Electrochemistry Applications of Redox Review l Oxidation reduction










- Slides: 30

Electrochemistry Applications of Redox

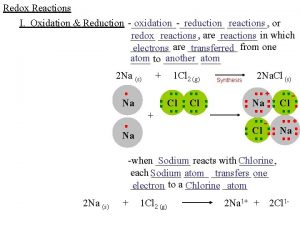
Review l Oxidation reduction reactions involve a transfer of electrons. l OIL- RIG l Oxidation Involves Gain l Reduction Involves Loss l LEO-GER l Lose Electrons Oxidation l Gain Electrons Reduction

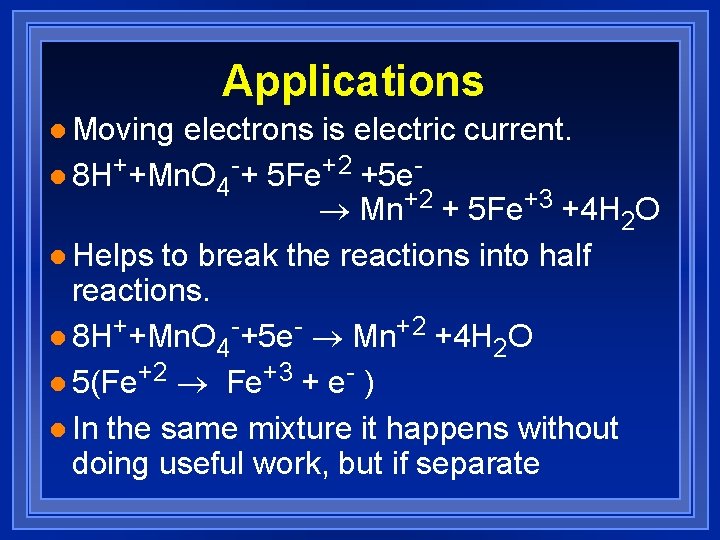
Applications l Moving electrons is electric current. l 8 H++Mn. O 4 -+ 5 Fe+2 +5 e® Mn+2 + 5 Fe+3 +4 H 2 O l Helps to break the reactions into half reactions. l 8 H++Mn. O 4 -+5 e- ® Mn+2 +4 H 2 O l 5(Fe+2 ® Fe+3 + e- ) l In the same mixture it happens without doing useful work, but if separate

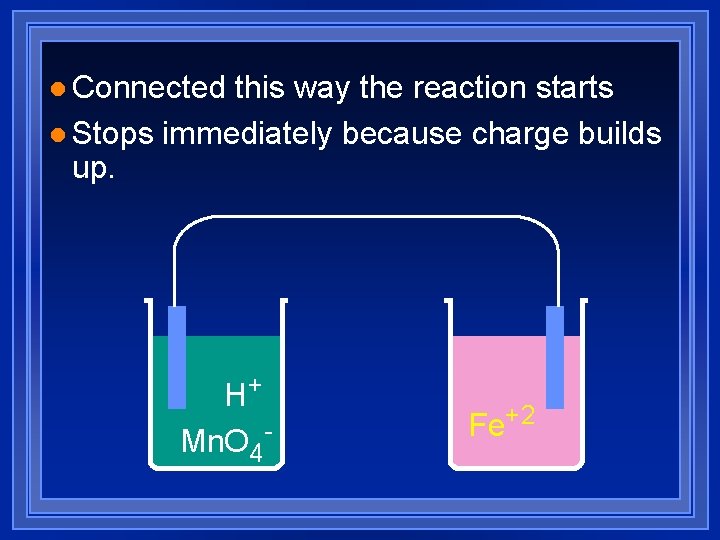
l Connected this way the reaction starts l Stops immediately because charge builds up. H+ Mn. O 4 - Fe+2

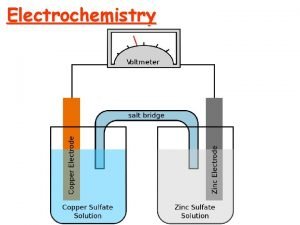
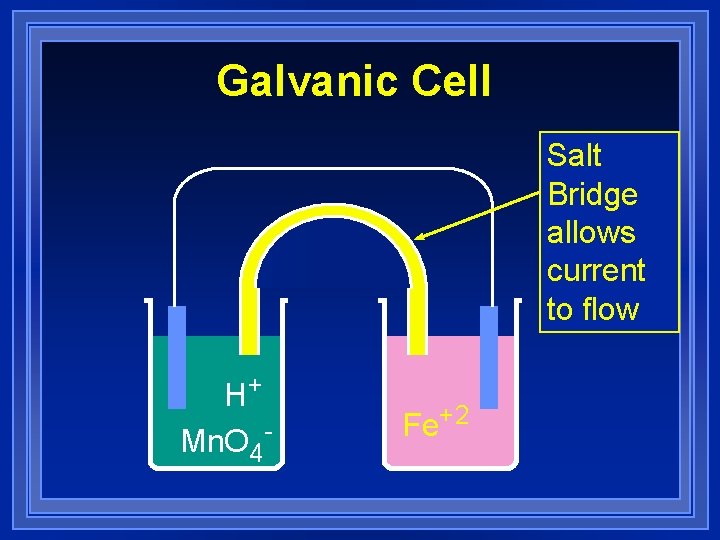
Galvanic Cell Salt Bridge allows current to flow H+ Mn. O 4 - Fe+2

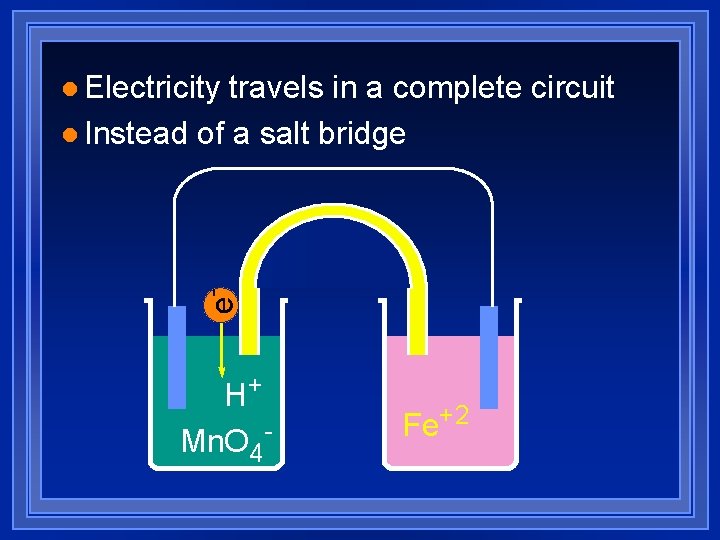
travels in a complete circuit l Instead of a salt bridge e- l Electricity H+ Mn. O 4 - Fe+2

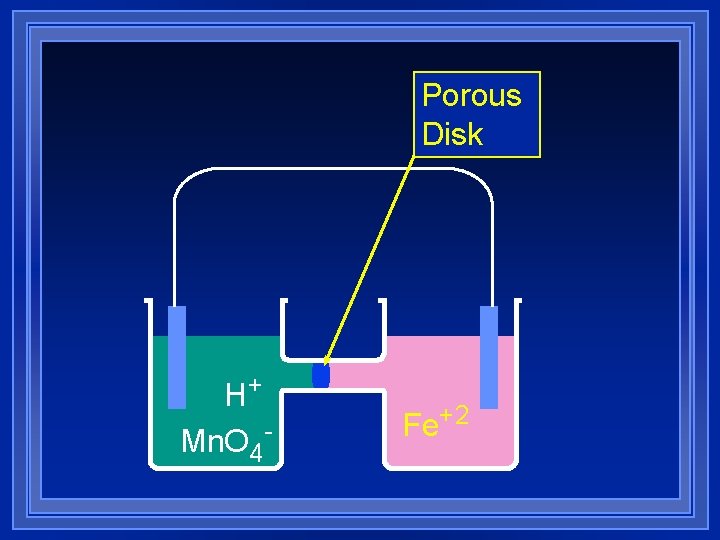
Porous Disk H+ Mn. O 4 - Fe+2

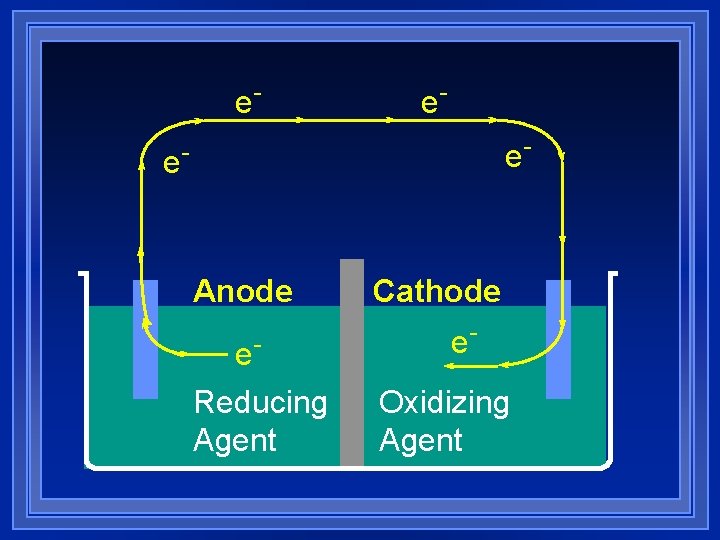
e- e- Anode e. Reducing Agent Cathode e. Oxidizing Agent

Cell Potential l Reducing agent pushes the electron. l Oxidizing agent pulls the electron. l The push or pull (“driving force”) is called the cell potential Ecell l Also called the electromotive force (emf) l Unit is the volt(V) l = 1 joule of work/coulomb of charge l Measured with a voltmeter

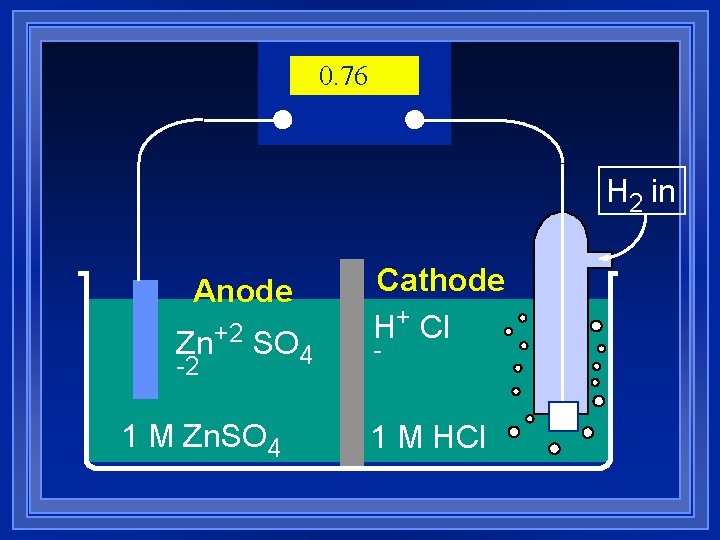
0. 76 H 2 in Anode Zn+2 SO 4 -2 1 M Zn. SO 4 Cathode H+ Cl - 1 M HCl

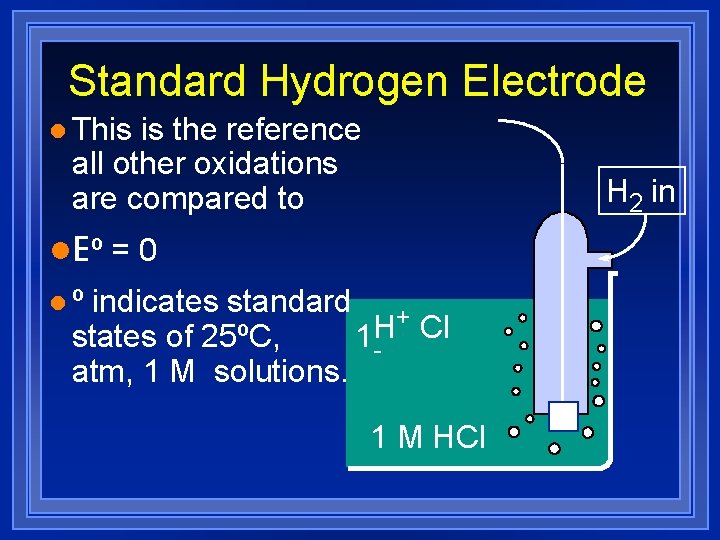
Standard Hydrogen Electrode l This is the reference all other oxidations are compared to H 2 in l. E º = 0 lº indicates standard + Cl states of 25ºC, 1 H atm, 1 M solutions. 1 M HCl

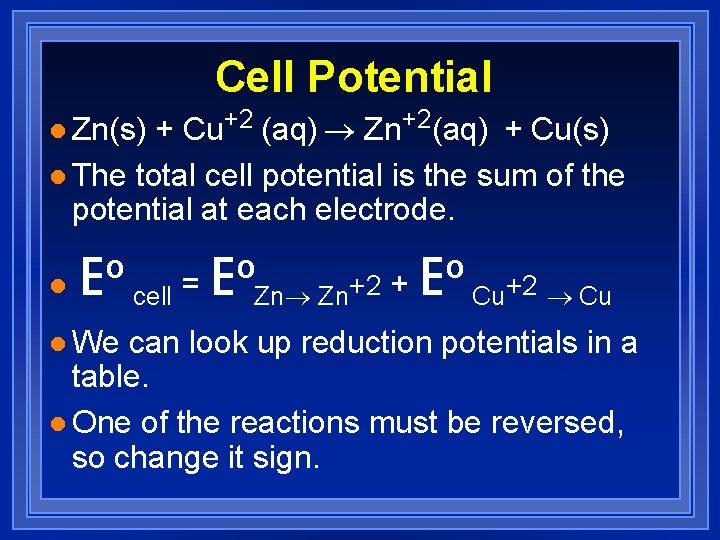
Cell Potential + Cu+2 (aq) ® Zn+2(aq) + Cu(s) l The total cell potential is the sum of the potential at each electrode. l Zn(s) l Eº cell = EºZn® Zn+2 + Eº Cu+2 ® Cu l We can look up reduction potentials in a table. l One of the reactions must be reversed, so change it sign.

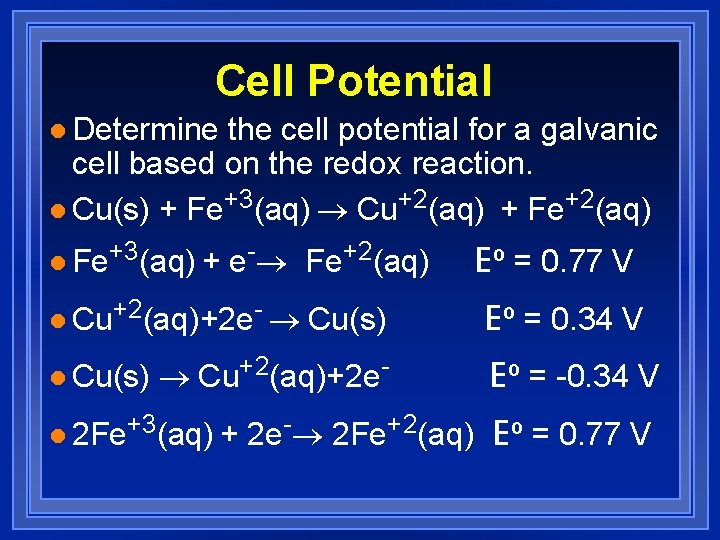
Cell Potential l Determine the cell potential for a galvanic cell based on the redox reaction. l Cu(s) + Fe+3(aq) ® Cu+2(aq) + Fe+2(aq) l Fe+3(aq) + e-® Fe+2(aq) Eº = 0. 77 V l Cu+2(aq)+2 e- ® Cu(s) Eº = 0. 34 V l Cu(s) ® Cu+2(aq)+2 e. Eº = -0. 34 V l 2 Fe+3(aq) + 2 e-® 2 Fe+2(aq) Eº = 0. 77 V

Line Notation solid½Aqueous½solid l Anode on the left½½Cathode on the right l Single line different phases. l Double line porous disk or salt bridge. l If all the substances on one side are aqueous, a platinum electrode is indicated. l For the last reaction l Cu(s)½Cu+2(aq)½½Fe+2(aq), Fe+3(aq)½Pt(s) l

Galvanic Cell l l 1) 2) 3) 4) The reaction always runs spontaneously in the direction that produced a positive cell potential. Four things for a complete description. Cell Potential Direction of flow Designation of anode and cathode Nature of all the componentselectrodes and ions

Practice l Completely describe the galvanic cell based on the following half-reactions under standard conditions. l Mn. O 4 - + 8 H+ +5 e- ® Mn+2 + 4 H 2 O Eº=1. 51 l Fe+3 +3 e- ® Fe(s) Eº=0. 036 V

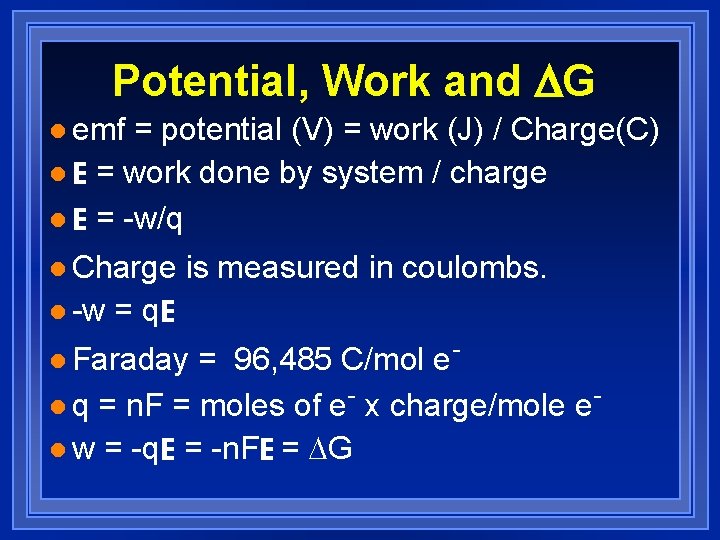
Potential, Work and DG l emf = potential (V) = work (J) / Charge(C) l E = work done by system / charge l E = -w/q l Charge l -w is measured in coulombs. = q. E = 96, 485 C/mol el q = n. F = moles of e- x charge/mole el w = -q. E = -n. FE = DG l Faraday

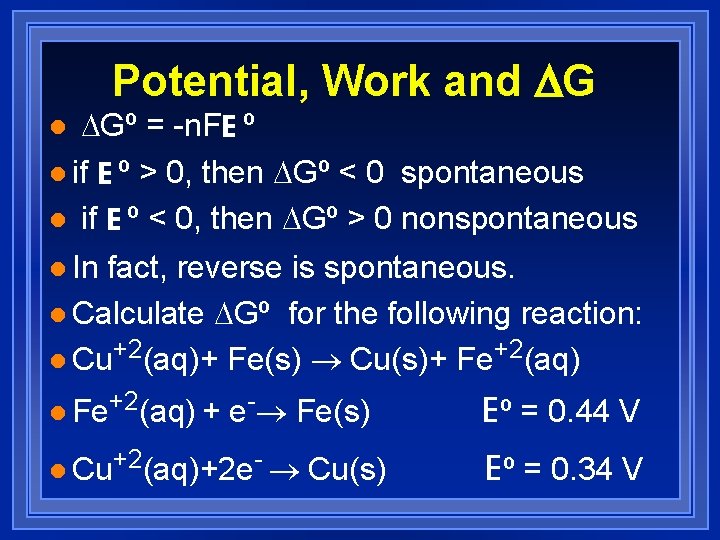
Potential, Work and DG DGº = -n. FE º l if E º > 0, then DGº < 0 spontaneous l if E º < 0, then DGº > 0 nonspontaneous l l In fact, reverse is spontaneous. l Calculate DGº for the following reaction: l Cu+2(aq)+ Fe(s) ® Cu(s)+ Fe+2(aq) l Fe+2(aq) + e-® Fe(s) l Cu+2(aq)+2 e- ® Cu(s) Eº = 0. 44 V Eº = 0. 34 V

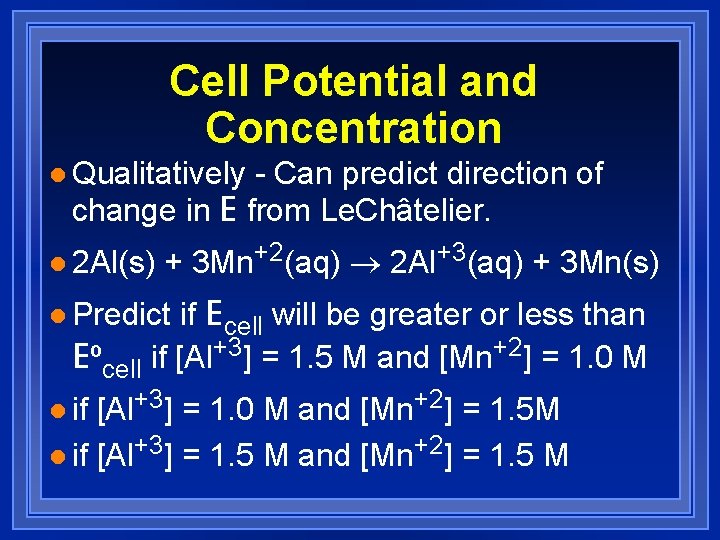
Cell Potential and Concentration l Qualitatively - Can predict direction of change in E from Le. Châtelier. l 2 Al(s) + 3 Mn+2(aq) ® 2 Al+3(aq) + 3 Mn(s) if Ecell will be greater or less than Eºcell if [Al+3] = 1. 5 M and [Mn+2] = 1. 0 M l Predict [Al+3] = 1. 0 M and [Mn+2] = 1. 5 M l if [Al+3] = 1. 5 M and [Mn+2] = 1. 5 M l if

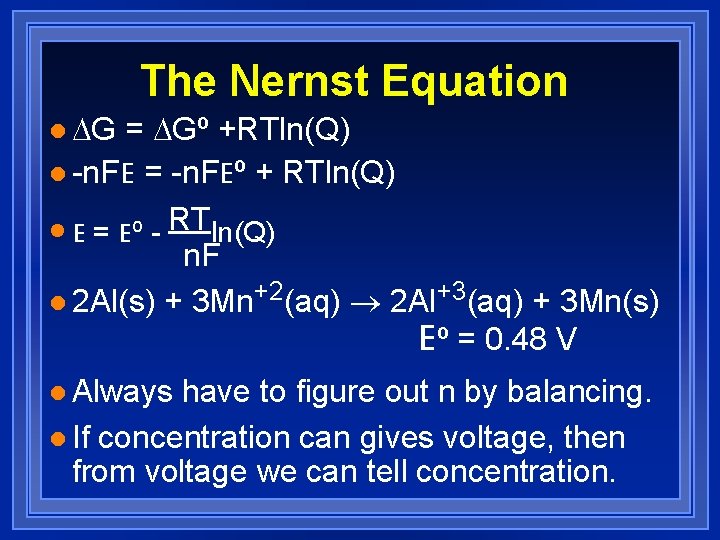
The Nernst Equation l DG = DGº +RTln(Q) l -n. FE = -n. FEº + RTln(Q) l E = Eº - RTln(Q) n. F l 2 Al(s) + 3 Mn+2(aq) ® 2 Al+3(aq) + 3 Mn(s) Eº = 0. 48 V l Always have to figure out n by balancing. l If concentration can gives voltage, then from voltage we can tell concentration.

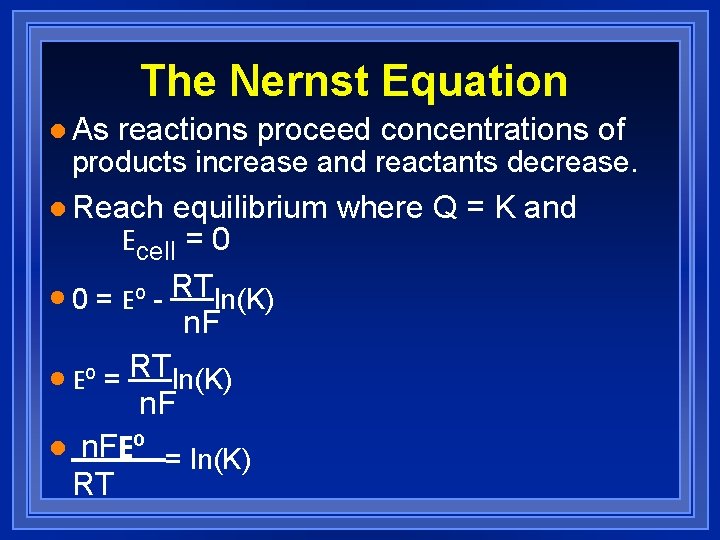
The Nernst Equation l As reactions proceed concentrations of products increase and reactants decrease. l Reach equilibrium where Q = K and Ecell = 0 l 0 = Eº - RTln(K) n. F l Eº = RTln(K) n. F l n. FEº = ln(K) RT

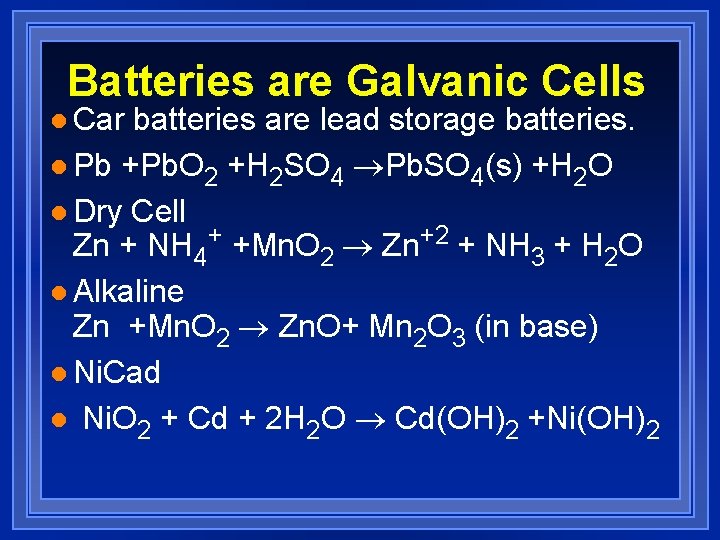
Batteries are Galvanic Cells l Car batteries are lead storage batteries. l Pb +Pb. O 2 +H 2 SO 4 ®Pb. SO 4(s) +H 2 O l Dry Cell Zn + NH 4+ +Mn. O 2 ® Zn+2 + NH 3 + H 2 O l Alkaline Zn +Mn. O 2 ® Zn. O+ Mn 2 O 3 (in base) l Ni. Cad l Ni. O 2 + Cd + 2 H 2 O ® Cd(OH)2 +Ni(OH)2

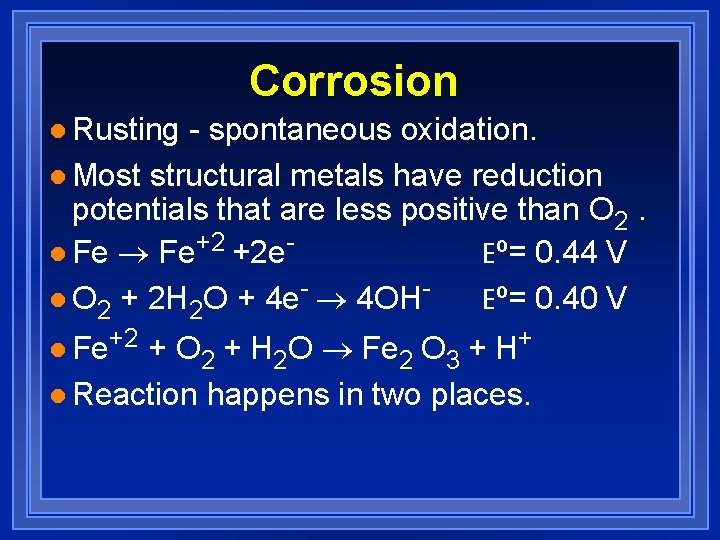
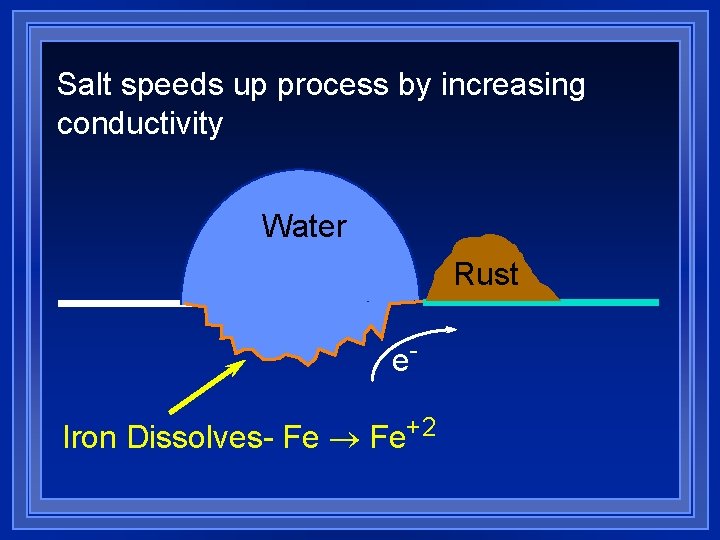
Corrosion l Rusting - spontaneous oxidation. l Most structural metals have reduction potentials that are less positive than O 2. l Fe ® Fe+2 +2 e. Eº= 0. 44 V l O 2 + 2 H 2 O + 4 e- ® 4 OHEº= 0. 40 V l Fe+2 + O 2 + H 2 O ® Fe 2 O 3 + H+ l Reaction happens in two places.

Salt speeds up process by increasing conductivity Water Rust e. Iron Dissolves- Fe ® Fe+2

Preventing Corrosion l Coating to keep out air and water. l Galvanizing - Putting on a zinc coat l Has a lower reduction potential, so it is more. easily oxidized. l Alloying with metals that form oxide coats. l Cathodic Protection - Attaching large pieces of an active metal like magnesium that get oxidized instead.

Electrolysis l Running a galvanic cell backwards. l Put a voltage bigger than the potential and reverse the direction of the redox reaction. l Used for electroplating.

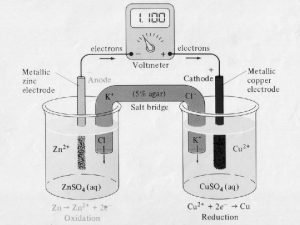
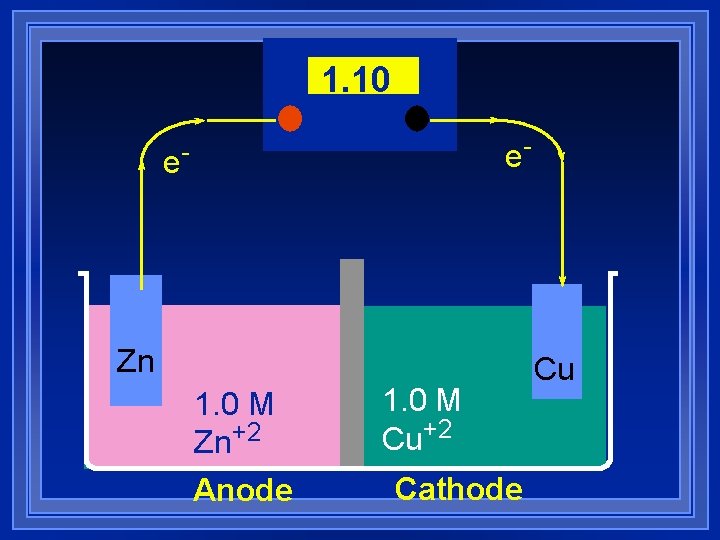
1. 10 e- e- Zn 1. 0 M Zn+2 Anode 1. 0 M Cu+2 Cathode Cu

e- A battery >1. 10 V Zn 1. 0 M Zn+2 Cathode 1. 0 M Cu+2 e- Cu Anode

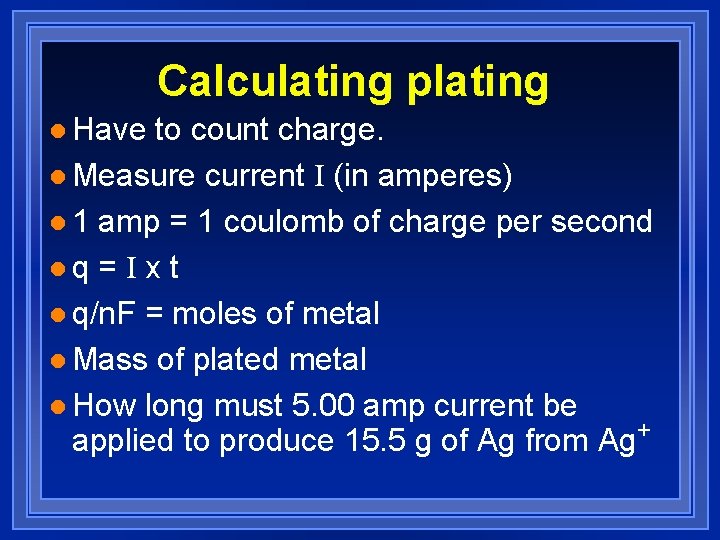
Calculating plating l Have to count charge. l Measure current I (in amperes) l 1 amp = 1 coulomb of charge per second lq = I x t l q/n. F = moles of metal l Mass of plated metal l How long must 5. 00 amp current be applied to produce 15. 5 g of Ag from Ag+

Other uses l Electroysis of water. l Seperating mixtures of ions. l More positive reduction potential means the reaction proceeds forward. l We want the reverse. l Most negative reduction potential is easiest to plate out of solution.
 Chapter 19 review oxidation-reduction reactions
Chapter 19 review oxidation-reduction reactions Chapter 19 review oxidation reduction reactions answers
Chapter 19 review oxidation reduction reactions answers Locos bird's beak
Locos bird's beak Cathode and anode reduction and oxidation
Cathode and anode reduction and oxidation Difference between oxidation number and charge
Difference between oxidation number and charge Oxidation-reduction quiz
Oxidation-reduction quiz Topic 19
Topic 19 Oxidation reduction leo ger
Oxidation reduction leo ger Examples of redox reaction
Examples of redox reaction Chapter 20 worksheet redox
Chapter 20 worksheet redox Balancing redox reactions
Balancing redox reactions Nernst equation simplified
Nernst equation simplified Milady chapter 20 vocabulary
Milady chapter 20 vocabulary Leo the lion says ger
Leo the lion says ger Corrosion
Corrosion Oxidation–reduction reactions
Oxidation–reduction reactions Leo says ger
Leo says ger Oxidation–reduction reactions
Oxidation–reduction reactions Oxidation reduction
Oxidation reduction Chemistry
Chemistry Oxidation
Oxidation Red cat an ox chemistry
Red cat an ox chemistry Oxidation number rukes
Oxidation number rukes 2kclo3 2kcl 3o2 oxidation and reduction
2kclo3 2kcl 3o2 oxidation and reduction Cathode oxidation or reduction
Cathode oxidation or reduction Aredox
Aredox Oxidation reduction reactions
Oxidation reduction reactions Self oxidation reduction reaction of aldehyde
Self oxidation reduction reaction of aldehyde Explain oxidation
Explain oxidation How to identify reducing and oxidizing agents
How to identify reducing and oxidizing agents Topic 9 oxidation-reduction
Topic 9 oxidation-reduction