DRUGELUTING STENTS WHICH AND TO WHOM Enrico Romagnoli


























- Slides: 52

DRUG-ELUTING STENTS: WHICH AND TO WHOM? Enrico Romagnoli MD, Ph. D Interventional Cardiology Unit, San Raffaele Hospital, Milan Torino 14 Novembre 2007

DES & restenosis Drug-eluting stents represent a major advance for reduction of restenosis Drug-eluting stents consistently have been shown to reduce the amount of late lumen loss in months following implantation represent a major advance for reduction of restenosis, compared to bare metal stent This difference translated in a marked reduction in restenosis rate and need for target vessel revascularization in randomized controlled trials

DES caveat ü Early DES trials were performed in lowrisk population ü End-points (usually late loss) were considered as the surrogate of clinical events ü Routine Angiographic follow-up not clinically driven ü DES versus BMS of similar design (not necessarily the best BMS)

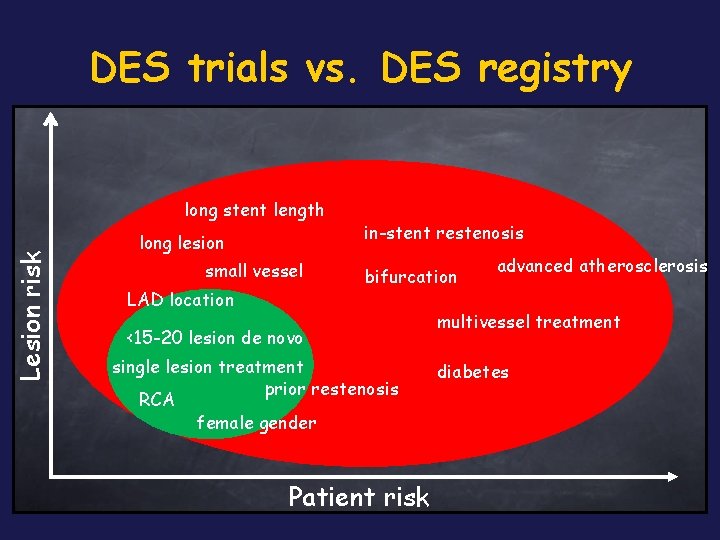
DES trials vs. DES registry Lesion risk long stent length long lesion small vessel LAD location in-stent restenosis bifurcation <15 -20 lesion de novo single lesion treatment prior restenosis RCA female gender Patient risk advanced atherosclerosis multivessel treatment diabetes

DES caveat ü Early DES trials were performed in lowrisk population ü End-points (usually late loss) were considered as the surrogate of clinical events ü Routine Angiographic follow-up not clinically driven ü DES versus BMS of similar design (not necessarily the best BMS)

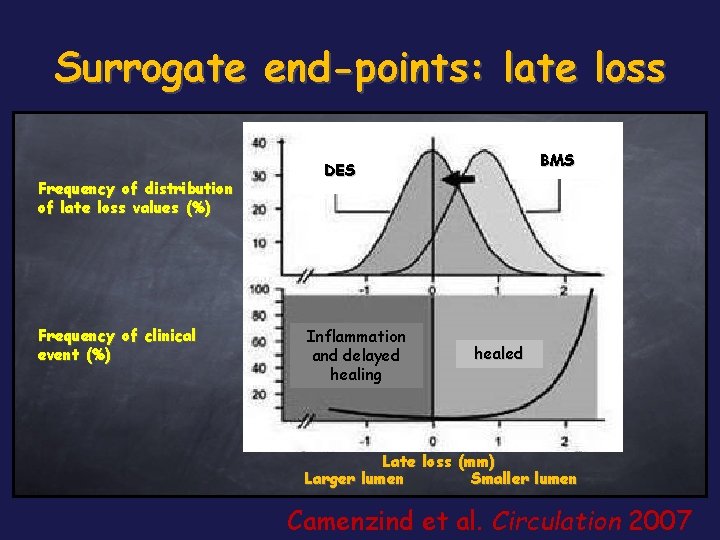
Surrogate end-points: late loss Frequency of distribution of late loss values (%) Frequency of clinical event (%) BMS DES Inflammation and delayed healing healed Late loss (mm) Larger lumen Smaller lumen Camenzind et al. Circulation 2007

Surrogate end-points: late loss There is no evidence of correlation between angiographic late lumen loss and clinical events The use of late loss as an end point tended to overestimate the difference in restenosis, and the derived effect seemed to be overemphasized with respect to the real risk. We don’t know the real clinical effect of late loss and its reliability as an end-point in direct comparative drug-eluting stent trials Agostoni et al. Am J Cardiol. 2006

DES caveat ü Early DES trials were performed in lowrisk population ü End-points (usually late loss) were considered as the surrogate of clinical events ü Routine Angiographic follow-up not clinically driven ü DES versus BMS of similar design (not necessarily the best BMS)

DES caveat ü Early DES trials were performed in lowrisk population ü End-points (usually late loss) were considered as the surrogate of clinical events ü Routine Angiographic follow-up not clinically driven ü DES versus BMS of similar design (not necessarily the best BMS)

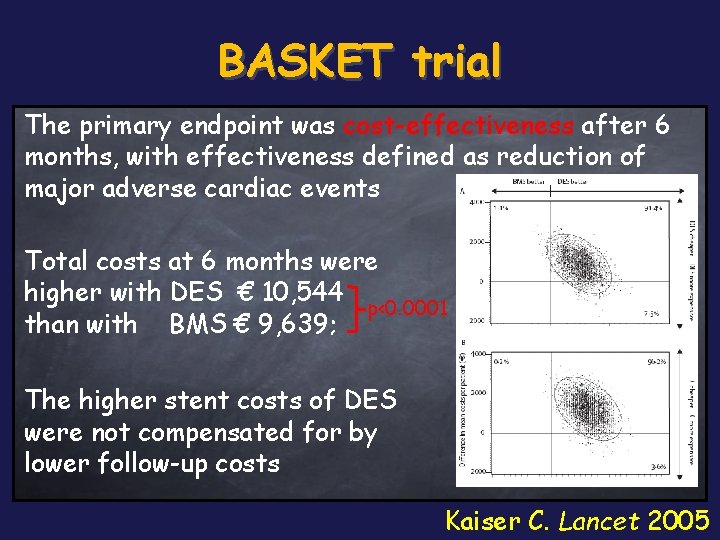
BASKET trial The primary endpoint was cost-effectiveness after 6 months, with effectiveness defined as reduction of major adverse cardiac events Total costs at 6 months were higher with DES € 10, 544 p<0. 0001 than with BMS € 9, 639; The higher stent costs of DES were not compensated for by lower follow-up costs Kaiser C. Lancet 2005

BASKET trial: 18 -month analysis In a real-world setting, use of DES in all patients is less cost effective than in studies with selected patients. Use of these stents could be restricted to patients in high-risk groups Brunner-La Rocca Lancet 2007

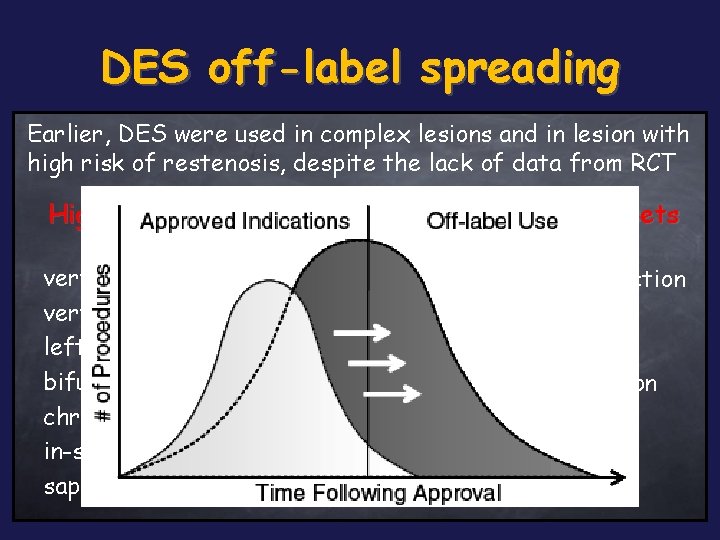
DES off-label spreading Earlier, DES were used in complex lesions and in lesion with high risk of restenosis, despite the lack of data from RCT High risk lesion subsets High risk patient subsets very small vessel/stent ! very long lesion ! left main disease bifurcation lesions chronic total occlusion in-stent restenosis !! saphenous vein graft acute myocardial infarction multivessel disease diabetics !!! severe LVEF dysfunction renal dysfunction

Off-label DES use ~80% off-label use of these devices in the real world” 18, 295 lesions/15, 157 patients EDITORIAL S Kaul, GA Diamond. www. cardiosource. com

Are DES adverse event rates increased with off-label use? EVENT registry N = 3, 323 NSTEMI with SES and PES, 55% off-label Off-label: 1. 6% On-label: 0. 9% P<0. 001 Off-label: 17. 5% On-label: 8. 9% Win HK JAMA 2007

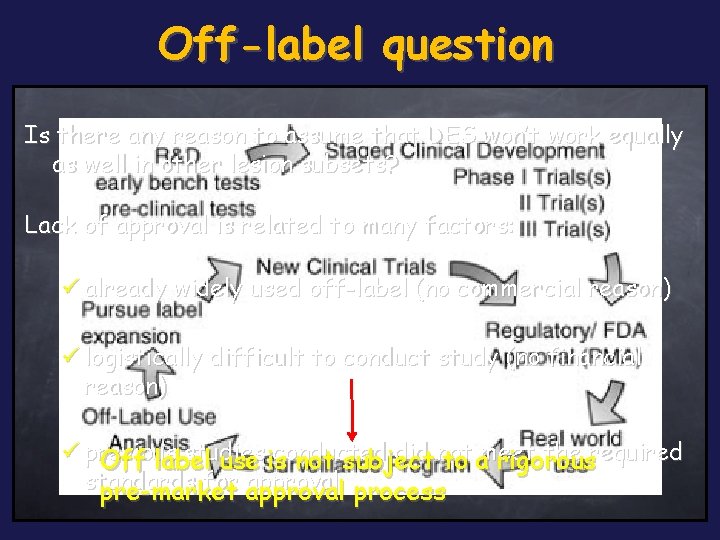
Off-label question Is there any reason to assume that DES won’t work equally as well in other lesion subsets? Lack of approval is related to many factors: ü already widely used off-label (no commercial reason) ü logistically difficult to conduct study (no financial reason) ü previous studies conducted did not the required Off label use is not subject to ameet rigorous standards for approval process pre-market

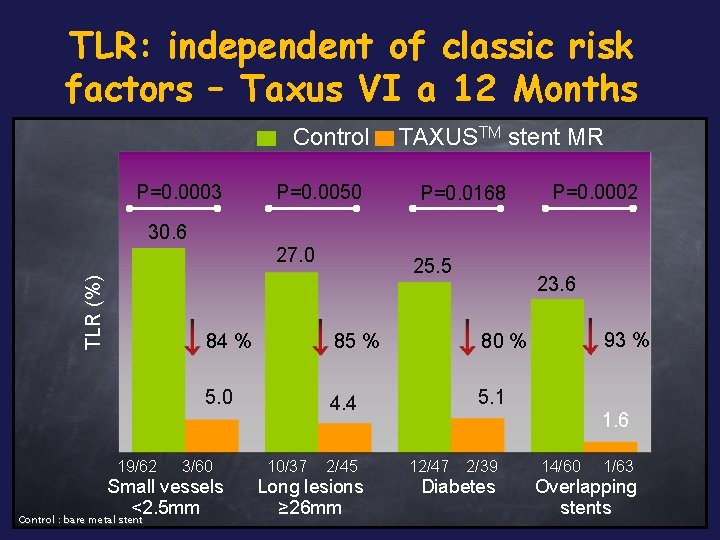
TLR: independent of classic risk factors – Taxus VI a 12 Months Control P=0. 0003 P=0. 0050 TAXUSTM stent MR P=0. 0168 P=0. 0002 30. 6 TLR (%) 27. 0 19/62 23. 6 84 % 85 % 80 % 5. 0 4. 4 5. 1 3/60 Small vessels <2. 5 mm Control : bare metal stent 25. 5 10/37 2/45 Long lesions ≥ 26 mm 93 % 1. 6 12/47 2/39 Diabetes 14/60 1/63 Overlapping stents

2006 DES storm

SCAAR registry Cumulative Risk 0. 10 0. 0 8 3 -year mortality increased 18% with DES 0. 0 6 0. 0 4 0. 0 2 0. 0 0 BMS DES RR: 1. 03(0. 84 -1. 26) RR: 1. 32 (1. 11 -1. 57) 0 0. 5 Time (years) 1. 0 1. 5 2. 0 2. 5 BMS 12880 12473 12354 12213 9298 5960 DES 5770 5604 5541 5468 3434 1776 Lagerqvist NEJM 2007 356; 1009 -19

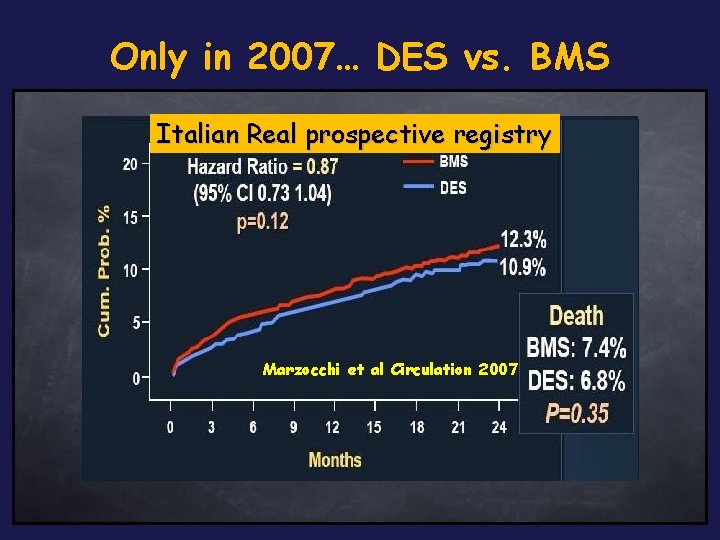
Only in 2007… DES vs. BMS Italian Real prospective registry DES in Ontario Stent registry 4. 9% 7. 1% P = 0. 03 Jensen LO JACC 2007 Wake forest experience Marzocchi et al Circulation 2007 Applegate AJC Western Denmark registry 2007 Tu JV NEJM 2007

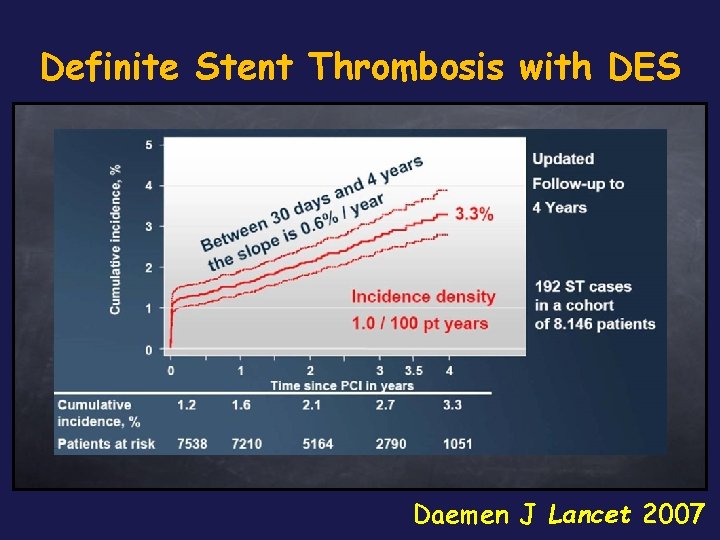
Definite Stent Thrombosis with DES Daemen J Lancet 2007

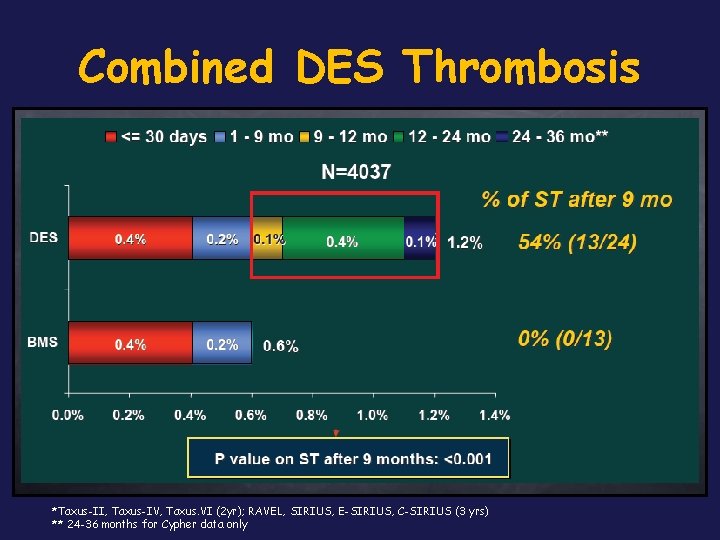
Combined DES Thrombosis *Taxus-II, Taxus-IV, Taxus. VI (2 yr); RAVEL, SIRIUS, E-SIRIUS, C-SIRIUS (3 yrs) ** 24 -36 months for Cypher data only

DES “a chi”? • Thesis: TLR reduction with no beneficial effect on death and re-infarction was observed in RCT • Antithesis: there are still concerns regarding generalization of DES to the population-at-large (potentially armful) • Synthesis: do we have to apply evidence based medicine?

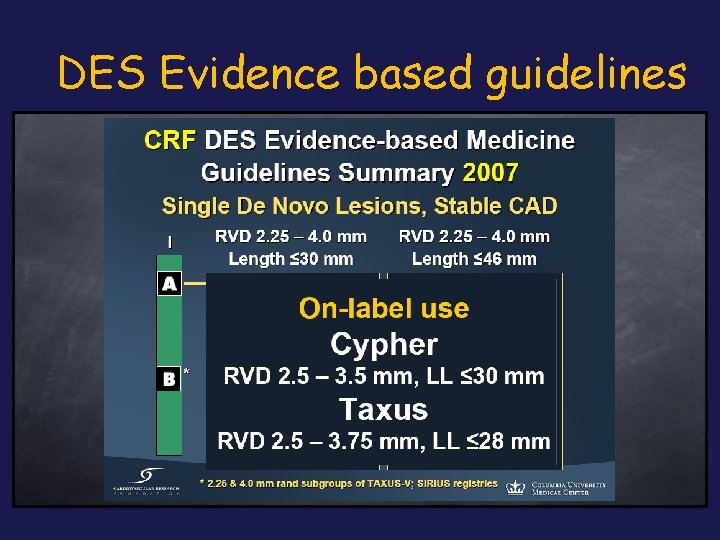
DES Evidence based guidelines

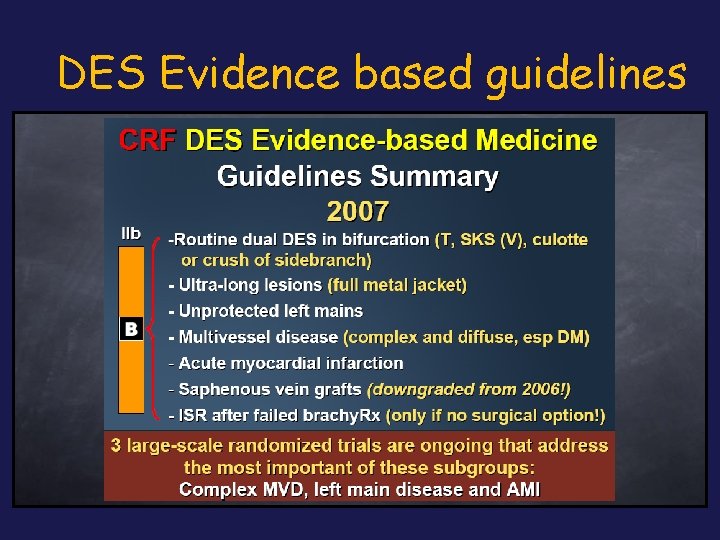
DES Evidence based guidelines

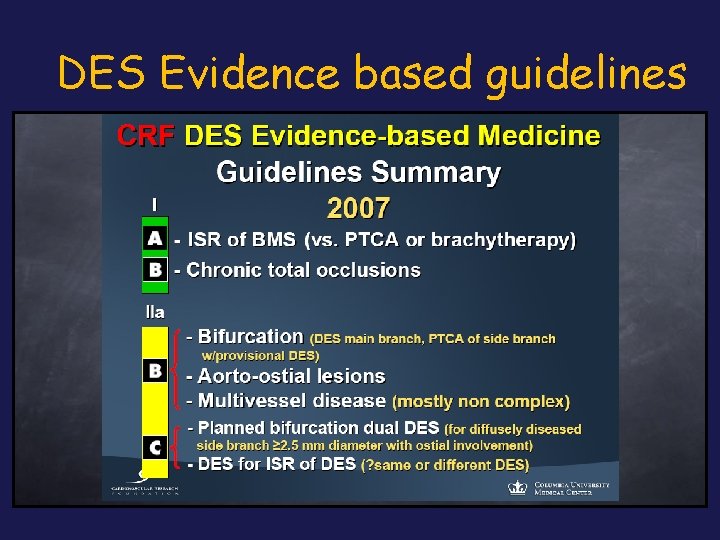
DES Evidence based guidelines

What is the Evidence? ü Randomized controlled trials (selective patients, underpowered) ü Observational Cohort registries (different strategies, unblinded, biased) ü Meta-analyses (meta-analysis have been proven wrong 35% of the time by large RCT!) ü Treatment strategy studies (biased, underpowered)

DES and Lesions/Patients Characteristics: the Fact üDES have been revolutionary in cardiology üFailure rates are increasing with increasing complexity of disease treated

In-stent restenosis – is still predictable?

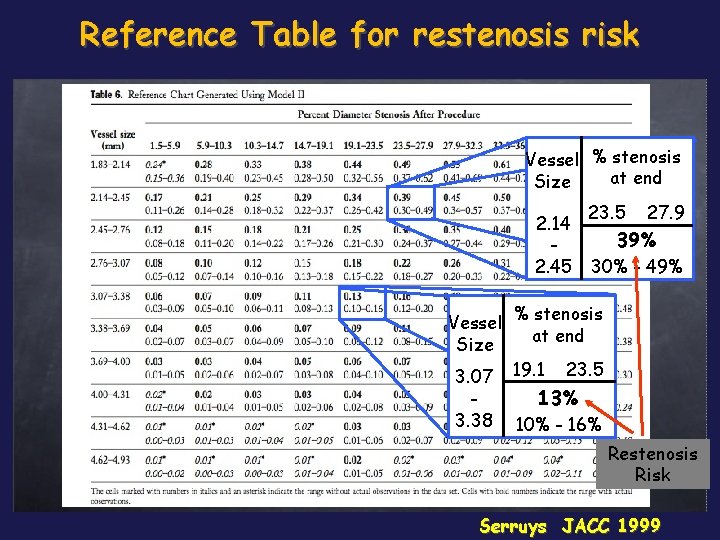
Reference Table for restenosis risk Vessel % stenosis at end Size 2. 14 2. 45 23. 5 27. 9 39% 30% - 49% Vessel % stenosis at end Size 3. 07 19. 1 23. 5 3. 38 13% 10% - 16% Restenosis Risk Serruys JACC 1999

BASKET trial: 18 -month analysis Used in all patients, drug-eluting High-risk stents arepatients not good value for money, even if prices were substantially reduced. Drug-eluting stents are cost effective in patients needing small vessel or bypass graft stenting, but not in those who require large native vessel stenting Brunner-La Rocca Lancet 2007

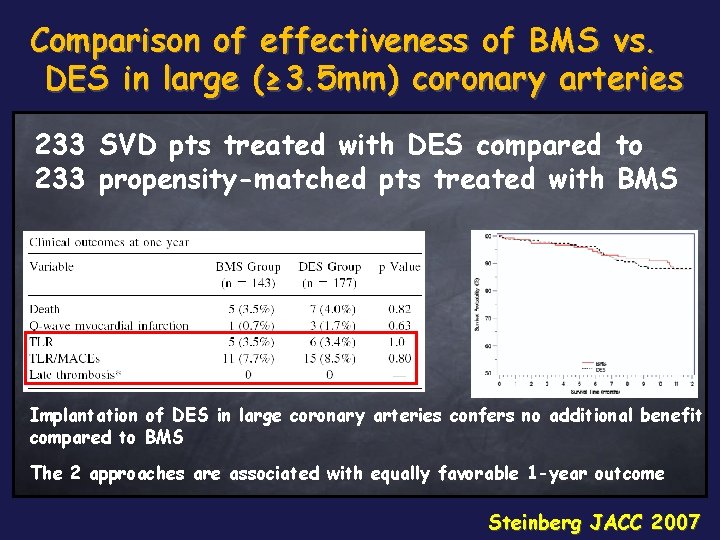
Comparison of effectiveness of BMS vs. DES in large (≥ 3. 5 mm) coronary arteries 233 SVD pts treated with DES compared to 233 propensity-matched pts treated with BMS Implantation of DES in large coronary arteries confers no additional benefit compared to BMS The 2 approaches are associated with equally favorable 1 -year outcome Steinberg JACC 2007

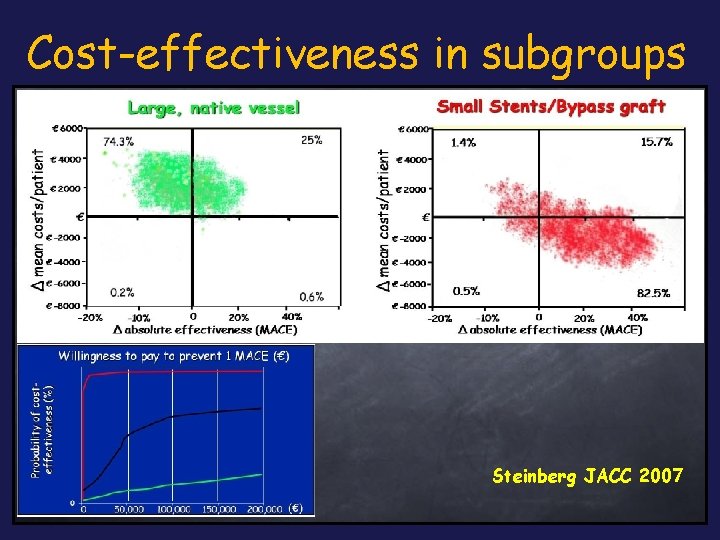
Cost-effectiveness in subgroups Steinberg JACC 2007

Restenosis is variable Adjusted for type of lesion, stent diameter and length Unadjusted SCAAR annual report 2007

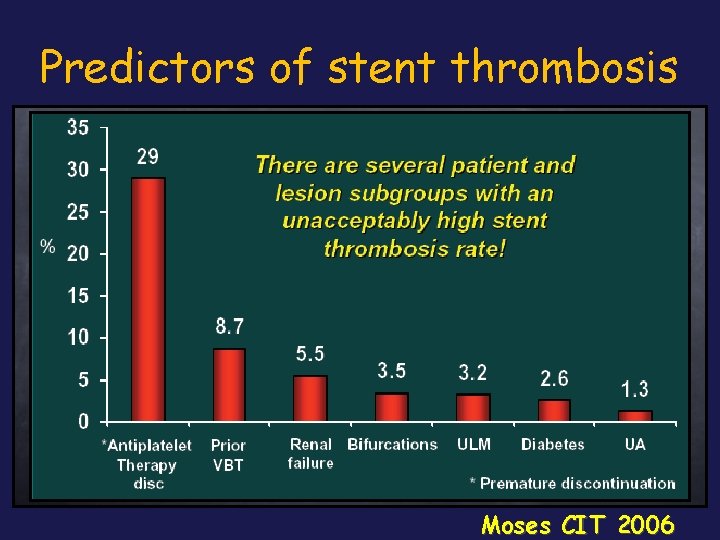
Predictors of stent thrombosis Moses CIT 2006

DES pending questions üISR lesion (esp. Diffuse proliferative and s/p VBT) üBifurcation (ostial side branch) üSVGs üLM disease (esp. Distal bifurcation) üAcute MI and thrombus-containing lesions

Quale DES?

DES Summary - 2007 in Italy: 10 DES With CE Mark in Yellow Manifacturer Name Drug Stent Material Polymer Axxion Bio. Matrix Paclitaxel Biolimus-A-9 Stainless Steel None Bioabsorbable Boston Scientific Taxus Liberté Promus Paclitaxel Everolimus Stainless Steel Cobalt Chromium Durable Cordis / J&J Cypher Select Cypher Neo Sirolimus Stainless Steel Cobalt Chromium Durable Eurocor Guidant/Abbott Genius Taxcor Champion Paclitaxel Everolimus Stainless Steel None Bioabsorbable Guidant/Abbott Medtronic Xience V Endeavor Everolimus Zotarolimus Cobalt Chromium Durable Sorin SMT Translumina Terumo Janus Infinnium Yukon Nobori Tacrolimus Paclitaxel Sirolimus Biolimus-A-9 Stainless Steel Stainless stel Stainless Steel None Bioabsorbable Npne Bioabsorbable Biosensors

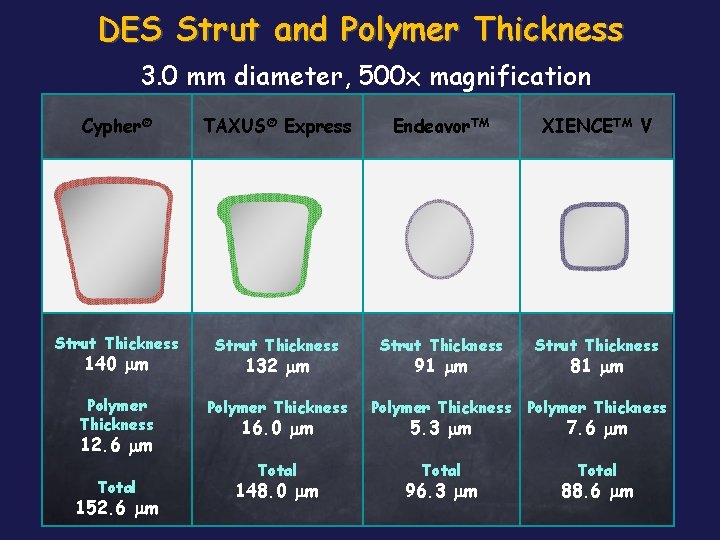
DES Strut and Polymer Thickness 3. 0 mm diameter, 500 x magnification Cypher® TAXUS® Express Endeavor. TM XIENCETM V Strut Thickness Polymer Thickness 140 mm 12. 6 mm Total 152. 6 mm 132 mm 16. 0 mm Total 148. 0 mm 91 mm 81 mm Polymer Thickness 5. 3 mm 7. 6 mm Total 96. 3 mm 88. 6 mm

Some DES do not work Quanam – Taxane (polymer sleeve) Guidant – Actinomycin D (polymer matrix) Guidant/Cook – Placlitaxel (direct application) Abbott – Batimastat (PC coating)

DES future directions

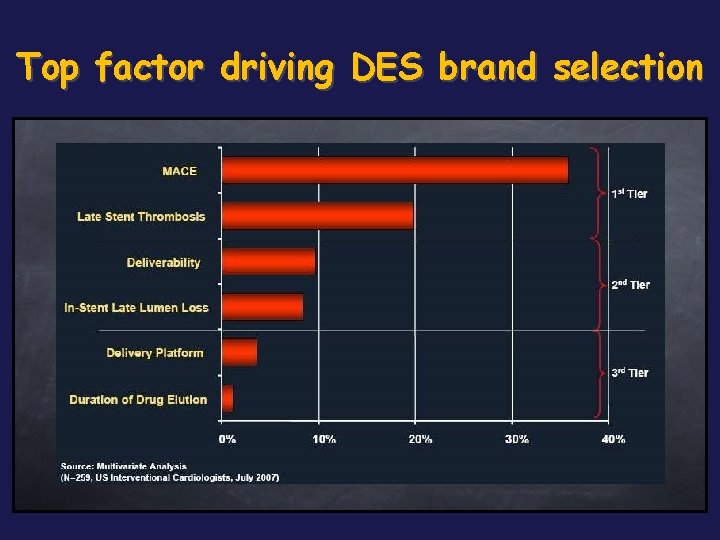
Top factor driving DES brand selection

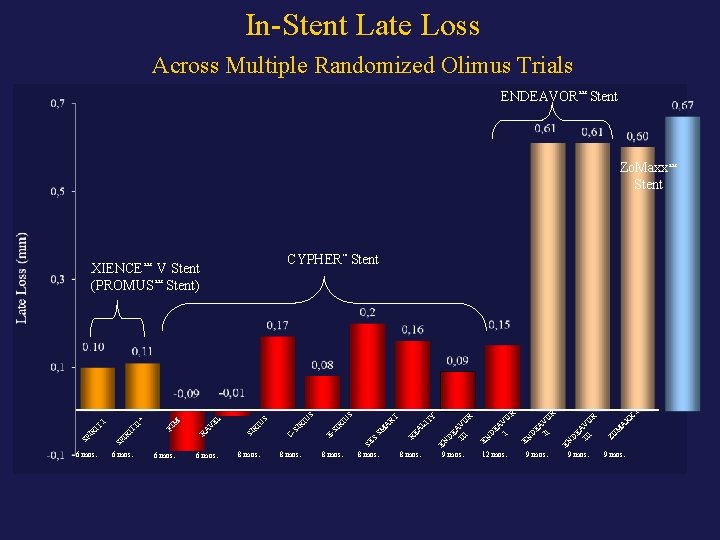
In-Stent Late Loss Across Multiple Randomized Olimus Trials ENDEAVOR™ Stent Zo. Maxx™ Stent XIENCE™ CYPHER™ Stent V Stent) I X 9 mos. A X 9 mos. M R 9 mos. EN EA II VO D EN O R V EA I D 12 mos. ZO 9 mos. EN EN D EA III VO R LI TY 8 mos. D EA III VO R 8 mos. RE A SM A RT IU S 8 mos. SE S 8 mos. SI R 8 mos. E- RI U U S EL 6 mos. CSI 6 mos. RI 6 mos. SI 6 mos. RA V M FI IT SP IR IT I II* S (PROMUS™

Does lesion characteristics dictate stent selection? Any specific suggestions? A) To limit restenosis B) To limit thrombosis C) To limit costs

What have we learned… about DES? ü Dual antiplatelet therapy is crucial for a longer duration then for BMS (how long? ) ü Risk of complication (death, MI, ST) are higher for patients receiving DES off-label vs on-label (is it different for BMS? ) ü Timing and mechanisms of stent thrombosis seem different than for BMS (which is the overall incidence? )

DES “Do the right choice…”

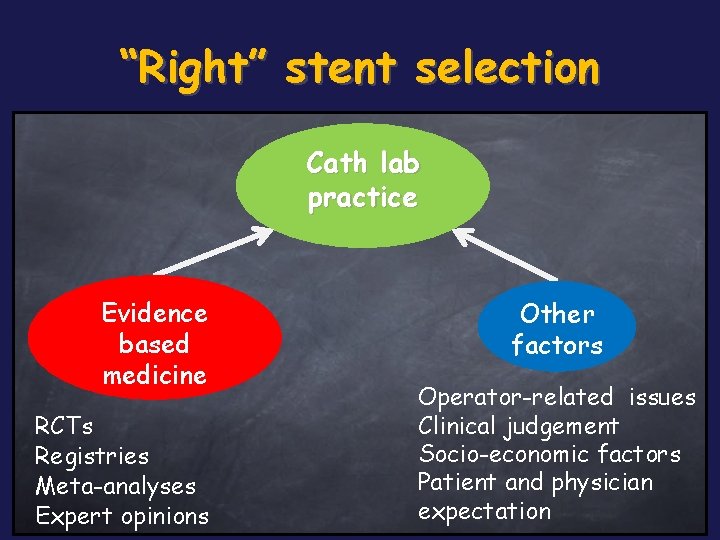
“Right” stent selection Cath lab practice Evidence based medicine RCTs Registries Meta-analyses Expert opinions Other factors Operator-related issues Clinical judgement Socio-economic factors Patient and physician expectation

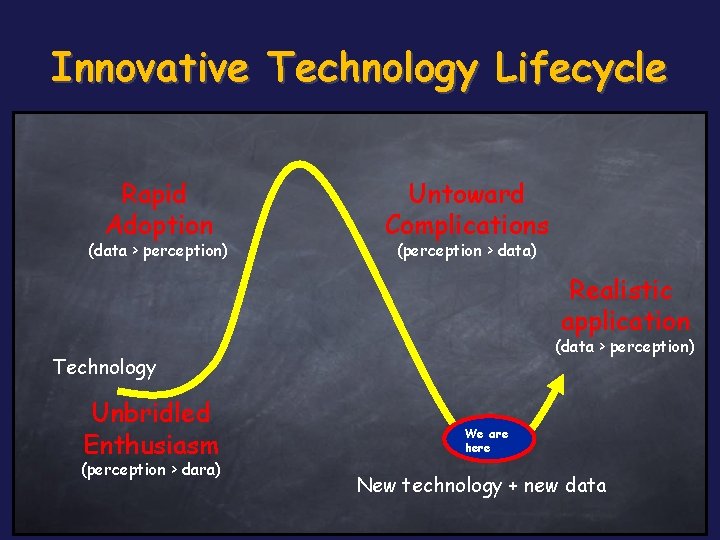
Innovative Technology Lifecycle Rapid Adoption (data > perception) Untoward Complications (perception > data) Realistic application (data > perception) Technology Unbridled Enthusiasm (perception > dara) We are here New technology + new data

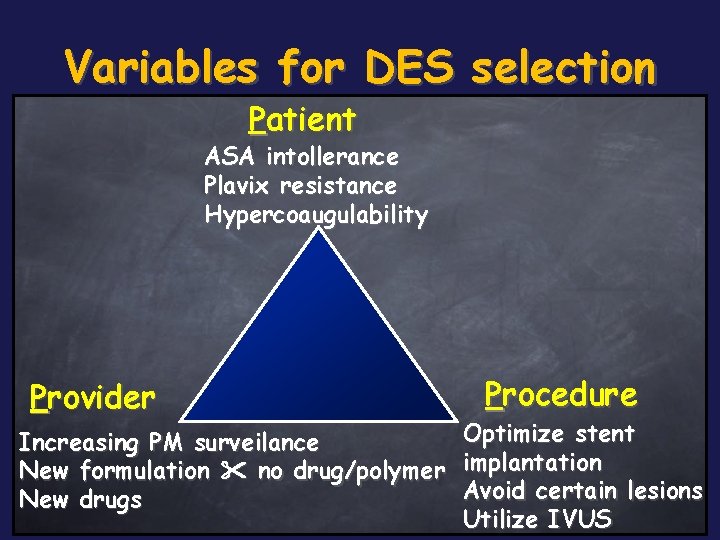
Variables for DES selection Patient ASA intollerance Plavix resistance Hypercoaugulability Provider Procedure Optimize stent Increasing PM surveilance New formulation no drug/polymer implantation Avoid certain lesions New drugs Utilize IVUS

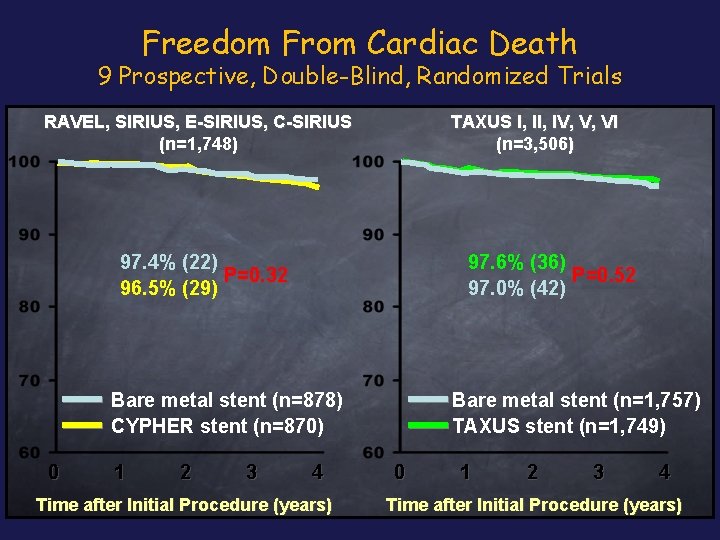
Freedom From Cardiac Death 9 Prospective, Double-Blind, Randomized Trials TAXUS I, IV, V, VI (n=3, 506) RAVEL, SIRIUS, E-SIRIUS, C-SIRIUS (n=1, 748) 97. 4% (22) P=0. 32 96. 5% (29) 97. 6% (36) P=0. 52 97. 0% (42) Bare metal stent (n=878) CYPHER stent (n=870) 0 1 2 3 4 Time after Initial Procedure (years) Bare metal stent (n=1, 757) TAXUS stent (n=1, 749) 0 1 2 3 4 Time after Initial Procedure (years)

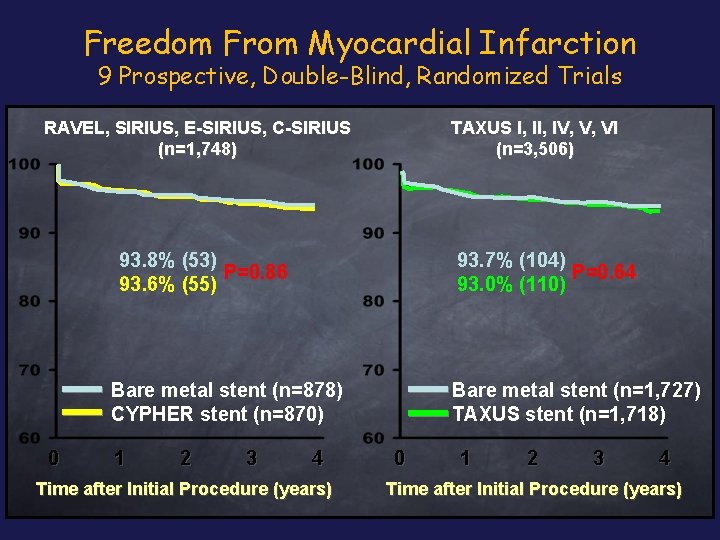
Freedom From Myocardial Infarction 9 Prospective, Double-Blind, Randomized Trials RAVEL, SIRIUS, E-SIRIUS, C-SIRIUS (n=1, 748) TAXUS I, IV, V, VI (n=3, 506) 93. 8% (53) P=0. 86 93. 6% (55) 93. 7% (104) P=0. 64 93. 0% (110) Bare metal stent (n=878) CYPHER stent (n=870) 0 1 2 3 4 Time after Initial Procedure (years) Bare metal stent (n=1, 727) TAXUS stent (n=1, 718) 0 1 2 3 4 Time after Initial Procedure (years)

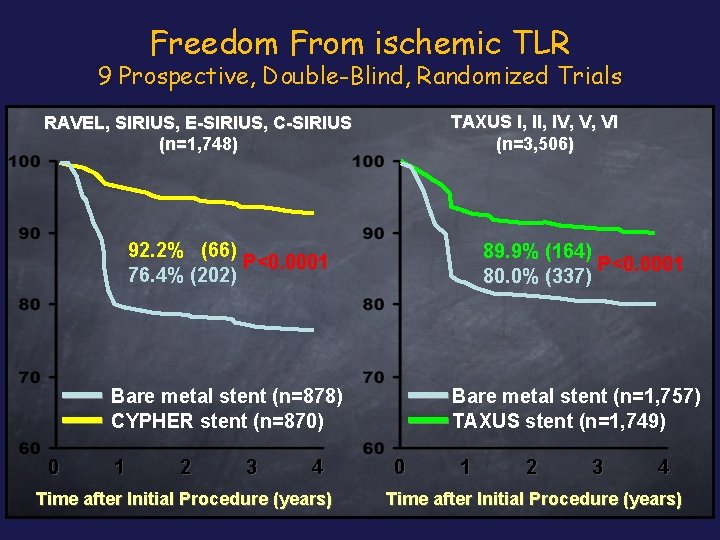
Freedom From ischemic TLR 9 Prospective, Double-Blind, Randomized Trials TAXUS I, IV, V, VI (n=3, 506) RAVEL, SIRIUS, E-SIRIUS, C-SIRIUS (n=1, 748) 92. 2% (66) P<0. 0001 76. 4% (202) 89. 9% (164) P<0. 0001 80. 0% (337) Bare metal stent (n=878) CYPHER stent (n=870) 0 1 2 3 4 Time after Initial Procedure (years) Bare metal stent (n=1, 757) TAXUS stent (n=1, 749) 0 1 2 3 4 Time after Initial Procedure (years)

For further slides on these topics please feel free to visit the metcardio. org website: http: //www. metcardio. org/slides. html
 Enrico romagnoli cardiologo
Enrico romagnoli cardiologo Francesca romagnoli mef
Francesca romagnoli mef Casellario romagnoli
Casellario romagnoli What who where why when who whom how
What who where why when who whom how Who says what in which channel to whom with what effect
Who says what in which channel to whom with what effect Relative pronoun which
Relative pronoun which Who whom whose
Who whom whose Whose adjective clause
Whose adjective clause Enrico ferro
Enrico ferro Enrico sciandrello
Enrico sciandrello Enrico fossa
Enrico fossa Enrico maria corsini
Enrico maria corsini Enrico fiaccadori
Enrico fiaccadori Attributskalierung
Attributskalierung Catia nannoni unibo
Catia nannoni unibo Enrico maranzana
Enrico maranzana Enrico quarantelli
Enrico quarantelli Seminarraum weimar
Seminarraum weimar Dr.boran
Dr.boran Enrico birkner dresden
Enrico birkner dresden Enrico duranti
Enrico duranti Fermi mondolfo
Fermi mondolfo Enrico bonanno
Enrico bonanno Enrico sbriccoli
Enrico sbriccoli Giovanni enrico pestalozzi
Giovanni enrico pestalozzi Enrico felcini
Enrico felcini Enrico hensel
Enrico hensel Via enrico fermi frattamaggiore
Via enrico fermi frattamaggiore Enrico dolza
Enrico dolza Fermi lecce
Fermi lecce Dr enrico flossmann
Dr enrico flossmann Thank you for your attention
Thank you for your attention Enrico stefani
Enrico stefani Ipia enrico fermi
Ipia enrico fermi Enrico ronchi
Enrico ronchi Cem yurtsev
Cem yurtsev Serenada privighetorii
Serenada privighetorii Paulette enrico iv
Paulette enrico iv Enrico e
Enrico e Suspiciuous
Suspiciuous Enrico petretto
Enrico petretto Enrico molisani
Enrico molisani Enrico e
Enrico e Enrico garcia
Enrico garcia Enrico corazzini pescara
Enrico corazzini pescara Istituto tecnico enrico fermi ozieri
Istituto tecnico enrico fermi ozieri Enrico franconi
Enrico franconi Enrico cecchi
Enrico cecchi Istituto comprensivo enrico fermi romano di lombardia
Istituto comprensivo enrico fermi romano di lombardia Erin heim
Erin heim Mrs foul
Mrs foul Adoloscense
Adoloscense Whose and whom
Whose and whom