DrugEluting Stents In AMI A Proven Strategy James






![One Year Composite Safety Endpoints* TAXUS (N=2257) EXPRESS (N=749) HR [95%CI] P Value Safety One Year Composite Safety Endpoints* TAXUS (N=2257) EXPRESS (N=749) HR [95%CI] P Value Safety](https://slidetodoc.com/presentation_image_h2/afe6b6d9bccae42d5be2879b1ebfe381/image-11.jpg)



- Slides: 29

Drug-Eluting Stents In AMI: A Proven Strategy? James Hermiller, MD, FACC, FSCAI St Vincent Hospital Indianapolis, IN

DISCLOSURES James Hermiller, MD Consulting Fees – Abbott Vascular, Boston Scientific Corporation, St. Jude Medical I intend to reference unlabeled/ unapproved uses of drugs or devices in my presentation. I intend to reference date and guidelines for drug-eluting stents.

Outline • Introduction • Randomized clinical trials • Registry studies • The guidelines • Summary

Introduction • Role of DES in the setting of STEMI has been uncertain due to: • DES associated delayed healing with thrombus and the concern about subsequent incomplete stent apposition • Does this lead to higher probability of stent thrombosis, MI and death with DES vs BMS • TLR and restenosis rates tend to be lower in STEMI vs. elective PCI patients • Outcomes from RCTs and registry studies of DES vs. BMS in STEMI have been conflicting

Introduction Stent Thrombosis • Stent Malapposition • Compliance DAP Restenosis

Premature Discontinuation of Thienopyridine Therapy After DES Implantation Spertus JA et al. Circulation 2006; 113: 2803 -9 Multicenter, prospective PREMIER registry in patients admitted with myocardial infarction -500 DES patients enrolled at 19 sites • -68500 DES patients enrolled at (14%) patients d/c thienopyridine % Mortality Between 30 Days and 1 Year 19 sites • 68 (14%) patients d/c thienopyridine Factors associated with premature Thienopyridine discontinuation -older age -lower socioeconomic status -preexisting cardiovascular disease -inadequate discharge instructions -lack of referral to cardiac rehab HR=9. 0 P<0. 001 HR=1. 5 P=0. 08

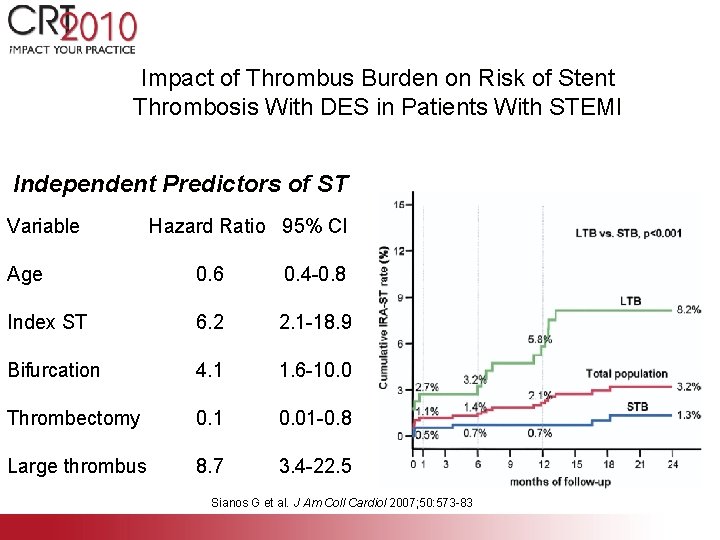
Impact of Thrombus Burden on Risk of Stent Thrombosis With DES in Patients With STEMI Independent Predictors of ST Variable Hazard Ratio 95% CI Age 0. 6 0. 4 -0. 8 Index ST 6. 2 2. 1 -18. 9 Bifurcation 4. 1 1. 6 -10. 0 Thrombectomy 0. 1 0. 01 -0. 8 Large thrombus 8. 7 3. 4 -22. 5 Sianos G et al. J Am Coll Cardiol 2007; 50: 573 -83

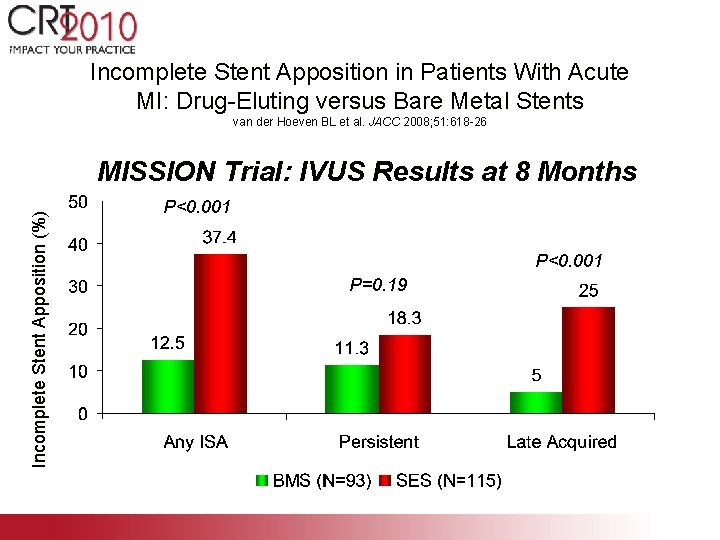
Incomplete Stent Apposition in Patients With Acute MI: Drug-Eluting versus Bare Metal Stents van der Hoeven BL et al. JACC 2008; 51: 618 -26 Incomplete Stent Apposition (%) MISSION Trial: IVUS Results at 8 Months P<0. 001 P=0. 19

Outline • Introduction • Randomized clinical trials • Registry studies • The guidelines • Summary

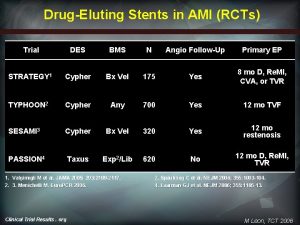
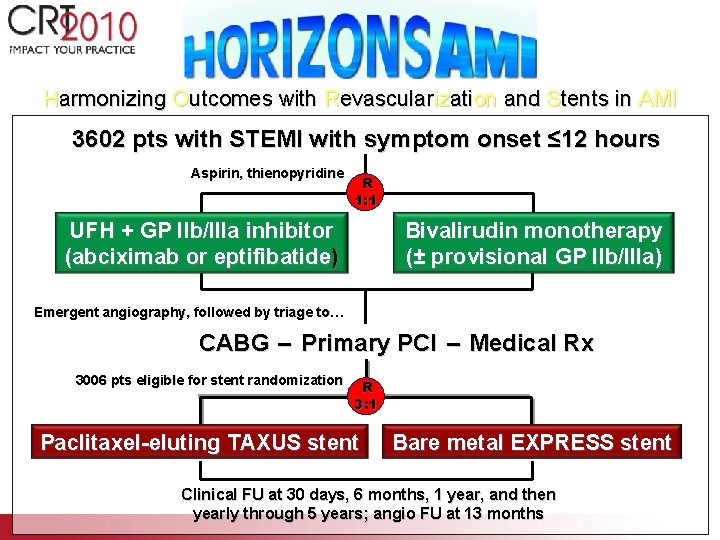
Harmonizing Outcomes with Revascularization and Stents in AMI 3602 pts with STEMI with symptom onset ≤ 12 hours Aspirin, thienopyridine R 1: 1 UFH + GP IIb/IIIa inhibitor (abciximab or eptifibatide) Bivalirudin monotherapy (± provisional GP IIb/IIIa) Emergent angiography, followed by triage to… CABG – Primary PCI – Medical Rx 3006 pts eligible for stent randomization R 3: 1 Paclitaxel-eluting TAXUS stent Bare metal EXPRESS stent Clinical FU at 30 days, 6 months, 1 year, and then yearly through 5 years; angio FU at 13 months
![One Year Composite Safety Endpoints TAXUS N2257 EXPRESS N749 HR 95CI P Value Safety One Year Composite Safety Endpoints* TAXUS (N=2257) EXPRESS (N=749) HR [95%CI] P Value Safety](https://slidetodoc.com/presentation_image_h2/afe6b6d9bccae42d5be2879b1ebfe381/image-11.jpg)
One Year Composite Safety Endpoints* TAXUS (N=2257) EXPRESS (N=749) HR [95%CI] P Value Safety MACE** 8. 1% 8. 0% 1. 02 [0. 76, 1. 36] 0. 92 Death, all-cause 3. 5% 0. 99 [0. 64, 1. 55] 0. 98 - Cardiac 2. 4% 2. 7% 0. 90 [0. 54, 1. 50] 0. 68 - Non cardiac 1. 1% 0. 8% 1. 32 [0. 54, 3. 22] 0. 55 Reinfarction 3. 7% 4. 5% 0. 81 [0. 54, 1. 21] 0. 31 - Q-wave 2. 0% 1. 9% 1. 07 [0. 59, 1. 94] 0. 83 - Non Q-wave 1. 8% 2. 7% 0. 68 [0. 39, 1. 17] 0. 16 Stent thrombosis† 3. 2% 3. 4% 0. 93 [0. 59, 1. 47] 0. 77 - ARC definite 2. 6% 3. 0% 0. 88 [0. 54, 1. 43] 0. 60 - ARC probable 0. 5% 0. 4% 1. 33 [0. 38, 4. 73] 0. 65 1. 0% 0. 7% 1. 52 [0. 58, 4. 00] 0. 39 Stroke *Kaplan-Meier estimates; **Primary safety endpoint; †ARC definite or probable Stone GW, et al. N Engl J Med. 2009; 360: 1946 – 59.

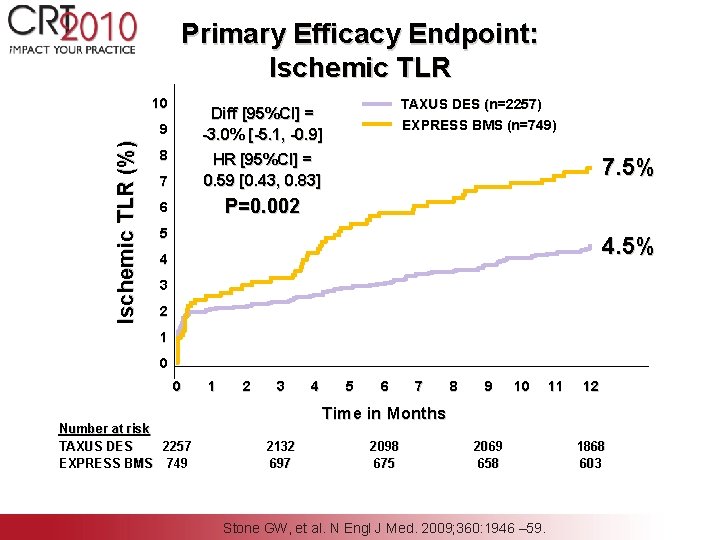
Primary Efficacy Endpoint: Ischemic TLR 10 9 Ischemic TLR (%) TAXUS DES (n=2257) EXPRESS BMS (n=749) Diff [95%CI] = -3. 0% [-5. 1, -0. 9] 8 7 HR [95%CI] = 0. 59 [0. 43, 0. 83] 6 P=0. 002 7. 5% 5 4. 5% 4 3 2 1 0 0 Number at risk TAXUS DES 2257 EXPRESS BMS 749 1 2 3 4 5 6 7 8 9 10 11 12 Time in Months 2132 697 2098 675 2069 658 Stone GW, et al. N Engl J Med. 2009; 360: 1946 – 59. 1868 603

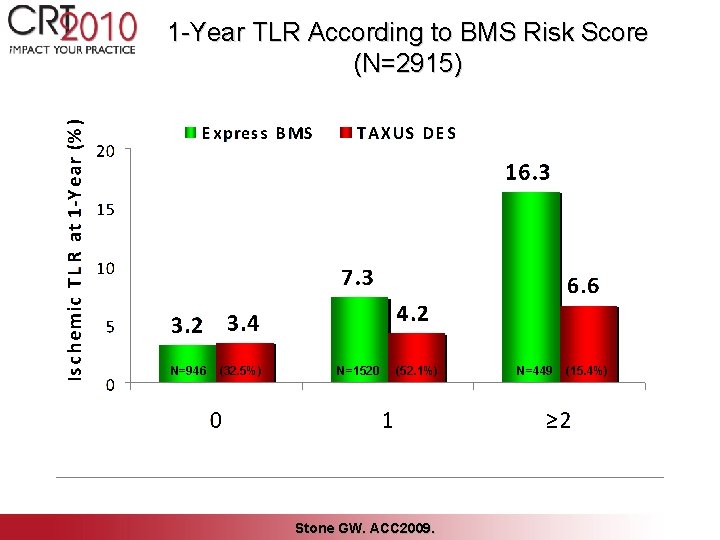
Multivariable Predictors of 1 -Year TLR (BMS Express patients, N=734) Variable Score HR (95% CI) P-value Total lesion length ≥ 40 mm 2 5. 28 [1. 73, 16. 15] 0. 004 Baseline RVD ≤ 3. 0 mm 1 3. 27 [1. 63, 6. 55] 0. 0008 Insulin-treated diabetes 1 3. 00 [1. 17, 7. 66] 0. 02 Lesion ulceration 1 3. 45 [1. 30, 9. 16] 0. 01 Killip class 2 -4 1 2. 50 [1. 20, 5. 20] 0. 01 Stone GW. ACC 2009.

1 -Year TLR According to BMS Risk Score (N=2915) N=946 (32. 5%) N=1520 (52. 1%) Stone GW. ACC 2009. N=449 (15. 4%)

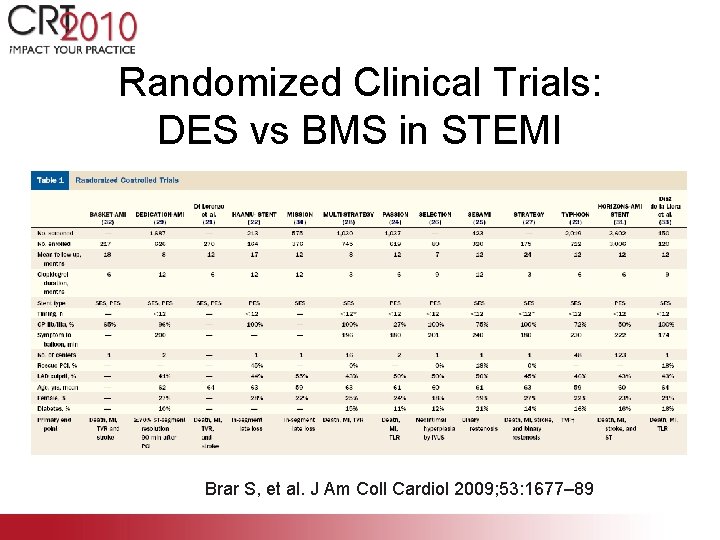
Randomized Clinical Trials: DES vs BMS in STEMI Brar S, et al. J Am Coll Cardiol 2009; 53: 1677– 89

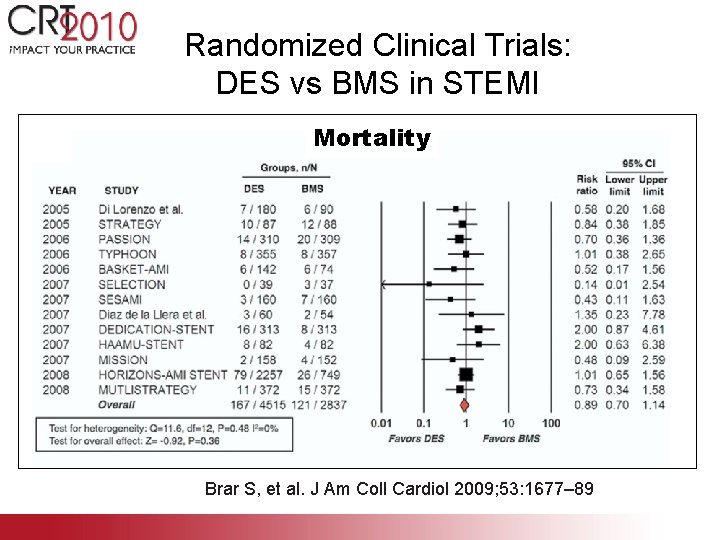
Randomized Clinical Trials: DES vs BMS in STEMI Mortality Brar S, et al. J Am Coll Cardiol 2009; 53: 1677– 89

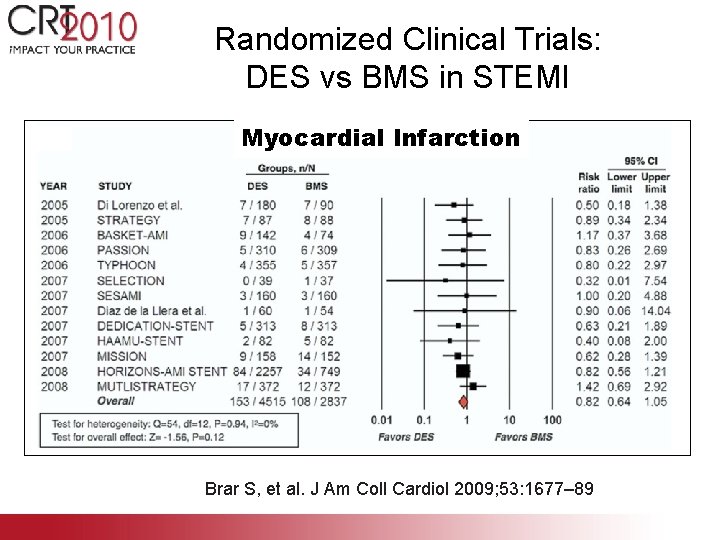
Randomized Clinical Trials: DES vs BMS in STEMI Myocardial Infarction Brar S, et al. J Am Coll Cardiol 2009; 53: 1677– 89

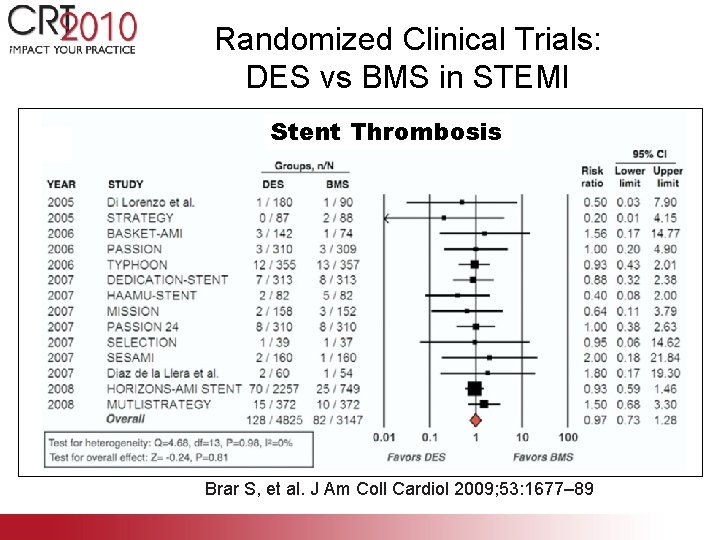
Randomized Clinical Trials: DES vs BMS in STEMI Stent Thrombosis Brar S, et al. J Am Coll Cardiol 2009; 53: 1677– 89

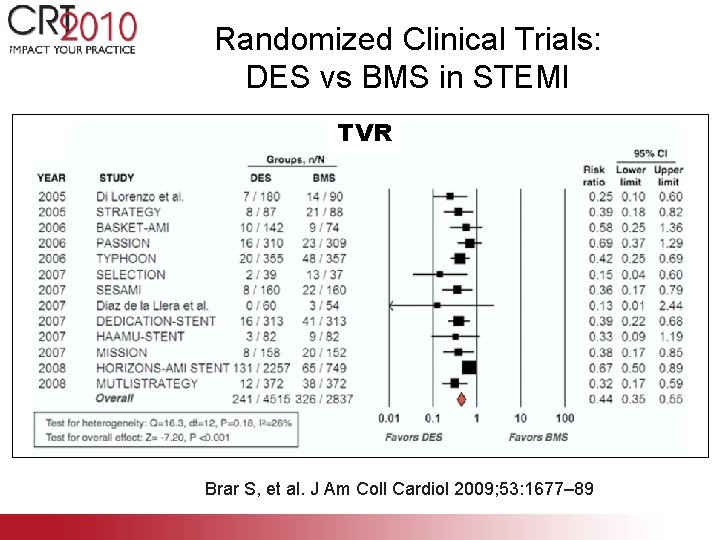
Randomized Clinical Trials: DES vs BMS in STEMI TVR Brar S, et al. J Am Coll Cardiol 2009; 53: 1677– 89

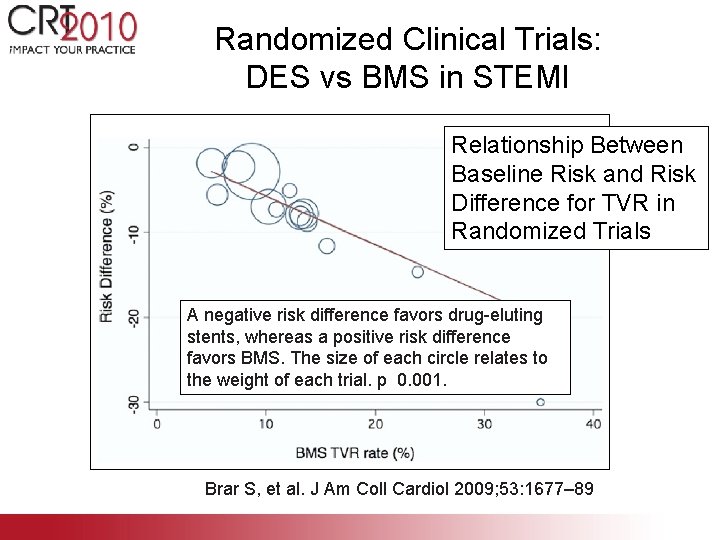
Randomized Clinical Trials: DES vs BMS in STEMI Relationship Between Baseline Risk and Risk Difference for TVR in Randomized Trials A negative risk difference favors drug-eluting stents, whereas a positive risk difference favors BMS. The size of each circle relates to the weight of each trial. p 0. 001. Brar S, et al. J Am Coll Cardiol 2009; 53: 1677– 89

Outline • Introduction • Randomized clinical trials • Registry studies • The guidelines • Summary

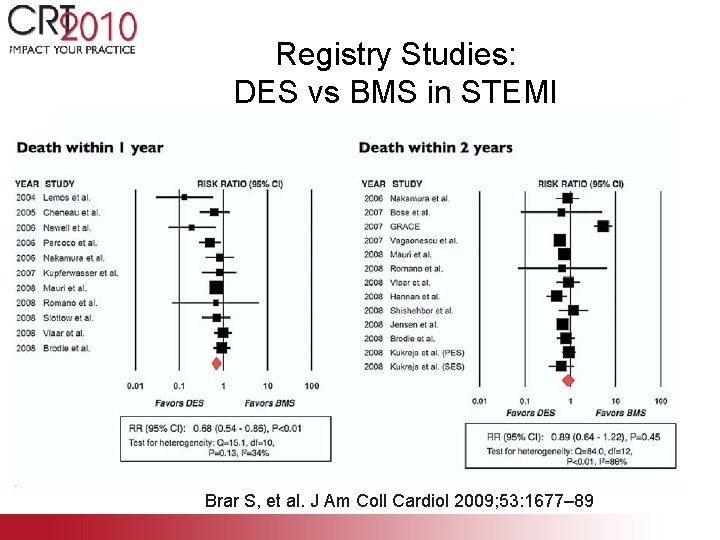
Registry Studies: DES vs BMS in STEMI Brar S, et al. J Am Coll Cardiol 2009; 53: 1677– 89

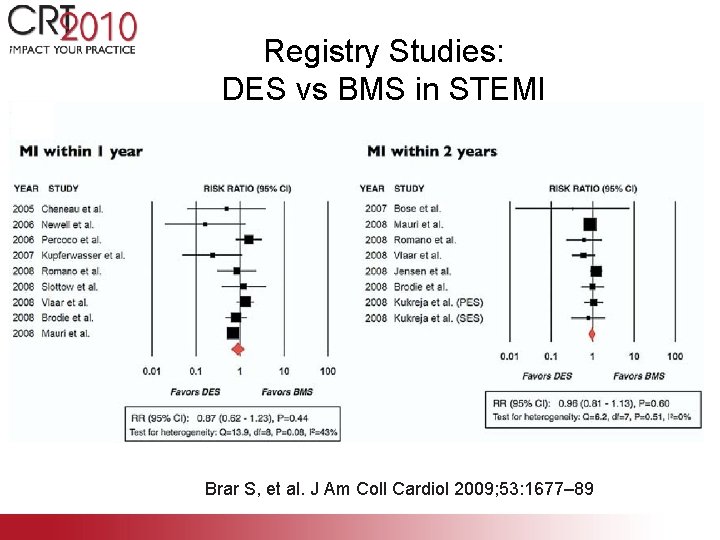
Registry Studies: DES vs BMS in STEMI Brar S, et al. J Am Coll Cardiol 2009; 53: 1677– 89

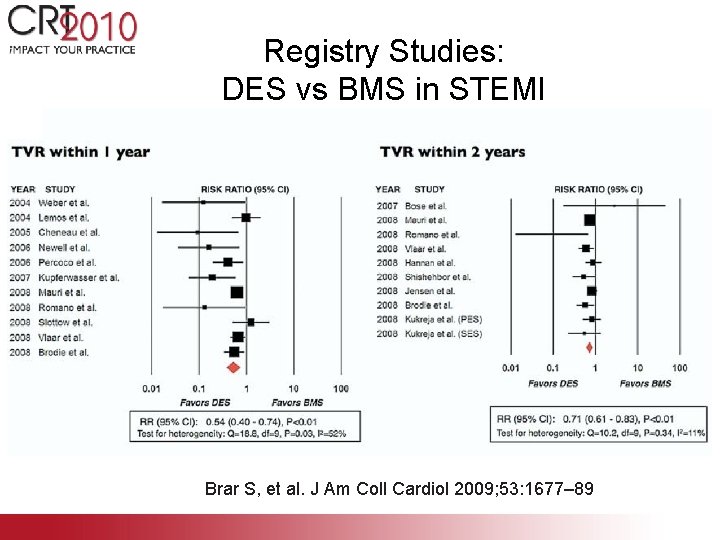
Registry Studies: DES vs BMS in STEMI Brar S, et al. J Am Coll Cardiol 2009; 53: 1677– 89

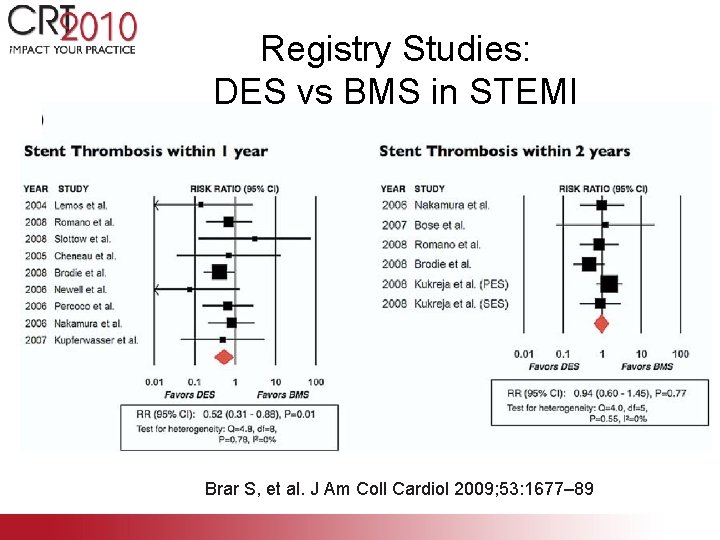
Registry Studies: DES vs BMS in STEMI Brar S, et al. J Am Coll Cardiol 2009; 53: 1677– 89

Outline • Introduction • Randomized clinical trials • Registry studies • The guidelines • Summary

Guidelines With ST-Elevation Myocardial Infarction (Updating the 2004 Guideline and 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients Kushner et al, J. Am. Coll. Cardiol. 2009; 54; 2205 -2241;

Guidelines With ST-Elevation Myocardial Infarction (Updating the 2004 Guideline and 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients “The major advantage of DES over BMS is a small reduction in TVR rates. Given cost considerations, it could be argued that selective use of DES to prevent restenosis and TVR in high-risk patients (i. e. , patients with diabetes) and in high-risk lesions (longer and smaller diameter stents) could be recommended” Kushner et al, J. Am. Coll. Cardiol. 2009; 54; 2205 -2241;

Summary • The utilization of DES vs BMS in STEMI associated with – No difference in mortality, recurrent MI, or stent thrombosis – Reduction in TLR and restenosis, particularly those with high risk of BMS restenosis (>1 – diabetic, lesion > 40 mm, RVD < 3 mm, diabetes, and lesion ulceration) • Current guidelines suggest DES in STEMI IIa recommendation • Careful determination of ability to remain on dual anti-platelet therapy critical (compliance, upcoming surgery, bleeding, and associated anti-coagulant therapy) • Compulsive implantation technique, ensuring adequate vasodilator given to prevent undersizing of stent, particularly with DES given higher propensity for subsequent malapposition
 Proven amazon course for $99
Proven amazon course for $99 Adopting proven technology instead of experimental
Adopting proven technology instead of experimental Proven in use
Proven in use Emc proven professional
Emc proven professional 4-5 triangle congruence sss and sas
4-5 triangle congruence sss and sas Allscripts tiger misys
Allscripts tiger misys Wisdom is justified by her deeds
Wisdom is justified by her deeds James russell odom and james clayton lawson
James russell odom and james clayton lawson Clay lawson and russell odom
Clay lawson and russell odom Goriot apo olvasonaplo
Goriot apo olvasonaplo Ami core measures
Ami core measures Lekw
Lekw Nothing ventured nothing gained origin
Nothing ventured nothing gained origin Pinagmumulan ng mga
Pinagmumulan ng mga Koncovka ami
Koncovka ami Cim
Cim Ami rotary dials
Ami rotary dials Ami meeting corpus
Ami meeting corpus Pharyngealisation
Pharyngealisation Asthma internist kenai peninsula
Asthma internist kenai peninsula Bios ami
Bios ami Ami quesada
Ami quesada Sachep
Sachep Nama ami
Nama ami Ami kodana
Ami kodana Ami els-collect
Ami els-collect Te ami
Te ami Vzor stroj i y
Vzor stroj i y Un vrai ami chanson
Un vrai ami chanson Mon ami albert
Mon ami albert