What is New for Drugeluting Stents Ron Waksman









- Slides: 42

What is New for Drug-eluting Stents? Ron Waksman, MD, FACC, FSCAI Professor of Medicine, (Cardiology) Georgetown University Director, Cardiovascular Research Advanced Education Med. Star Heart &Vascular Institute, Washington DC

Ron Waksman, MD

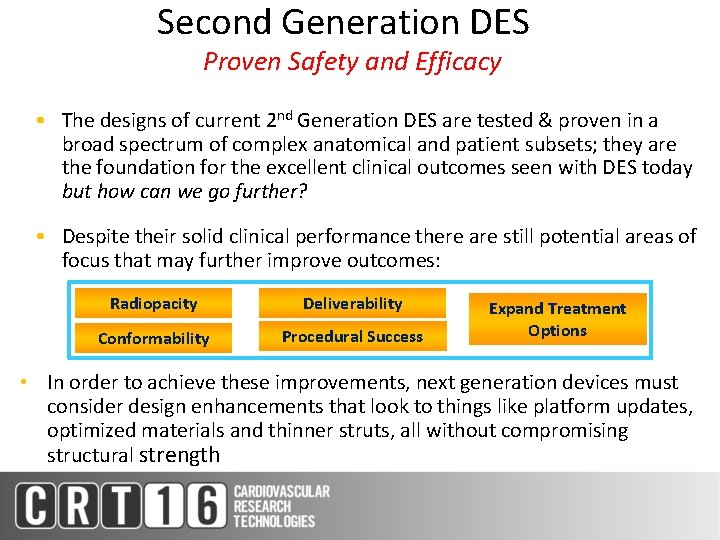
Second Generation DES Proven Safety and Efficacy • The designs of current 2 nd Generation DES are tested & proven in a broad spectrum of complex anatomical and patient subsets; they are the foundation for the excellent clinical outcomes seen with DES today but how can we go further? • Despite their solid clinical performance there are still potential areas of focus that may further improve outcomes: Radiopacity Deliverability Conformability Procedural Success Expand Treatment Options • In order to achieve these improvements, next generation devices must consider design enhancements that look to things like platform updates, optimized materials and thinner struts, all without compromising structural strength

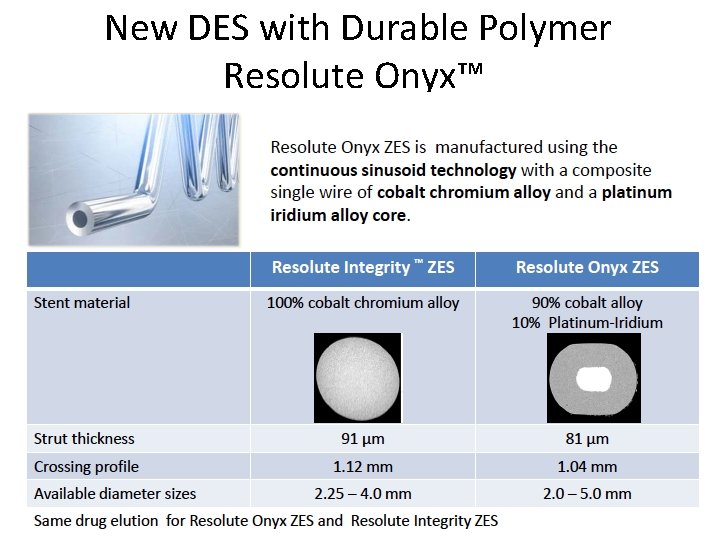
New DES with Durable Polymer Resolute Onyx™

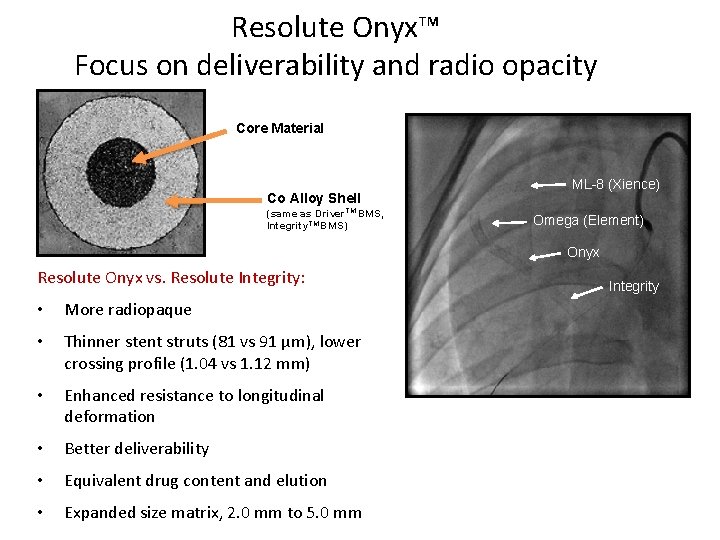
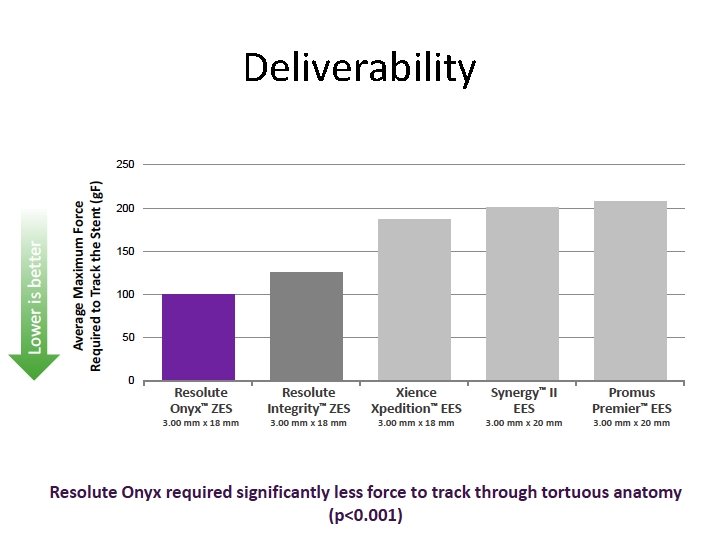
Resolute Onyx™ Focus on deliverability and radio opacity Core Material Co Alloy Shell (same as Driver TM BMS, Integrity. TM BMS) ML-8 (Xience) Omega (Element) Onyx Resolute Onyx vs. Resolute Integrity: • More radiopaque • Thinner stent struts (81 vs 91 µm), lower crossing profile (1. 04 vs 1. 12 mm) • Enhanced resistance to longitudinal deformation • Better deliverability • Equivalent drug content and elution • Expanded size matrix, 2. 0 mm to 5. 0 mm Integrity

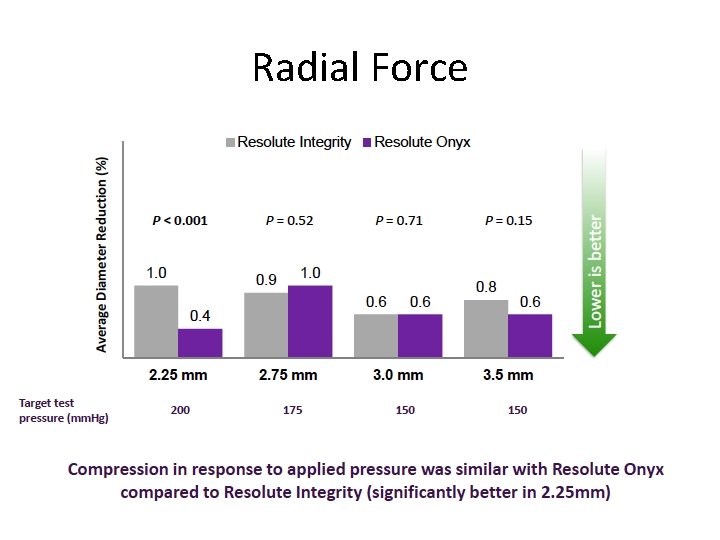
Radial Force

Deliverability

BIODEGRADABLE POLYMERS DES NEW DES 2016 BIODEGRADABL E DES SCAFFOLDS POLYMERS FREE

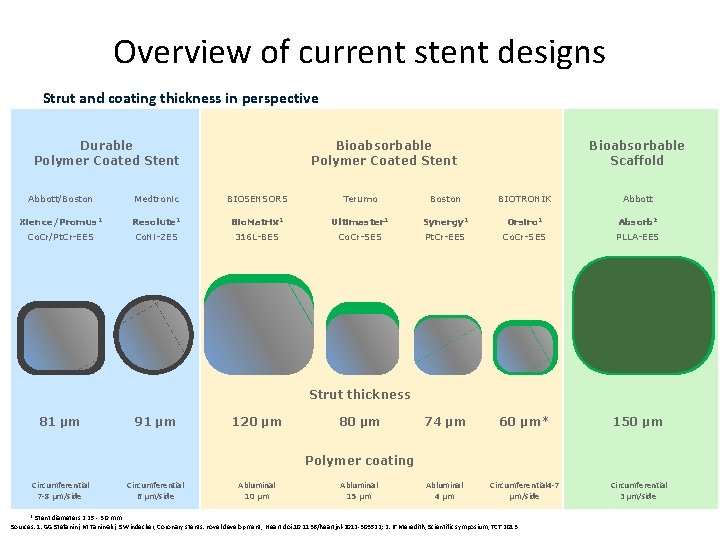
Overview of current stent designs Strut and coating thickness in perspective Bioabsorbable Polymer Coated Stent Durable Polymer Coated Stent Bioabsorbable Scaffold Abbott/Boston Medtronic BIOSENSORS Terumo Boston BIOTRONIK Abbott Xience/Promus 1 Resolute 1 Bio. Matrix 1 Ultimaster 1 Synergy 1 Orsiro 1 Absorb 2 Co. Cr/Pt. Cr-EES Co. Ni-ZES 316 L-BES Co. Cr-SES Pt. Cr-EES Co. Cr-SES PLLA-EES 74 µm 60 µm* 150 µm Abluminal 4 µm Circumferential 4 -7 µm/side Circumferential 3 µm/side Strut thickness 81 µm 91 µm 120 µm 80 µm Polymer coating Circumferential 7 -8 µm/side Circumferential 6 µm/side Abluminal 10 µm Abluminal 15 µm * Stent diameters 2. 25 - 3. 0 mm Sources: 1: GG Stefanini, M Taniwaki, S Windecker, Coronary stents: novel development, Heart doi: 10. 1136/heartjnl-2012 -303522; 2: IT Meredith, Scientific symposium, TCT 2013


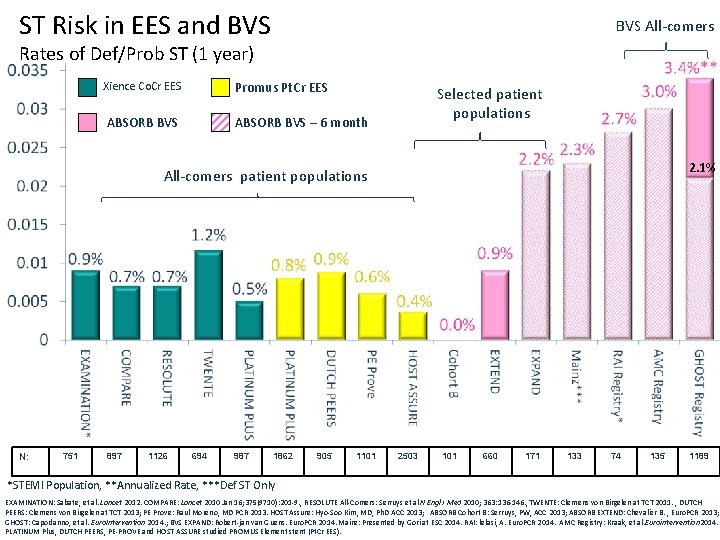
ST Risk in EES and BVS All-comers Rates of Def/Prob ST (1 year) Promus Pt. Cr EES Xience Co. Cr EES ABSORB BVS Selected patient populations ABSORB BVS – 6 month 2. 1% All-comers patient populations N: 751 897 1126 694 987 1862 905 1101 2503 101 660 171 133 74 135 1189 *STEMI Population, **Annualized Rate, ***Def ST Only EXAMINATION: Sabate, et al. Lancet 2012. COMPARE: Lancet 2010 Jan 16; 375(9710): 201 -9. , RESOLUTE All-Comers: Serruys et al N Engl J Med 2010; 363: 136 -146. , TWENTE: Clemens von Birgelen at TCT 2011. , DUTCH PEERS: Clemens von Birgelen at TCT 2013; PE Prove: Raul Moreno, MD PCR 2013. HOST Assure: Hyo-Soo Kim, MD, Ph. D ACC 2013; ABSORB Cohort B: Serruys, PW, ACC 2013; ABSORB EXTEND: Chevalier B. , Euro. PCR 2013; GHOST: Capodanno, et al. Euro. Intervention 2014. ; BVS EXPAND: Robert-jan van Guens. Euro. PCR 2014. Mainz: Presented by Gori at ESC 2014. RAI: lelasi, A. Euro. PCR 2014. AMC Registry: Kraak, et al. Eurointervention 2014. PLATINUM Plus, DUTCH PEERS, PE-PROVE and HOST ASSURE studied PROMUS Element stent (Pt. Cr EES).

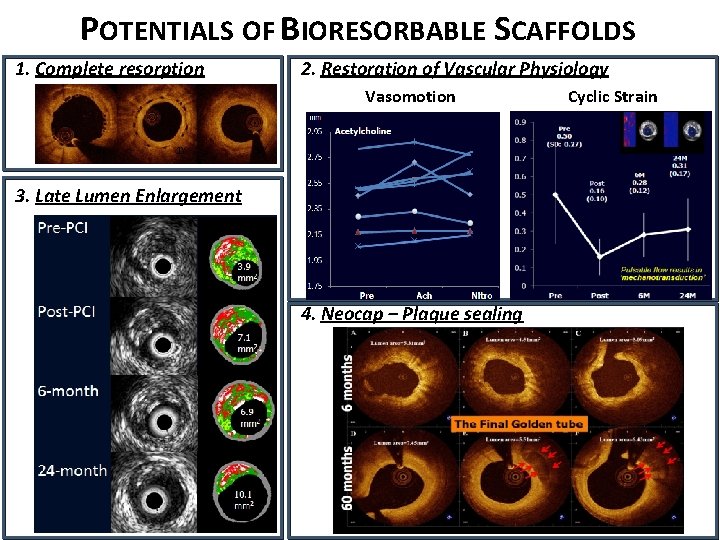
POTENTIALS OF BIORESORBABLE SCAFFOLDS 1. Complete resorption 2. Restoration of Vascular Physiology Vasomotion BL 1 Y 5 Y 3. Late Lumen Enlargement 4. Neocap – Plaque sealing Cyclic Strain

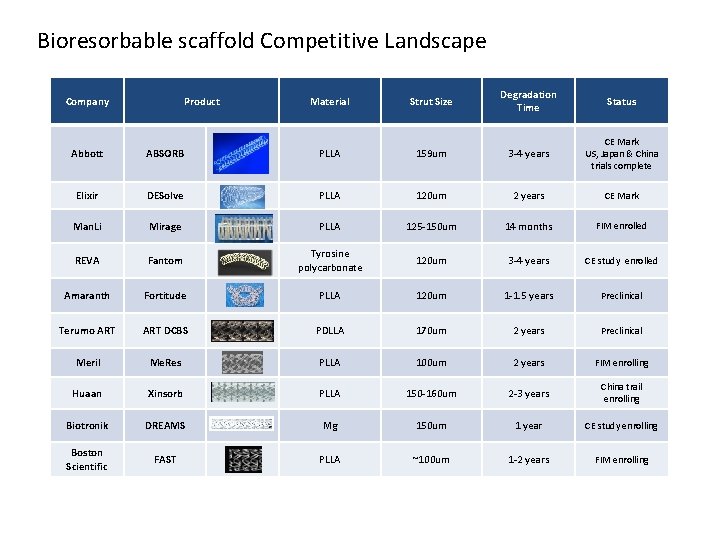
Bioresorbable scaffold Competitive Landscape Company Product Material Strut Size Degradation Time Status Abbott ABSORB PLLA 159 um 3 -4 years CE Mark US, Japan & China trials complete Elixir DESolve PLLA 120 um 2 years CE Mark Man. Li Mirage PLLA 125 -150 um 14 months FIM enrolled REVA Fantom Tyrosine polycarbonate 120 um 3 -4 years CE study enrolled Amaranth Fortitude PLLA 120 um 1 -1. 5 years Preclinical Terumo ART DCBS PDLLA 170 um 2 years Preclinical Meril Me. Res PLLA 100 um 2 years FIM enrolling Huaan Xinsorb PLLA 150 -160 um 2 -3 years China trail enrolling Biotronik DREAMS Mg 150 um 1 year CE study enrolling Boston Scientific FAST PLLA ~100 um 1 -2 years FIM enrolling

ABSORB III NCT 01751906 2, 250 pts with up to 2 de novo lesions in different epicardial vessels enrolled, with follow-up for at least 5 years, at up to 122 US and non-US sites 2, 000 pts randomized 2: 1 ABSORB v XIENCE (+50 lead-in pts and 200 pt non-randomized angio/IVUS/OCT/VM FU cohort) RVD: 2. 50 - 3. 75 mm; Lesion length: ≤ 24 mm Scaffold diameters: 2. 5, 3. 0 and 3. 5 mm Scaffold lengths: 12, 18, and 28 mm Primary endpoint was met (n=2, 000): TLF at 1 year (powered for noninferiority) US approval Pending

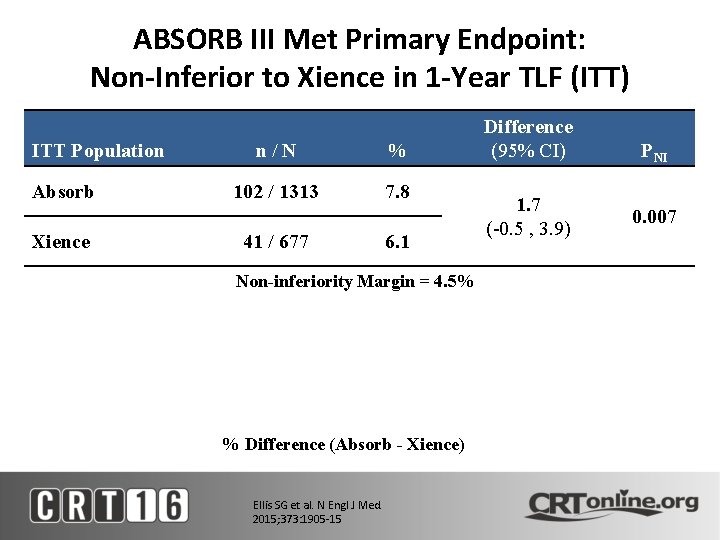
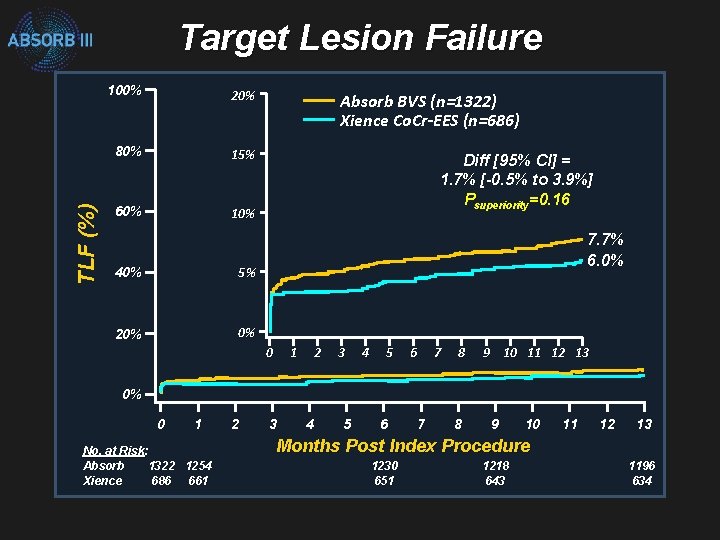
ABSORB III Met Primary Endpoint: Non-Inferior to Xience in 1 -Year TLF (ITT) ITT Population Absorb Xience n/N % 102 / 1313 7. 8 41 / 677 6. 1 Non-inferiority Margin = 4. 5% % Difference (Absorb - Xience) Ellis SG et al. N Engl J Med. 2015; 373: 1905 -15 Difference (95% CI) 1. 7 (-0. 5 , 3. 9) PNI 0. 007

TLF (%) Target Lesion Failure 100% 20% 80% 15% 60% 10% 40% 5% 20% 0% Absorb BVS (n=1322) Xience Co. Cr-EES (n=686) Diff [95% CI] = 1. 7% [-0. 5% to 3. 9%] Psuperiority=0. 16 7. 7% 6. 0% 0 1 2 3 4 5 6 7 8 9 10 11 12 13 0% 0 1 No. at Risk: Absorb 1322 1254 Xience 686 661 2 3 4 5 6 7 8 9 10 11 12 13 Months Post Index Procedure 1230 651 1218 643 1196 634

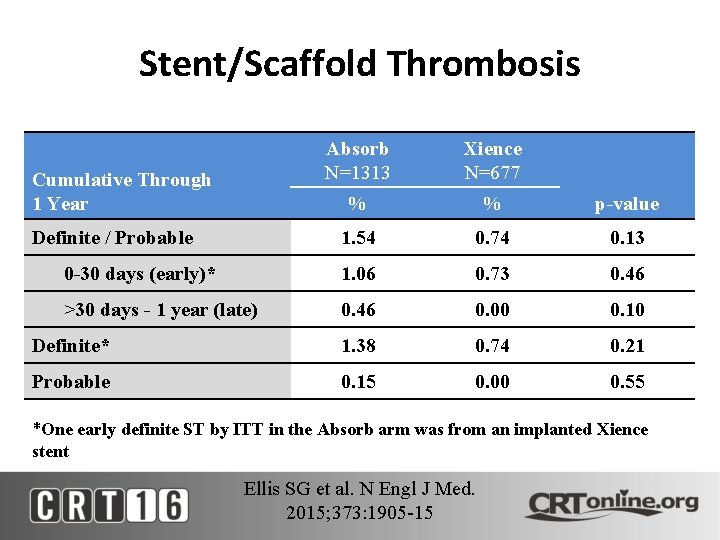
Stent/Scaffold Thrombosis Absorb N=1313 Xience N=677 % % p-value 1. 54 0. 74 0. 13 0 -30 days (early)* 1. 06 0. 73 0. 46 >30 days - 1 year (late) 0. 46 0. 00 0. 10 Definite* 1. 38 0. 74 0. 21 Probable 0. 15 0. 00 0. 55 Cumulative Through 1 Year Definite / Probable *One early definite ST by ITT in the Absorb arm was from an implanted Xience stent Ellis SG et al. N Engl J Med. 2015; 373: 1905 -15

Absorb Worldwide Commercial Usage: >125, 000 Patients and >200, 000 Implants in over 100 Countries Will it be available in the US? FDA Circulatory Systems Device Panel March 15, 2016 Approved

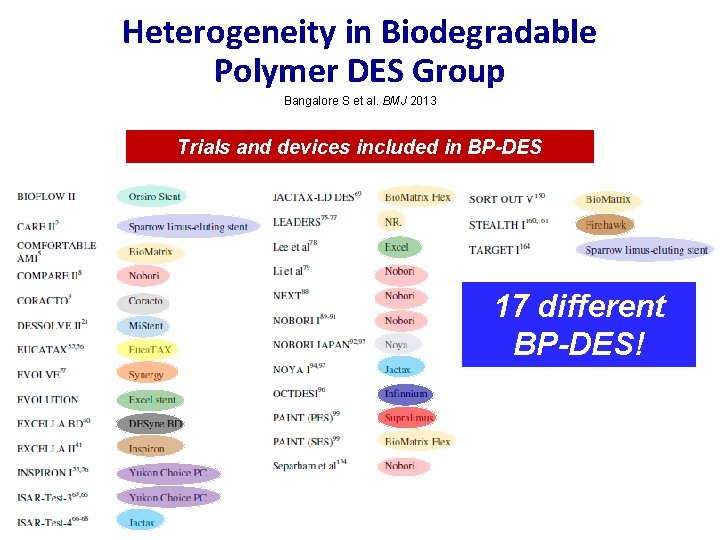
Heterogeneity in Biodegradable Polymer DES Group Bangalore S et al. BMJ 2013 Trials and devices included in BP-DES 17 different BP-DES!

Are DES with Biodegradable Polymers are Better than DES with Durable Polymer ?

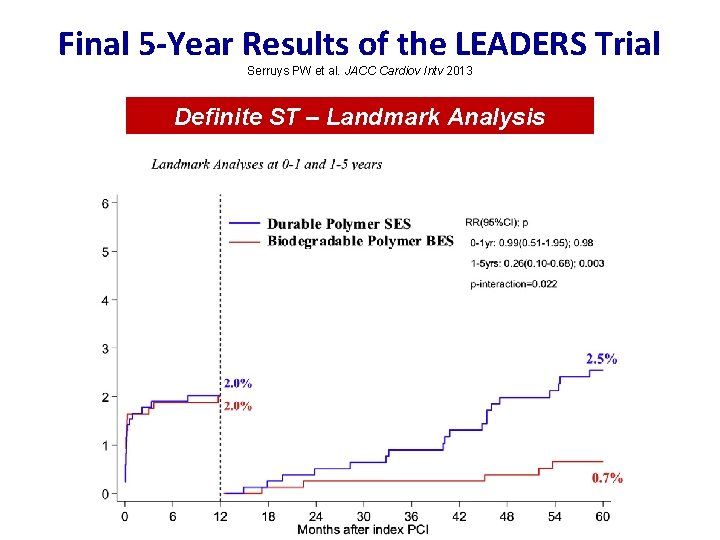
Final 5 -Year Results of the LEADERS Trial Serruys PW et al. JACC Cardiov Intv 2013 Definite ST – Landmark Analysis

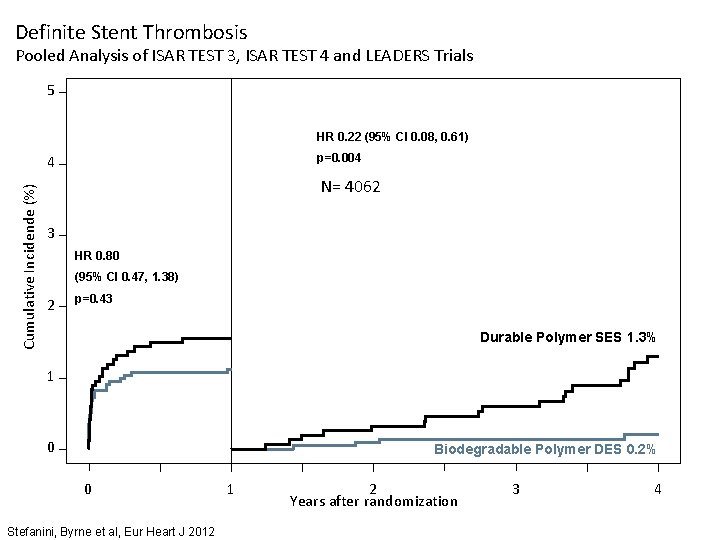
Definite Stent Thrombosis Pooled Analysis of ISAR TEST 3, ISAR TEST 4 and LEADERS Trials 5 HR 0. 22 (95% CI 0. 08, 0. 61) p=0. 004 Cumulative Incidende (%) 4 N= 4062 3 HR 0. 80 (95% CI 0. 47, 1. 38) 2 p=0. 43 Durable Polymer SES 1. 3% 1 0 Biodegradable Polymer DES 0. 2% 0 Stefanini, Byrne et al, Eur Heart J 2012 1 2 Years after randomization 3 4

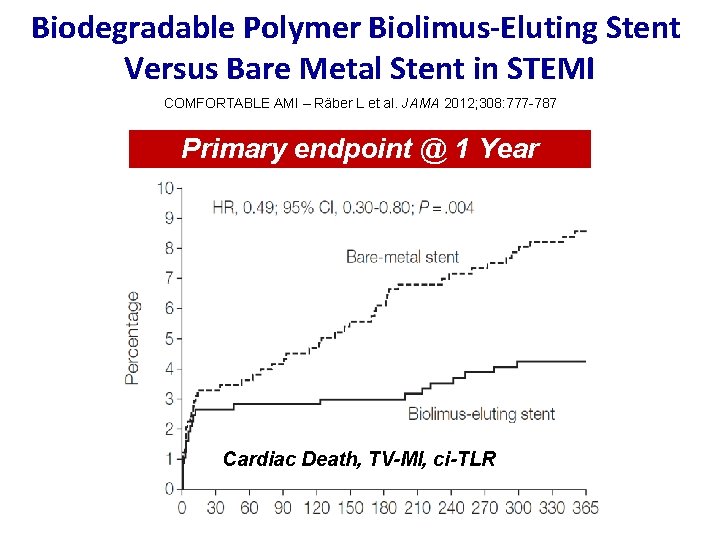
Biodegradable Polymer Biolimus-Eluting Stent Versus Bare Metal Stent in STEMI COMFORTABLE AMI – Räber L et al. JAMA 2012; 308: 777 -787 Primary endpoint @ 1 Year Cardiac Death, TV-MI, ci-TLR

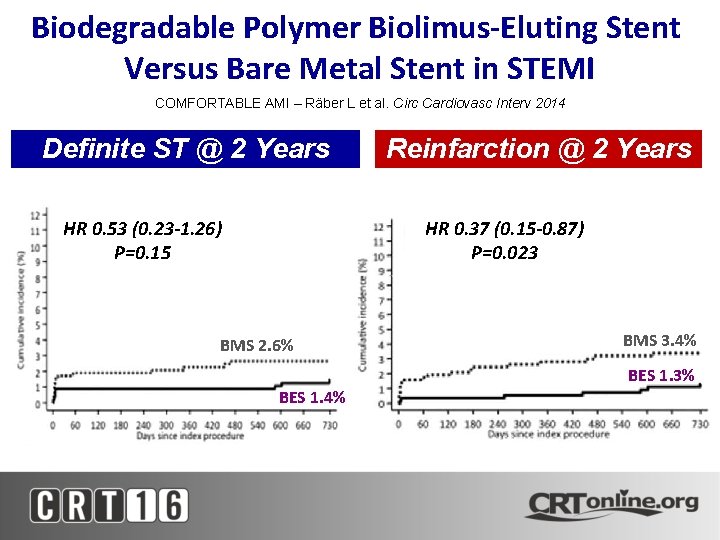
Biodegradable Polymer Biolimus-Eluting Stent Versus Bare Metal Stent in STEMI COMFORTABLE AMI – Räber L et al. Circ Cardiovasc Interv 2014 Definite ST @ 2 Years HR 0. 53 (0. 23 -1. 26) P=0. 15 Reinfarction @ 2 Years HR 0. 37 (0. 15 -0. 87) P=0. 023 BMS 2. 6% BMS 3. 4% BES 1. 3% BES 1. 4%

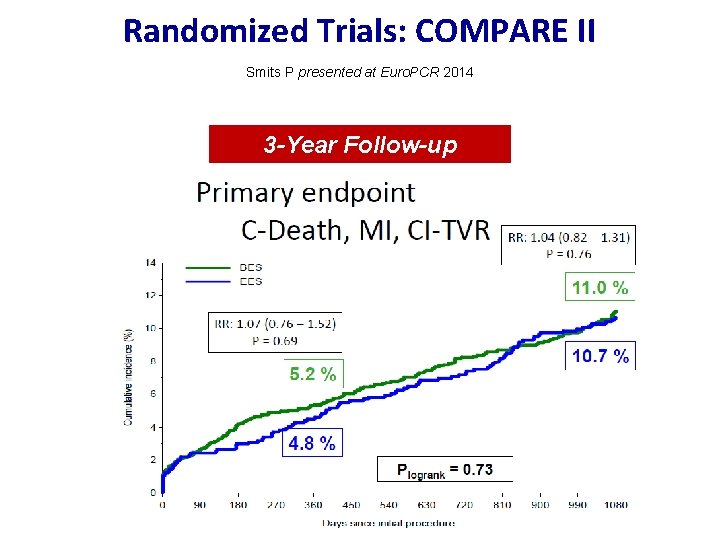
Randomized Trials: COMPARE II Smits P presented at Euro. PCR 2014 3 -Year Follow-up

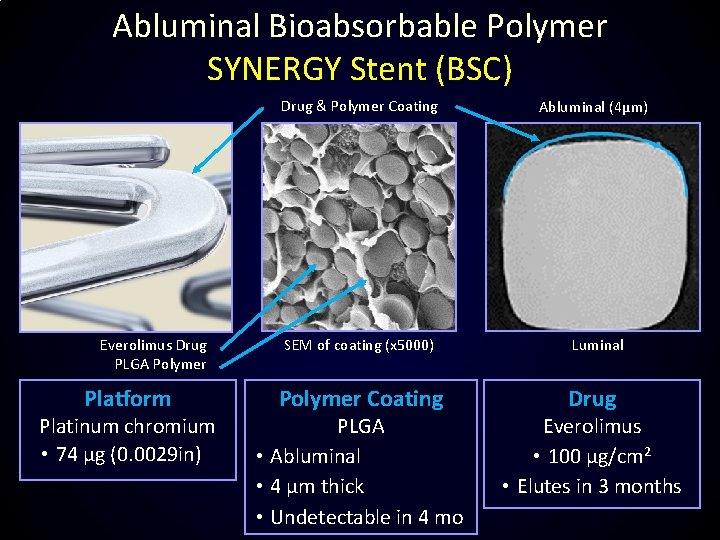
Abluminal Bioabsorbable Polymer SYNERGY Stent (BSC) Everolimus Drug PLGA Polymer Platform Platinum chromium • 74 μg (0. 0029 in) Drug & Polymer Coating Abluminal (4μm) SEM of coating (x 5000) Luminal Polymer Coating PLGA • Abluminal • 4 µm thick • Undetectable in 4 mo Drug Everolimus • 100 μg/cm 2 • Elutes in 3 months

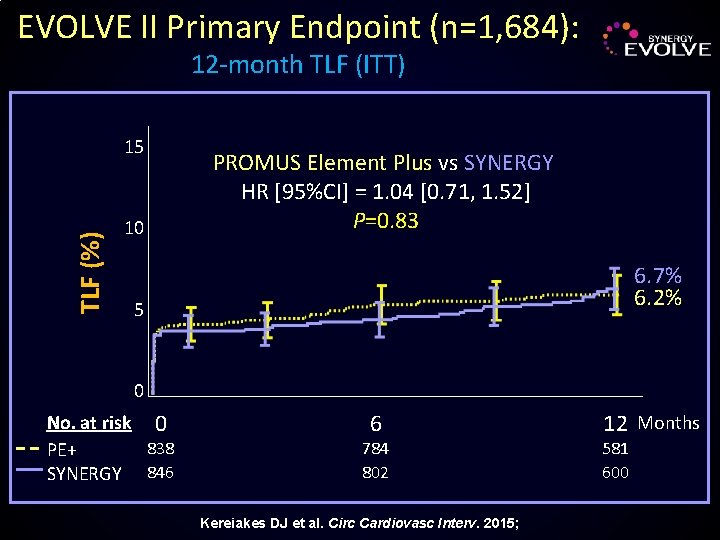
EVOLVE II Primary Endpoint (n=1, 684): 12 -month TLF (ITT) TLF (%) 15 10 PROMUS Element Plus vs SYNERGY HR [95%CI] = 1. 04 [0. 71, 1. 52] P=0. 83 6. 7% 6. 2% 5 0 No. at risk 0 838 PE+ SYNERGY 846 6 784 802 Kereiakes DJ et al. Circ Cardiovasc Interv. 2015; 12 Months 581 600

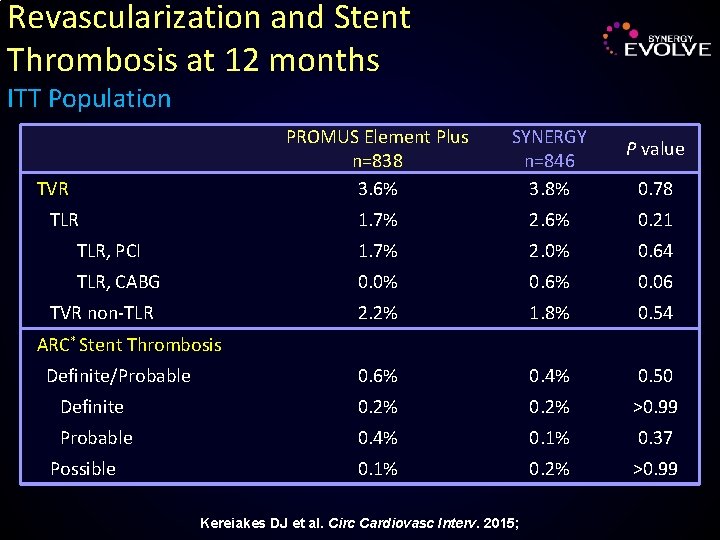
Revascularization and Stent Thrombosis at 12 months ITT Population PROMUS Element Plus n=838 3. 6% SYNERGY n=846 3. 8% 1. 7% 2. 6% 0. 21 TLR, PCI 1. 7% 2. 0% 0. 64 TLR, CABG 0. 0% 0. 6% 0. 06 2. 2% 1. 8% 0. 54 0. 6% 0. 4% 0. 50 Definite 0. 2% >0. 99 Probable 0. 4% 0. 1% 0. 37 0. 1% 0. 2% >0. 99 TVR TLR TVR non-TLR P value 0. 78 ARC* Stent Thrombosis Definite/Probable Possible Kereiakes DJ et al. Circ Cardiovasc Interv. 2015;

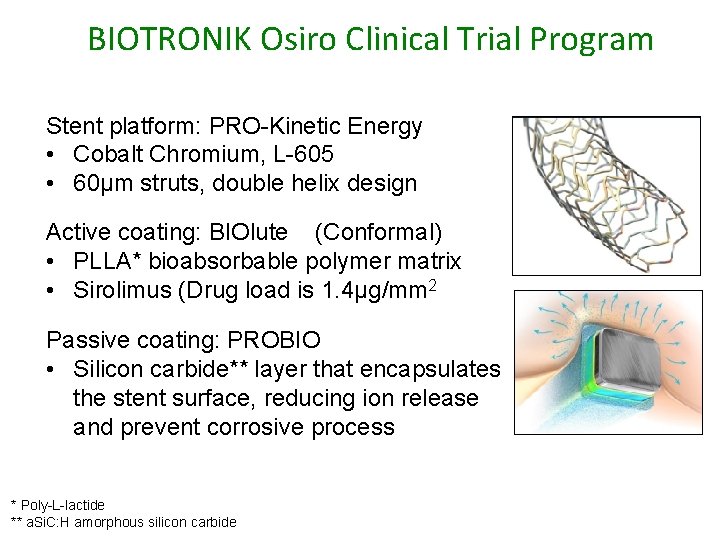
BIOTRONIK Osiro Clinical Trial Program Stent platform: PRO-Kinetic Energy • Cobalt Chromium, L-605 • 60µm struts, double helix design Active coating: BIOlute (Conformal) • PLLA* bioabsorbable polymer matrix • Sirolimus (Drug load is 1. 4µg/mm 2 Passive coating: PROBIO • Silicon carbide** layer that encapsulates the stent surface, reducing ion release and prevent corrosive process * Poly-L-lactide ** a. Si. C: H amorphous silicon carbide

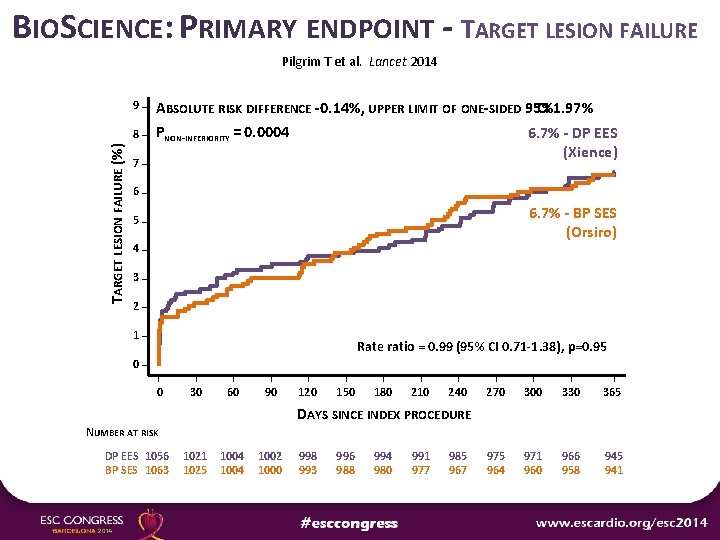
BIOSCIENCE: PRIMARY ENDPOINT - TARGET LESION FAILURE Pilgrim T et al. Lancet 2014 9 TARGET LESION FAILURE (%) 8 7 ABSOLUTE RISK DIFFERENCE -0. 14%, UPPER LIMIT OF ONE-SIDED 95% CI 1. 97% PNON-INFERIORITY = 0. 0004 6. 7% - DP EES (Xience) 6 6. 7% - BP SES (Orsiro) 5 4 3 2 1 Rate ratio = 0. 99 (95% CI 0. 71 -1. 38), p=0. 95 0 0 30 60 90 120 150 180 210 240 270 300 330 365 975 964 971 960 966 958 945 941 DAYS SINCE INDEX PROCEDURE NUMBER AT RISK DP EES 1056 BP SES 1063 1021 1025 1004 1002 1000 998 993 996 988 994 980 991 977 985 967

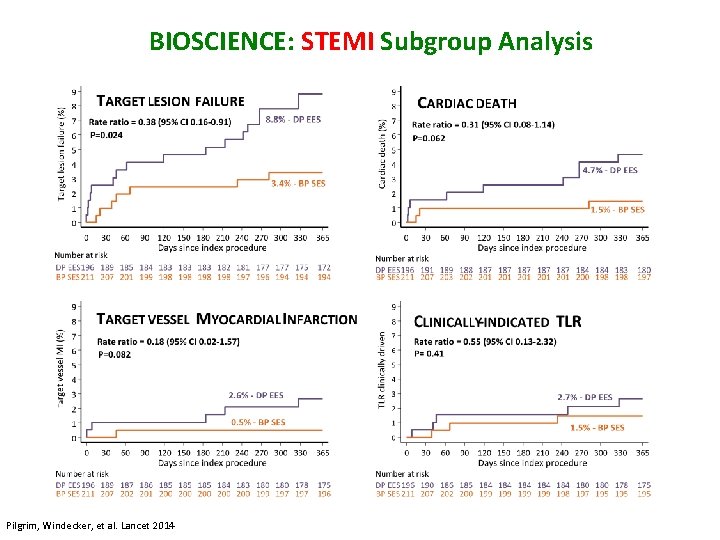
BIOSCIENCE: STEMI Subgroup Analysis Pilgrim, Windecker, et al. Lancet 2014

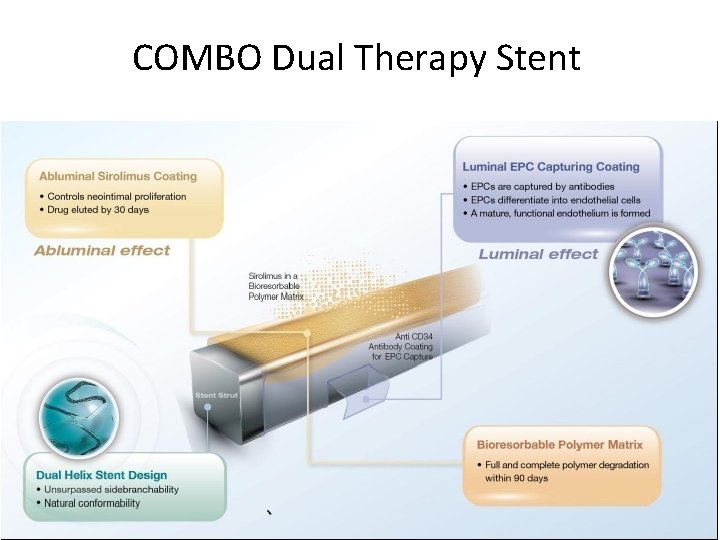
COMBO Dual Therapy Stent

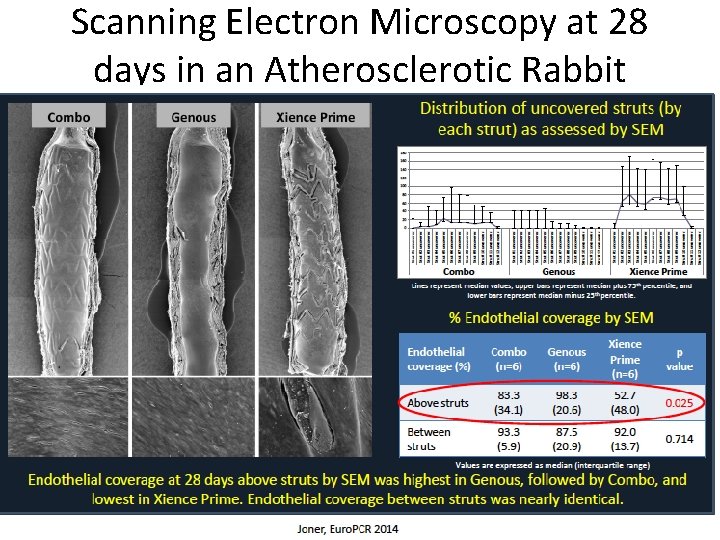
Scanning Electron Microscopy at 28 days in an Atherosclerotic Rabbit Model

Building a Polymer Free DES Pros and Cons of Current Approaches Bioabsorbable Polymers • Ability to extend drug elution to ensure efficacy • Need fast degradation (<3 m) to enable reduced DAPT • Potential concern about inflammation and biocompatibility during polymer degradation Polymer Free Nanotechnology • Potential to inhibit restenosis and cell proliferation without the use of a polymer • Concern about speed of elution (fast) and efficacy

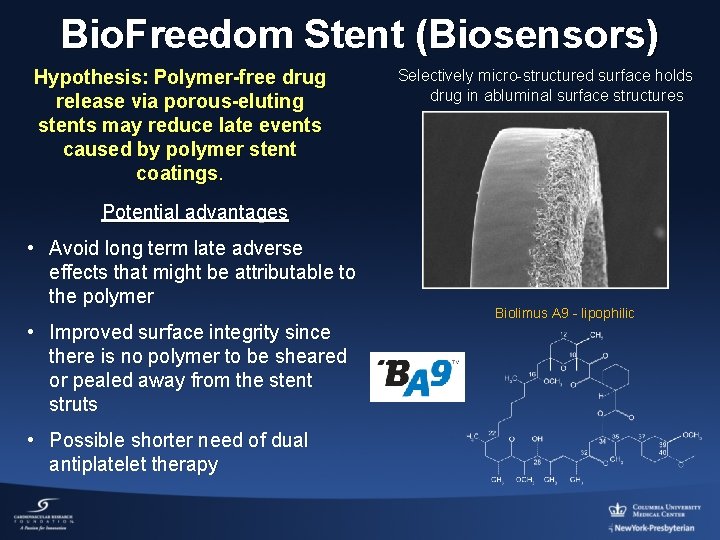
Bio. Freedom Stent (Biosensors) Hypothesis: Polymer-free drug release via porous-eluting stents may reduce late events caused by polymer stent coatings. Selectively micro-structured surface holds drug in abluminal surface structures Potential advantages • Avoid long term late adverse effects that might be attributable to the polymer • Improved surface integrity since there is no polymer to be sheared or pealed away from the stent struts • Possible shorter need of dual antiplatelet therapy Biolimus A 9 - lipophilic

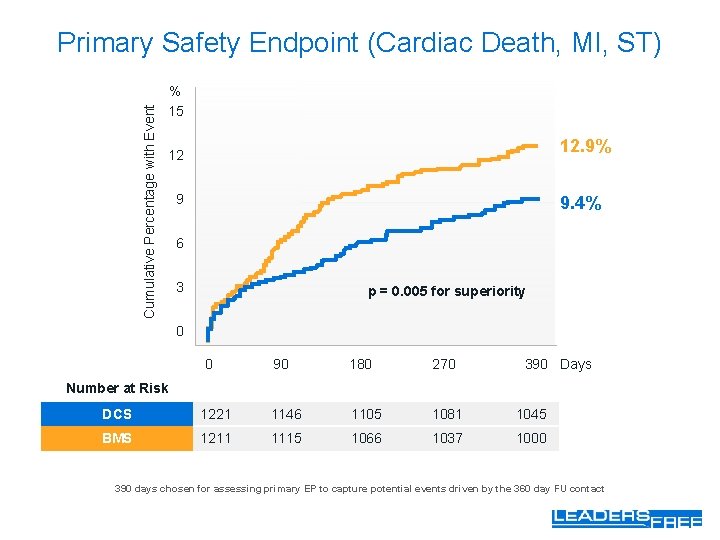
Cumulative Percentage with Event Primary Safety Endpoint (Cardiac Death, MI, ST) % 15 12. 9% 12 9 9. 4% 6 3 p = 0. 005 for superiority 0 0 90 180 270 390 Days Number at Risk DCS 1221 1146 1105 1081 1045 BMS 1211 1115 1066 1037 1000 390 days chosen for assessing primary EP to capture potential events driven by the 360 day FU contact

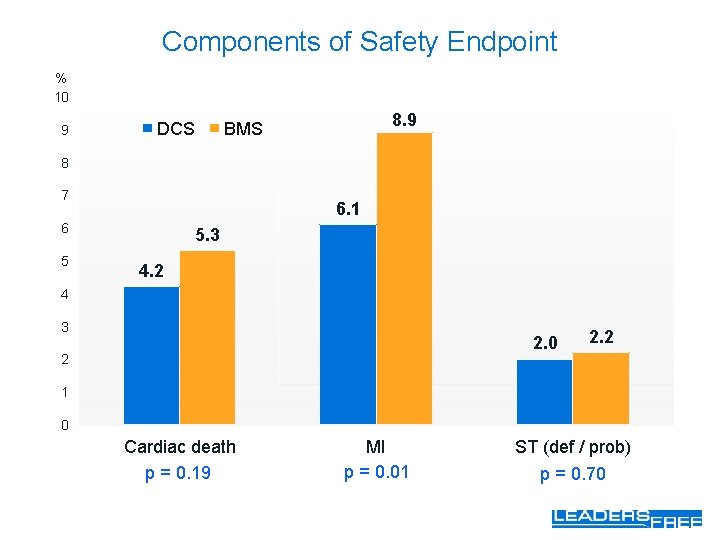
Components of Safety Endpoint % 10 9 DCS 8. 9 BMS 8 7 6. 1 6 5 5. 3 4. 2 4 3 2. 0 2 2. 2 1 0 Cardiac death p = 0. 19 MI p = 0. 01 ST (def / prob) p = 0. 70


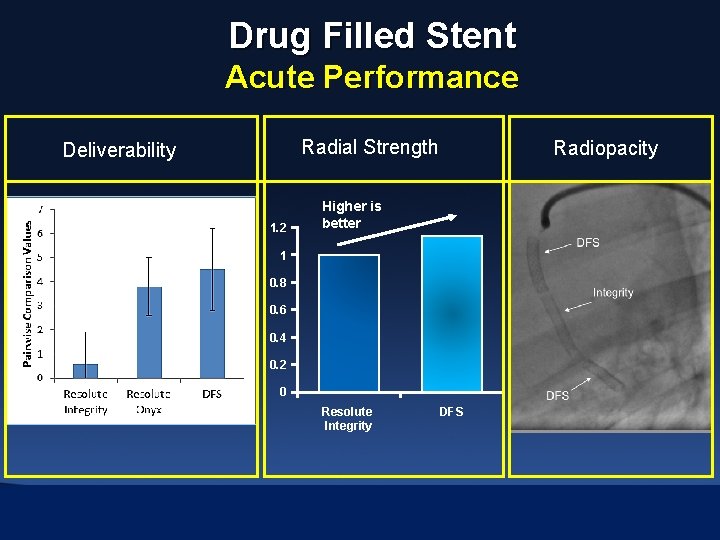
Drug Filled Stent Acute Performance Radial Strength Deliverability 1. 2 Radiopacity Higher is better 1 0. 8 0. 6 0. 4 0. 2 0 Resolute Integrity DFS

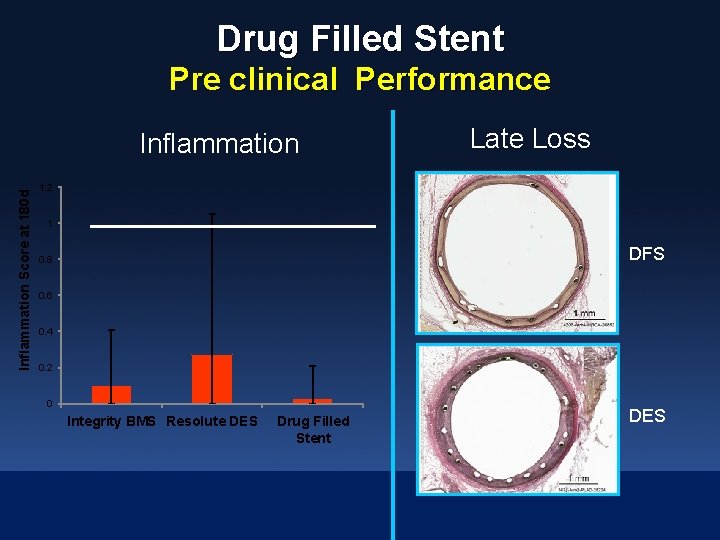
Drug Filled Stent Pre clinical Performance Inflammation Score at 180 d Inflammation Late Loss 1. 2 1 DFS 0. 8 0. 6 0. 4 0. 2 0 Integrity BMS Resolute DES Drug Filled Stent DES

Summary • New Drug Eluting stents are available now • They are thinner and more deliverable • Focus have shifted to Biodegradable Polymers, Polymer free, and biodegradable scaffolds. • At one year these new DES are non-inferior to best in class second generation DES. • The role of of fully biodegradable scaffold, depends on long term superiority on the best in class DES. • It remain to be seen if these emerging DES will have long term clinical impact

Thank you for your attention
 Simha sethumadhavan
Simha sethumadhavan För och nackdelar med firo
För och nackdelar med firo Matematisk modellering eksempel
Matematisk modellering eksempel Cks
Cks Särskild löneskatt för pensionskostnader
Särskild löneskatt för pensionskostnader Texter för hinduer tantra
Texter för hinduer tantra Lyckans minut erik lindorm analys
Lyckans minut erik lindorm analys Inköpsprocessen steg för steg
Inköpsprocessen steg för steg Kolposkopi, px
Kolposkopi, px Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning Varians formel
Varians formel Datorkunskap för nybörjare
Datorkunskap för nybörjare Tack för att ni har lyssnat
Tack för att ni har lyssnat Vad står k.r.å.k.a.n för
Vad står k.r.å.k.a.n för Läkarutlåtande för livränta
Läkarutlåtande för livränta Treserva lathund
Treserva lathund Tack för att ni lyssnade
Tack för att ni lyssnade Påbyggnader för flakfordon
Påbyggnader för flakfordon Egg för emanuel
Egg för emanuel Tobinskatten för och nackdelar
Tobinskatten för och nackdelar Atmosfr
Atmosfr Vanlig celldelning
Vanlig celldelning Verifikationsplan
Verifikationsplan Personlig tidbok fylla i
Personlig tidbok fylla i Rutin för avvikelsehantering
Rutin för avvikelsehantering Myndigheten för delaktighet
Myndigheten för delaktighet Presentera för publik crossboss
Presentera för publik crossboss Fuktmätningar i betong enlig rbk
Fuktmätningar i betong enlig rbk Fspos
Fspos Var 1721 för stormaktssverige
Var 1721 för stormaktssverige Tack för att ni har lyssnat
Tack för att ni har lyssnat Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Referat mall
Referat mall Varför kallas perioden 1918-1939 för mellankrigstiden?
Varför kallas perioden 1918-1939 för mellankrigstiden? Karttecken brant
Karttecken brant Fimbrietratt
Fimbrietratt Vätsketryck formel
Vätsketryck formel Enheter för massa
Enheter för massa Elektronik för barn
Elektronik för barn Borra hål för knoppar
Borra hål för knoppar Bris för vuxna
Bris för vuxna Mat för idrottare
Mat för idrottare A gastrica
A gastrica