DrugEluting Stents In Depth What are the FDA













- Slides: 15

Drug-Eluting Stents In Depth: What are the FDA Requirements for Label Expansion? by Ashley B. Boam, MSBE Interventional Cardiology Devices Branch FDA/CDRH/ODE/DCD February 22, 2010 DHHS/FDA

DISCLOSURES Ashley Boam I have no real or apparent conflicts of interest to report.

Typical “On-Label” Population n de novo lesions n single or dual vessel disease n length < 30 mm n diameters 2. 25/2. 5 – 3. 5/4. 0 mm n no major side branches (≥ 2. 0 mm) n not ostial, CTO, SVG, no thrombus n etc. DHHS/FDA

Possible Label Expansions n Treatment of vessels that are: – Diameters – 2. 25 mm or 4. 0 mm – Lengths - >30 mm – Bifurcations – Left main n Treatment of patients that are: – STEMI – non. STEMI ACS DHHS/FDA

Smaller, wider, longer n To date, most randomized studies limited to 2. 53. 5 mm diameter and <30 mm length – Approach for 2. 25 mm and 4. 0 mm diameters was single-arm study compared to performance goal based on historical data – Now that several approved DES available, include these diameters in randomized trial n For lesion lengths >30 mm to ~36 mm, singlearm study compared to performance goal; once longer stent lengths available, include in randomized cohort DHHS/FDA

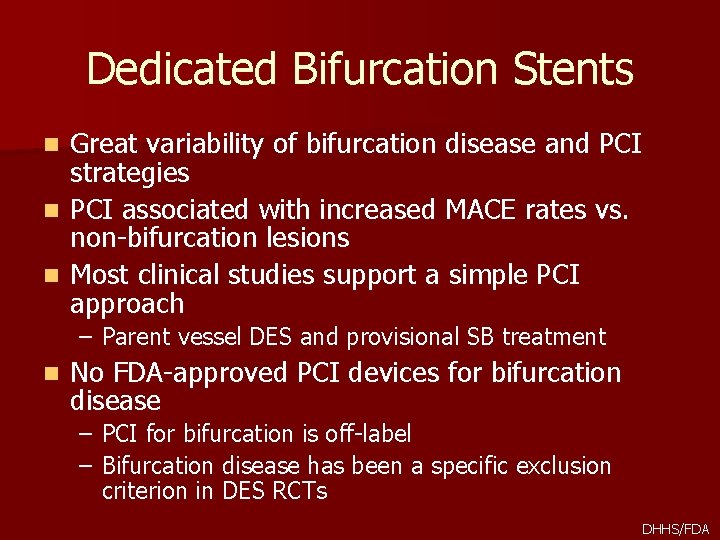
Dedicated Bifurcation Stents Great variability of bifurcation disease and PCI strategies n PCI associated with increased MACE rates vs. non-bifurcation lesions n Most clinical studies support a simple PCI approach n – Parent vessel DES and provisional SB treatment n No FDA-approved PCI devices for bifurcation disease – PCI for bifurcation is off-label – Bifurcation disease has been a specific exclusion criterion in DES RCTs DHHS/FDA

Dedicated Bifurcation Stents n RCT needed – No established OPC or performance goal – Multiple anatomic, procedural, and patient covariates requiring adjustment precludes a non-randomized trial n Proposed Control group: Approved DES in parent vessel with provisional SB treatment – Justify alternative approaches for specific Medina lesion subsets – Clearly define strategy for SB treatment in protocol § SB imaging only or functional assessment by FFR? – Specify & justify other techniques: e. g. , kissing balloon DHHS/FDA

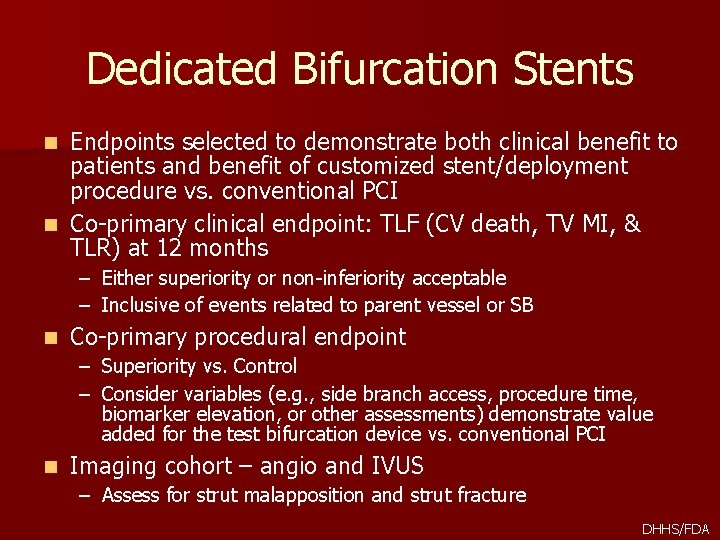
Dedicated Bifurcation Stents Endpoints selected to demonstrate both clinical benefit to patients and benefit of customized stent/deployment procedure vs. conventional PCI n Co-primary clinical endpoint: TLF (CV death, TV MI, & TLR) at 12 months n – Either superiority or non-inferiority acceptable – Inclusive of events related to parent vessel or SB n Co-primary procedural endpoint – Superiority vs. Control – Consider variables (e. g. , side branch access, procedure time, biomarker elevation, or other assessments) demonstrate value added for the test bifurcation device vs. conventional PCI n Imaging cohort – angio and IVUS – Assess for strut malapposition and strut fracture DHHS/FDA

DES for Left Main Important high-risk population for which a standard of care exists – CABG n Challenges in LM studies include: n – Identification of the patient population § all LM § with or without MVD § non-bifurcations – Selection of primary endpoint § MACCE composite (as in SYNTAX)? § split MACCE? – – n primary - death, MI, stroke secondary – revascularization More discussion tomorrow at FDA Town Hall DHHS/FDA

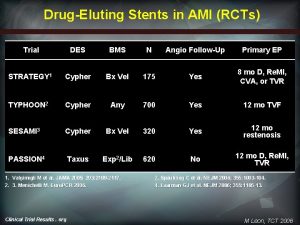
DES in STEMI Several previous small studies in STEMI n HORIZONS – large global RCT of DES vs. BMS in STEMI population (presented TCT 2008) n 12 month outcomes n – Efficacy – significant reduction in ischemic TLR (7. 5% BMS vs. 4. 5% Taxus) – Safety - no differences in MACE, all-cause mortality, death + reinfarct Outcomes fit with known pathology in STEMI n Relatively small differences predicted between DES and BMS n – – – larger diameter vessels shorter, more focal lesions non-stenotic lesions DHHS/FDA

Next Trials in STEMI n Currently not approved indication for DES n If Taxus gains indication for STEMI: – RCT vs. Taxus would be acceptable n If no DES with indication for STEMI – Equipoise still exists? – Consider RCT vs. BMS n In general, longer term outcomes (>1 year) and role of DAPT still of interest DHHS/FDA

DES for NSTEMI ACS n UA/NSTEMI – subset of ACS associated with an increased risk of cardiac death and subsequent MI n Defined by ECG ST-segment depression or prominent Twave inversion and/or positive biomarkers of necrosis (e. g. , troponin) in the absence of ST-segment elevation and with clinical evidence of ischemia n Move to troponin as “preferred biomarker” provides opportunity for new look at the population for trials of PCI with DES n Consider troponin+ patients with non-thrombotic lesions as target patient population DHHS/FDA

Trials for DES in NSTEMI n FDA is open to proposals; suggestions below n For DES already approved for stable patients – Single arm study of T+ patients (non-thrombotic lesions) – Comparison to PG based on results in stable patients + clinically acceptable margin – Alternative: Use of data from all-comers postmarket studies using above approach (if hypotheses prespecified under IDE) DHHS/FDA

Trials for DES in NSTEMI n For new DES – RCT – enroll stable and T+ patients (non-thrombotic lesions) – Control for stable patients is approved DES – Compare T+ patients to approved DES and PG – PG based on published studies of DES PCI in ACS (ACUITY, TRITON, others? ) 12 month primary endpoint still relevant in both cases n Also plan to assess: n – Follow-up >1 year – Impact of DAPT > 1 year DHHS/FDA

Contact Information Ashley Boam ashley. boam@fda. hhs. gov 301 -796 -6341 DHHS/FDA
 Insidan region jh
Insidan region jh Total internal reflection in a semicircular glass block
Total internal reflection in a semicircular glass block Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ độ dài liên kết
độ dài liên kết Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Phối cảnh
Phối cảnh điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Ng-html
Ng-html Hệ hô hấp
Hệ hô hấp Các số nguyên tố là gì
Các số nguyên tố là gì đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới