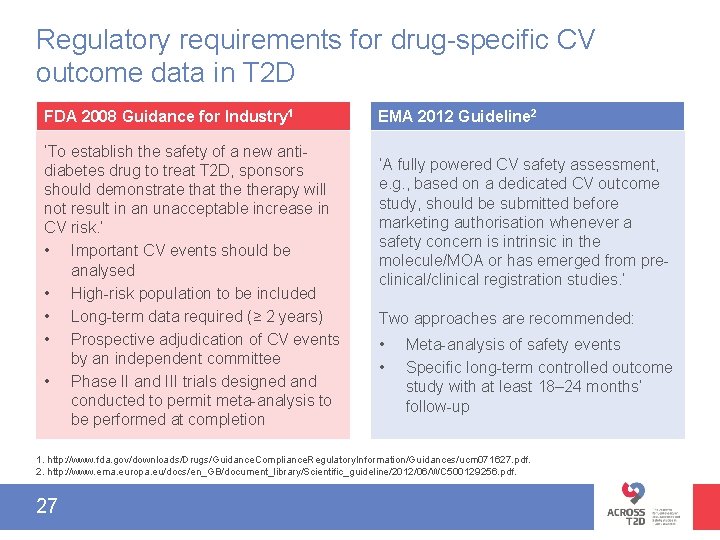
Regulatory requirements for drug-specific CV outcome data in T 2 D FDA 2008 Guidance for Industry 1 ‘To establish the safety of a new antidiabetes drug to treat T 2 D, sponsors should demonstrate that therapy will not result in an unacceptable increase in CV risk. ’ • Important CV events should be analysed • High-risk population to be included • Long-term data required (≥ 2 years) • Prospective adjudication of CV events by an independent committee • Phase II and III trials designed and conducted to permit meta-analysis to be performed at completion EMA 2012 Guideline 2 ‘A fully powered CV safety assessment, e. g. , based on a dedicated CV outcome study, should be submitted before marketing authorisation whenever a safety concern is intrinsic in the molecule/MOA or has emerged from preclinical/clinical registration studies. ’ Two approaches are recommended: • • Meta-analysis of safety events Specific long-term controlled outcome study with at least 18– 24 months’ follow-up 1. http: //www. fda. gov/downloads/Drugs/Guidance. Compliance. Regulatory. Information/Guidances/ucm 071627. pdf. 2. http: //www. ema. europa. eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC 500129256. pdf. 27

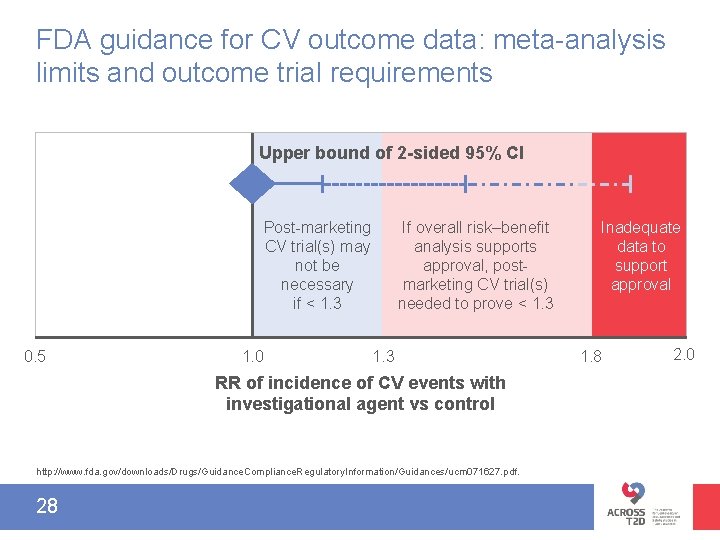
FDA guidance for CV outcome data: meta-analysis limits and outcome trial requirements Upper bound of 2 -sided 95% CI Post-marketing CV trial(s) may not be necessary if < 1. 3 0. 5 1. 0 If overall risk–benefit analysis supports approval, postmarketing CV trial(s) needed to prove < 1. 3 RR of incidence of CV events with investigational agent vs control http: //www. fda. gov/downloads/Drugs/Guidance. Compliance. Regulatory. Information/Guidances/ucm 071627. pdf. 28 Inadequate data to support approval 1. 8 2. 0

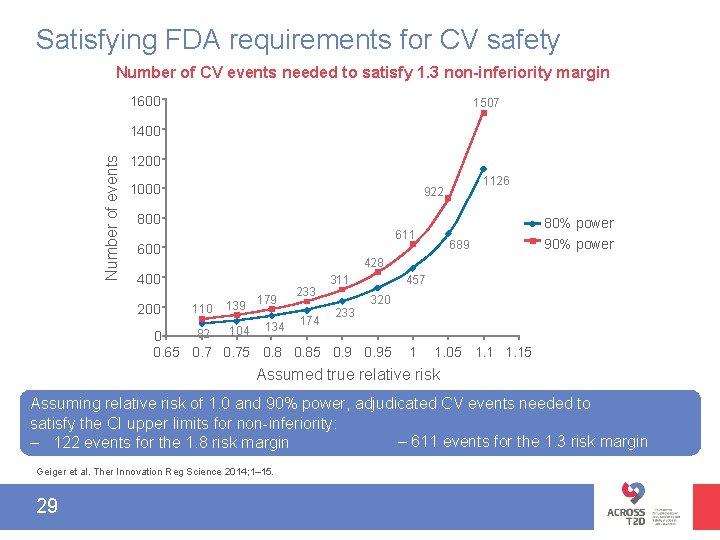
Satisfying FDA requirements for CV safety Number of CV events needed to satisfy 1. 3 non-inferiority margin 1600 1507 Number of events 1400 1200 1000 1126 922 800 80% power 611 600 689 90% power 428 400 200 110 139 179 134 233 174 457 311 233 320 104 82 0 0. 65 0. 75 0. 85 0. 95 1 1. 05 1. 15 Assumed true relative risk Assuming relative risk of 1. 0 and 90% power, adjudicated CV events needed to satisfy the CI upper limits for non-inferiority: – 611 events for the 1. 3 risk margin – 122 events for the 1. 8 risk margin Geiger et al. Ther Innovation Reg Science 2014; 1– 15. 29

Metformi n CVOT interpretation FDA mandate 30 SU CV safety TZD

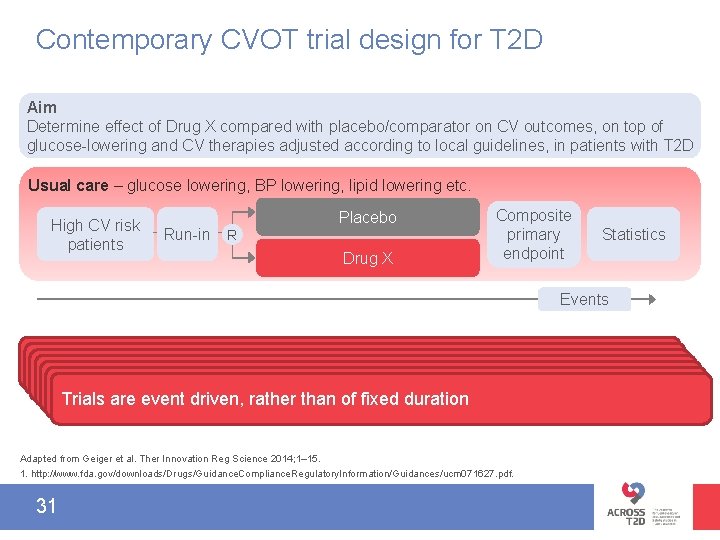
Contemporary CVOT trial design for T 2 D Aim Determine effect of Drug X compared with placebo/comparator on CV outcomes, on top of glucose-lowering and CV therapies adjusted according to local guidelines, in patients with T 2 D Usual care – glucose lowering, BP lowering, lipid lowering etc. High CV risk patients Run-in Placebo R Drug X Composite primary endpoint Statistics Events 1 FDA mandates that CV safety be demonstrated in high CV risk population All patients are on a usual care background (to control diabetes and CV risk Run-in helps to establish patient adherence to long-term treatment and FU As adjustment of background therapy is encouraged, CVOTs not designed to FDA recommends 3 P-MACE as primary CV endpoint or expanded factors); investigators encouraged to adjust therapy following local guidelines assess impact of a difference in Hb. A 1 Sequential statistical testing (superiority tested only if non-inferiority established) 1 c between study arms 4 P-MACE (e. g. , including hospitalisation for unstable angina pectoris) Trials are event driven, rather than of fixed duration Adapted from Geiger et al. Ther Innovation Reg Science 2014; 1– 15. 1. http: //www. fda. gov/downloads/Drugs/Guidance. Compliance. Regulatory. Information/Guidances/ucm 071627. pdf. 31

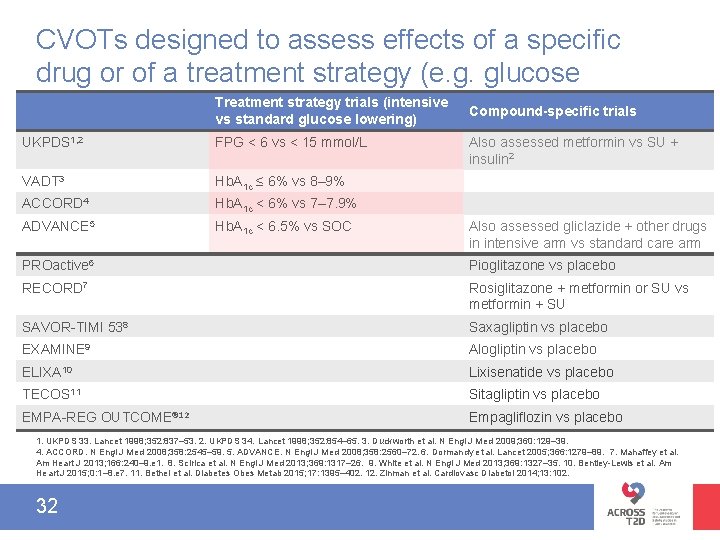
CVOTs designed to assess effects of a specific drug or of a treatment strategy (e. g. glucose Treatment strategy trials (intensive Compound-specific trials lowering) vs standard glucose lowering) UKPDS 1, 2 FPG < 6 vs < 15 mmol/L VADT 3 Hb. A 1 c ≤ 6% vs 8– 9% ACCORD 4 Hb. A 1 c < 6% vs 7– 7. 9% ADVANCE 5 Hb. A 1 c < 6. 5% vs SOC Also assessed metformin vs SU + insulin 2 Also assessed gliclazide + other drugs in intensive arm vs standard care arm PROactive 6 Pioglitazone vs placebo RECORD 7 Rosiglitazone + metformin or SU vs metformin + SU SAVOR-TIMI 538 Saxagliptin vs placebo EXAMINE 9 Alogliptin vs placebo ELIXA 10 Lixisenatide vs placebo TECOS 11 Sitagliptin vs placebo EMPA-REG OUTCOME® 12 Empagliflozin vs placebo 1. UKPDS 33. Lancet 1998; 352: 837– 53. 2. UKPDS 34. Lancet 1998; 352: 854– 65. 3. Duckworth et al. N Engl J Med 2009; 360: 129– 39. 4. ACCORD. N Engl J Med 2008; 358: 2545– 59. 5. ADVANCE. N Engl J Med 2008; 358: 2560– 72. 6. Dormandy et al. Lancet 2005; 366: 1279– 89. 7. Mahaffey et al. Am Heart J 2013; 166: 240– 9. e 1. 8. Scirica et al. N Engl J Med 2013; 369: 1317– 26. 9. White et al. N Engl J Med 2013; 369: 1327– 35. 10. Bentley-Lewis et al. Am Heart J 2015; 0: 1– 8. e 7. 11. Bethel et al. Diabetes Obes Metab 2015; 17: 1395– 402. 12. Zinman et al. Cardiovasc Diabetol 2014; 13: 102. 32

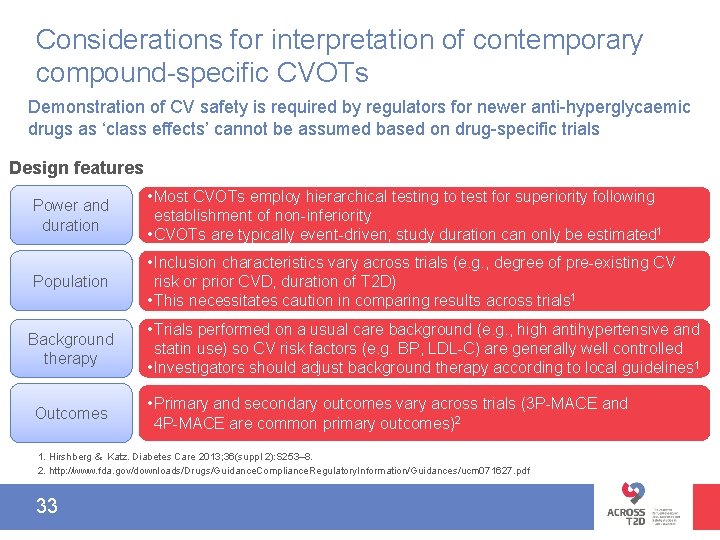
Considerations for interpretation of contemporary compound-specific CVOTs Demonstration of CV safety is required by regulators for newer anti-hyperglycaemic drugs as ‘class effects’ cannot be assumed based on drug-specific trials Design features Power and duration • Most CVOTs employ hierarchical testing to test for superiority following establishment of non-inferiority • CVOTs are typically event-driven; study duration can only be estimated 1 Population • Inclusion characteristics vary across trials (e. g. , degree of pre-existing CV risk or prior CVD, duration of T 2 D) • This necessitates caution in comparing results across trials 1 Background therapy Outcomes • Trials performed on a usual care background (e. g. , high antihypertensive and statin use) so CV risk factors (e. g. BP, LDL-C) are generally well controlled • Investigators should adjust background therapy according to local guidelines 1 • Primary and secondary outcomes vary across trials (3 P-MACE and 4 P-MACE are common primary outcomes)2 1. Hirshberg & Katz. Diabetes Care 2013; 36(suppl 2): S 253– 8. 2. http: //www. fda. gov/downloads/Drugs/Guidance. Compliance. Regulatory. Information/Guidances/ucm 071627. pdf. 33

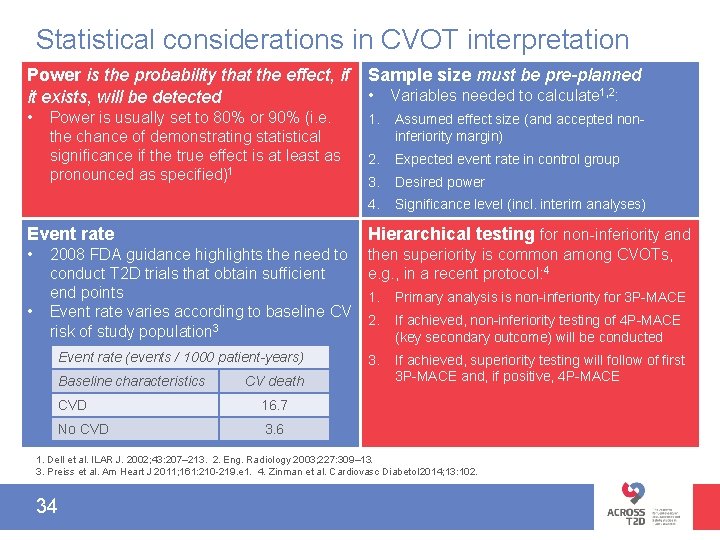
Statistical considerations in CVOT interpretation Power is the probability that the effect, if it exists, will be detected Sample size must be pre-planned • 1. Assumed effect size (and accepted noninferiority margin) 2. Expected event rate in control group 3. Desired power 4. Significance level (incl. interim analyses) Power is usually set to 80% or 90% (i. e. the chance of demonstrating statistical significance if the true effect is at least as pronounced as specified)1 • Variables needed to calculate 1, 2: Event rate Hierarchical testing for non-inferiority and • then superiority is common among CVOTs, e. g. , in a recent protocol: 4 • 2008 FDA guidance highlights the need to conduct T 2 D trials that obtain sufficient end points Event rate varies according to baseline CV risk of study population 3 Event rate (events / 1000 patient-years) Baseline characteristics CV death CVD 16. 7 No CVD 3. 6 1. Primary analysis is non-inferiority for 3 P-MACE 2. If achieved, non-inferiority testing of 4 P-MACE (key secondary outcome) will be conducted 3. If achieved, superiority testing will follow of first 3 P-MACE and, if positive, 4 P-MACE 1. Dell et al. ILAR J. 2002; 43: 207– 213. 2. Eng. Radiology 2003; 227: 309– 13. 3. Preiss et al. Am Heart J 2011; 161: 210 -219. e 1. 4. Zinman et al. Cardiovasc Diabetol 2014; 13: 102. 34

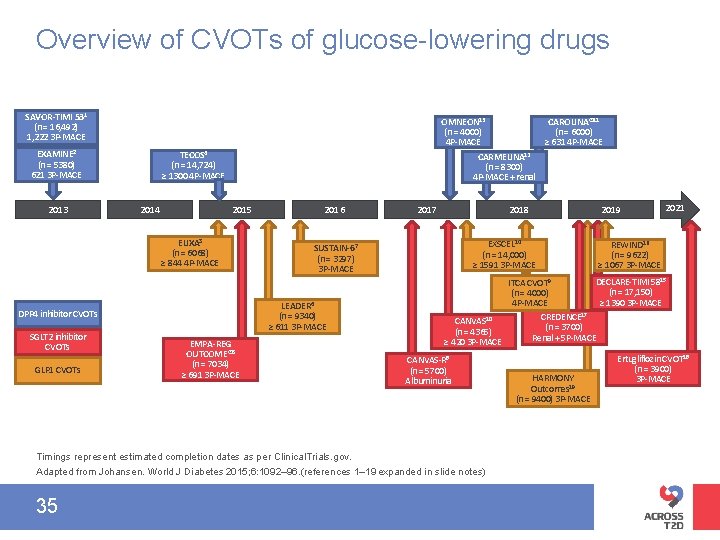
Overview of CVOTs of glucose-lowering drugs SAVOR-TIMI 531 (n = 16, 492) 1, 222 3 P-MACE EXAMINE 2 (n = 5380) 621 3 P-MACE 2013 OMNEON 13 CAROLINA® 11 (n = 4000) (n = 6000) 4 P-MACE ≥ 631 4 P-MACE CARMELINA 12 (n = 8300) 4 P-MACE + renal TECOS 4 (n = 14, 724) ≥ 1300 4 P-MACE 2014 2015 ELIXA 3 (n = 6068) ≥ 844 4 P-MACE GLP 1 CVOTs EMPA-REG OUTCOME® 5 (n = 7034) ≥ 691 3 P-MACE 2017 2018 EXSCEL 14 (n = 14, 000) ≥ 1591 3 P-MACE SUSTAIN-67 (n = 3297) 3 P-MACE LEADER 6 (n = 9340) ≥ 611 3 P-MACE DPP 4 inhibitor CVOTs SGLT 2 inhibitor CVOTs 2016 2021 REWIND 16 (n = 9622) ≥ 1067 3 P-MACE DECLARE-TIMI 5815 ITCA CVOT 9 (n = 17, 150) (n = 4000) ≥ 1390 3 P-MACE 4 P-MACE CREDENCE 17 CANVAS 10 (n = 3700) (n = 4365) Renal + 5 P-MACE ≥ 420 3 P-MACE CANVAS-R 8 (n = 5700) Albuminuria Timings represent estimated completion dates as per Clinical. Trials. gov. Adapted from Johansen. World J Diabetes 2015; 6: 1092– 96. (references 1– 19 expanded in slide notes) 35 2019 HARMONY Outcomes 19 (n = 9400) 3 P-MACE Ertugliflozin CVOT 18 (n = 3900) 3 P-MACE

Module C: Summary • Metformin exerts various CV effects – There is some evidence to suggest a CV benefit but there is a paucity of evidence from long-term CVOTs 1 • There is limited evidence evaluating the CV safety of profile of SUs 2, 3 – There is a need for evaluation of the CV safety of SUs in a long-term dedicated CVOT • The long-term CV safety profile of drugs within the TZD class remains controversial, emphasising the need for compounds within the same class to demonstrate CV safety 4 • The 2008 FDA guidance requires that new diabetes drugs demonstrate CV safety via meta-analysis and CVOT 5 • There are many considerations in interpreting the results of contemporary drug-specific CVOTs designed to assess the effect of a specific drug, added to background usual care, on MACE 6 1. Boussageaon et al. PLo. S Med. 2012; 9: e 1001204. 2. UKPDS 34. Lancet 1998; 352: : 837– 53. 3. ADVANCE. New Engl J Med 2008; 358: 2560– 72. 4. Rosenson et al. Am Heart J. 2012; 164: 672– 80. 5. FDA Guidance for Industry. 6. http: //www. fda. gov/downloads/Drugs/Guidance. Compliance. Regulatory. Information/Guidances/ucm 071627. pdf. 36