DENSITY WHAT IS THE DIFFERENCE MASS VOLUME DENSITY




















- Slides: 35

DENSITY

WHAT IS THE DIFFERENCE?

MASS, VOLUME & DENSITY • Mass and volume are physical properties. Together they are used to find the DENSITY of an object! • What is Volume? • What is Mass?

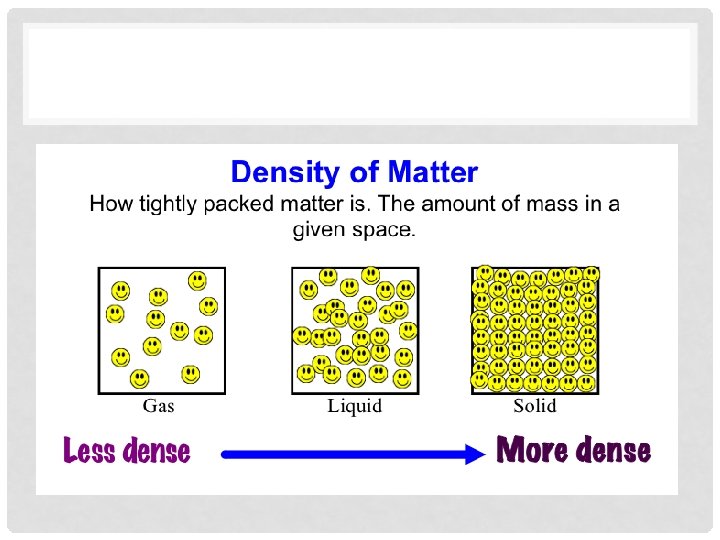
WHAT IS DENSITY? • Density is a comparison of how much matter there is in a certain amount of space. • The amount of matter per unit of space • More matter in less space= more dense • Less matter in more space= less dense

WHAT DOES THAT REALLY MEAN? • How tightly packed the matter in an object is

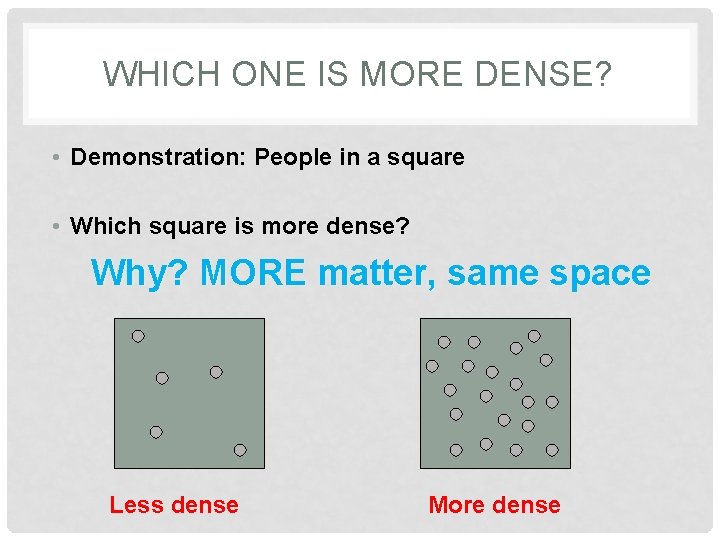
WHICH ONE IS MORE DENSE? • Demonstration: People in a square • Which square is more dense? Why? MORE matter, same space Less dense More dense

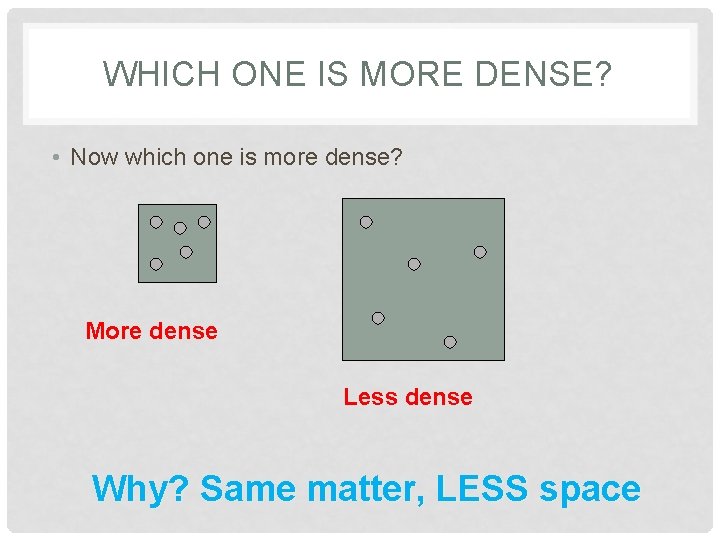
WHICH ONE IS MORE DENSE? • Now which one is more dense? More dense Less dense Why? Same matter, LESS space

YOU TRY • How do I make the front of the classroom MORE dense? LESS dense?

THINKING LIKE A COLLEGE SCHOLARCLAIM, EVIDENCE, REASONING • Claim: level one response stating the answer in a complete sentence. No proof, just answer. • Evidence: State the evidence in sentence form. (What is your proof? Your data? ) • Reasoning: • Answers the question “How do you know? ” • Contains your claim and evidence and explains them. • Shows you understand the question.

BOTH TOWNS TAKE ARE 500, 000 SQUARE FEET. WHICH ONE IS MORE DENSE? WHY? Claim: The city of waterloo is more dense. Evidence: Both cities take up the same amount of space, but Waterloo has more people in that space. Reasoning: An object that is more dense has more matter per unit of space. Waterloo has over 98, 000 more people tightly packed into the same amount of space, therefore Waterloo is more dense than Motley.

ONE ELEVATOR IS 20 SQ. FEET AND ANOTHER ELEVATOR IS 10 SQ. FEET. THEY BOTH HAVE 8 PEOPLE IN THEM. WHICH IS MORE DENSE? • Claim: Elevator 2 is more dense. • Evidence: Elevator 2 has the same amount of people in a smaller amount of space, so the matter is more tightly packed. • Reasoning: The more tightly packed together matter is, the more dense the object is. In Elevator 2, there is the same amount of matter in a smaller space, therefore elevator 2 is more dense.

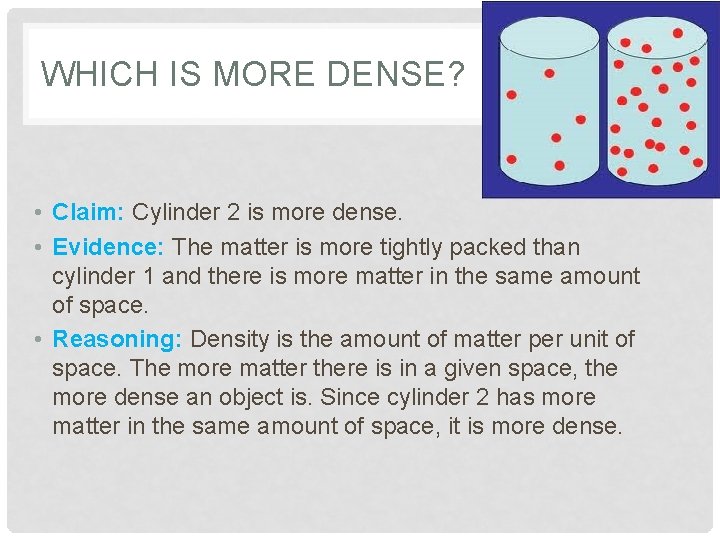
WHICH IS MORE DENSE? • Claim: Cylinder 2 is more dense. • Evidence: The matter is more tightly packed than cylinder 1 and there is more matter in the same amount of space. • Reasoning: Density is the amount of matter per unit of space. The more matter there is in a given space, the more dense an object is. Since cylinder 2 has more matter in the same amount of space, it is more dense.

PRACTICE!



DENSITY- LET’S CALCULATE!!!

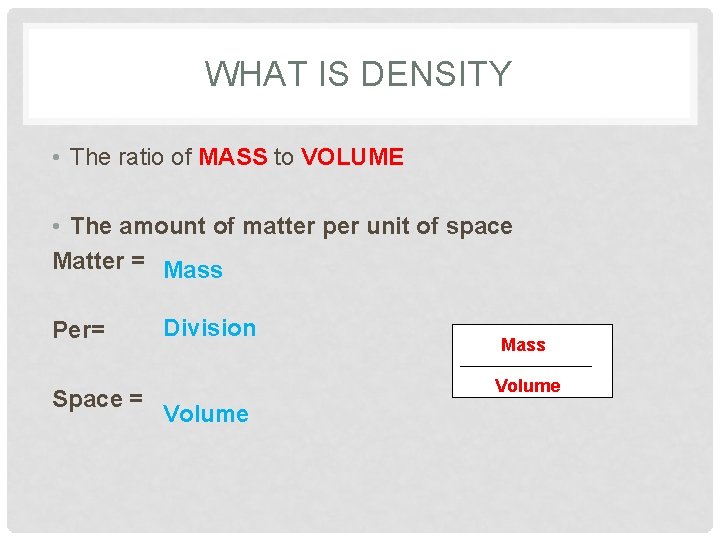
WHAT IS DENSITY • The ratio of MASS to VOLUME • The amount of matter per unit of space Matter = Mass Per= Division Mass ____________ Space = Volume

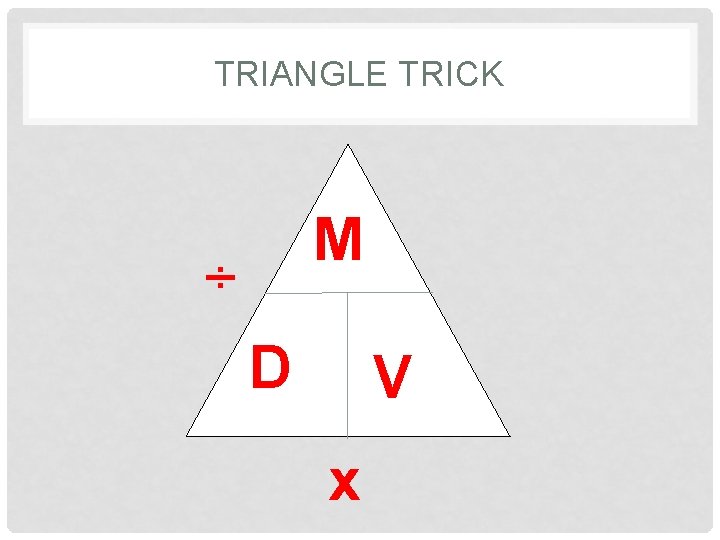
TRIANGLE TRICK M ÷ D V x

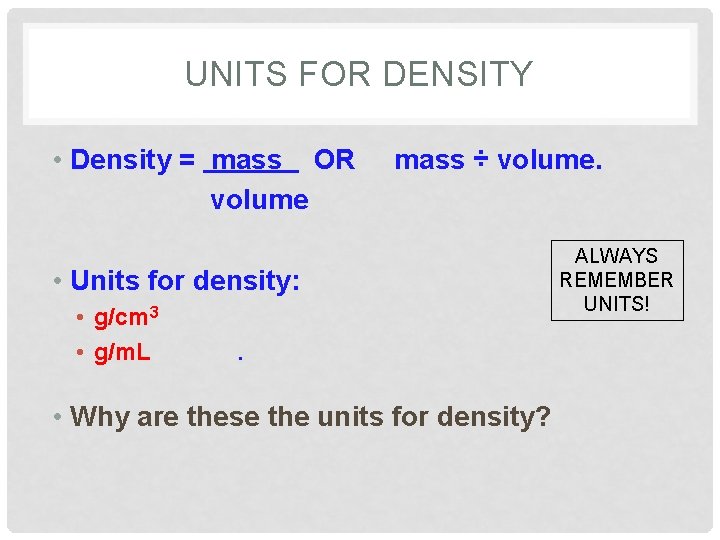
UNITS FOR DENSITY • Density = mass OR volume mass ÷ volume. • Units for density: • g/cm 3 • g/m. L . • Why are these the units for density? ALWAYS REMEMBER UNITS!

STEPS FOR CALCULATING DENSITY 1) Write the equation 2) Label 3) Plug in and solve

LET’S TRY A DENSITY PROBLEM TOGETHER • Frank has a paper clip. It has a mass of 9 g and a volume of 3 cm 3. What is its density? • • D= m/v M= 9 g, V=3 cm 3 D= 9 g/3 cm 3 D= 3 g/cm 3 • Frank also has an eraser. It has a mass of 42 g, and a volume of 7 m. L. What is its density? • • D= m/v M= 42 g, V=7 m. L D= 42 g/7 m. L D= 6 g/m. L

PRACTICE TIME

EXIT TICKET 1) Osmium is a very dense metal. What is its density if 50 g of the metal occupies a volume of 2 cm 3? 2) Which object is the most dense? Why? • Object 1: 1. 4 g/m. L • Object 2: 7 g/m. L • Object 3: 0. 3 g/m. L • Object 4: 24 g/m. L • Object 5: 1. 04 g/m. L


WORK ON THESE PROBLEMS WITH YOUR NEIGHBOR. • Jack has a rock. The rock has a mass of 6 g and a volume of 3 cm 3. What is the density of the rock? • Jill has a gel pen. The gel pen has a mass of 8 g and a volume of 2 cm 3. What is the density of the rock?


CALCULATING DENSITY WITH WATER DISPLACEMENT

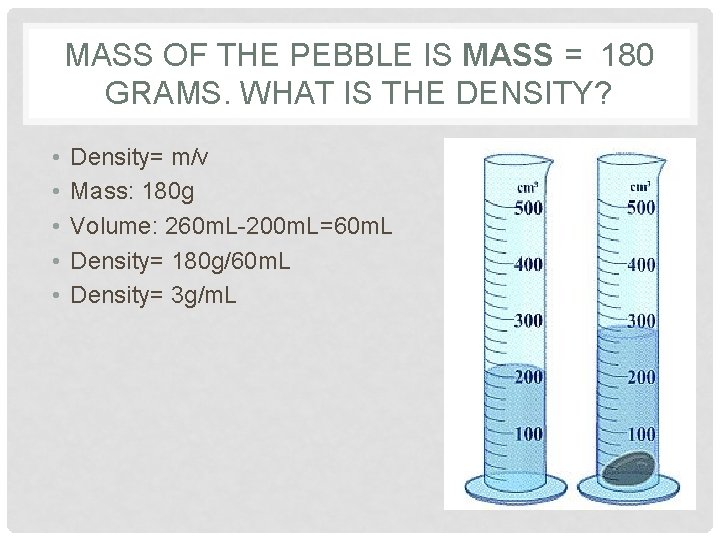
MASS OF THE PEBBLE IS MASS = 180 GRAMS. WHAT IS THE DENSITY? • • • Density= m/v Mass: 180 g Volume: 260 m. L-200 m. L=60 m. L Density= 180 g/60 m. L Density= 3 g/m. L

EXIT QUESTION • The mass of an object is 90 grams. You fill a graduated cylinder with 20 m. L of water. You drop the object into the graduated cylinder and the water rises to 25 m. L. What is the density of the object?

LIQUID LAYERS • If you pour together liquids that don’t mix and have different densities, they will form liquid layers. • The liquid with the highest density will be on the bottom. • The liquid with the lowest density will be on the top.

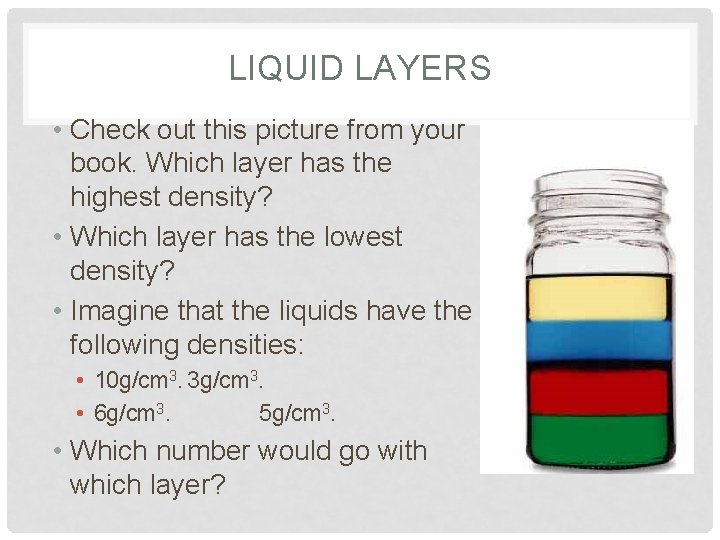
LIQUID LAYERS • Check out this picture from your book. Which layer has the highest density? • Which layer has the lowest density? • Imagine that the liquids have the following densities: • 10 g/cm 3. 3 g/cm 3. • 6 g/cm 3. 5 g/cm 3. • Which number would go with which layer?

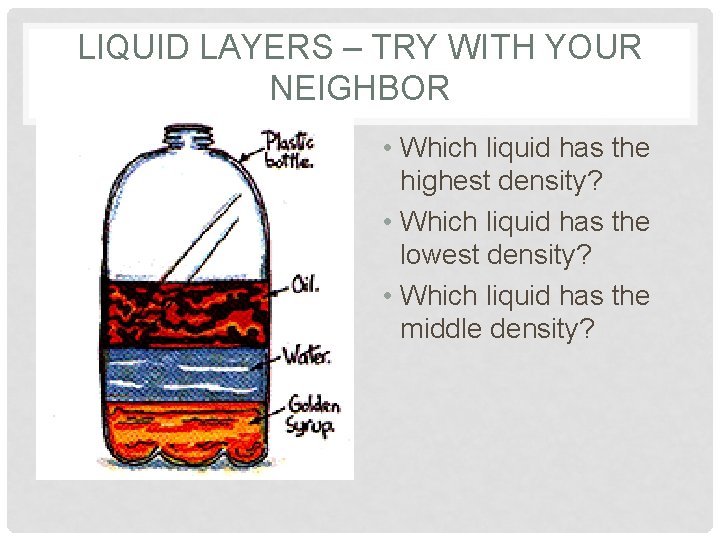
LIQUID LAYERS – TRY WITH YOUR NEIGHBOR • Which liquid has the highest density? • Which liquid has the lowest density? • Which liquid has the middle density?

LIQUID LAYERS – TRY ON YOUR OWN! • Imagine that the liquids on the right have the following densities: • 15 g/cm 3 • 3 g/cm 3 • 7 g/cm 3 10 g/cm 3 9 g/cm 3 12 g/cm 3 • Match the colors to the correct densities. 3 g/cm 3 7 g/cm 3 9 g/cm 3 10 g/cm 3 12 g/cm 3 15 g/cm 3

REVIEW • What is the formula for density? • What happens if you pour together liquids that have different densities? • Will the liquid on the top have the highest or lowest density? • Will the liquid on the bottom have the highest or lowest density?

SUPER SCIENTIST QUESTION OF THE DAY • Jake has a book, a ruler, and a balance. • How can Jake find the density of the book with the tools he has?