Clinical Diagnostic Therapeutic importance of Enzymes and isoenzymes






- Slides: 38

Clinical (Diagnostic & Therapeutic ) importance of Enzymes and isoenzymes

Objectives • List the clinically important enzymes and isoenzymes. • State which of the enzymes and isoenzymes are found in which tissues • Outline different ways of measuring plasma enzyme • Describe plasma enzyme changes in different diseases.

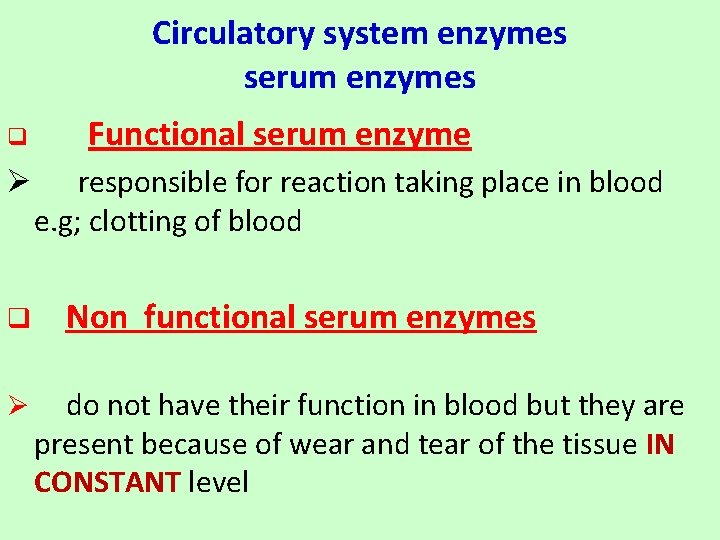
Circulatory system enzymes serum enzymes q Functional serum enzyme Ø responsible for reaction taking place in blood e. g; clotting of blood q Non functional serum enzymes Ø do not have their function in blood but they are present because of wear and tear of the tissue IN CONSTANT level

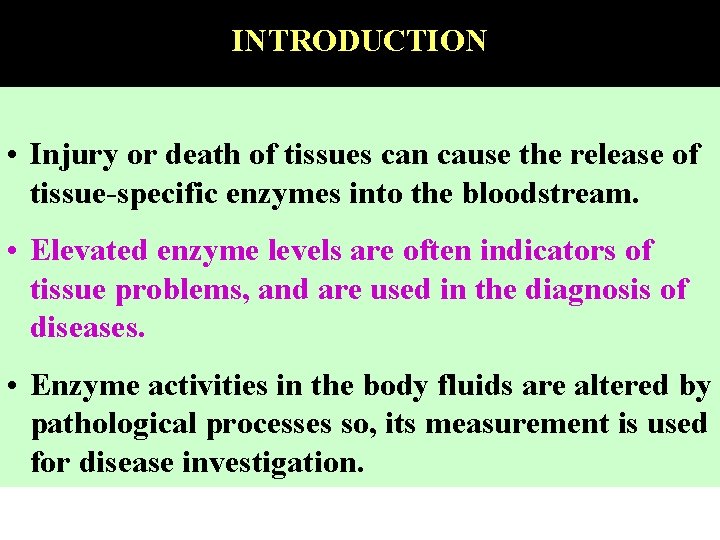
INTRODUCTION • Injury or death of tissues can cause the release of tissue-specific enzymes into the bloodstream. • Elevated enzyme levels are often indicators of tissue problems, and are used in the diagnosis of diseases. • Enzyme activities in the body fluids are altered by pathological processes so, its measurement is used for disease investigation.

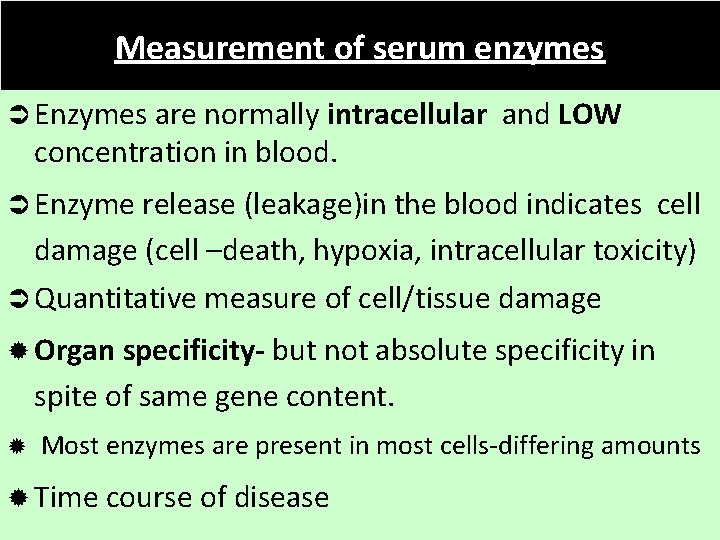
Measurement of serum enzymes Ü Enzymes are normally intracellular and LOW concentration in blood. Ü Enzyme release (leakage)in the blood indicates cell damage (cell –death, hypoxia, intracellular toxicity) Ü Quantitative measure of cell/tissue damage ® Organ specificity- but not absolute specificity in spite of same gene content. ® Most enzymes are present in most cells-differing amounts ® Time course of disease

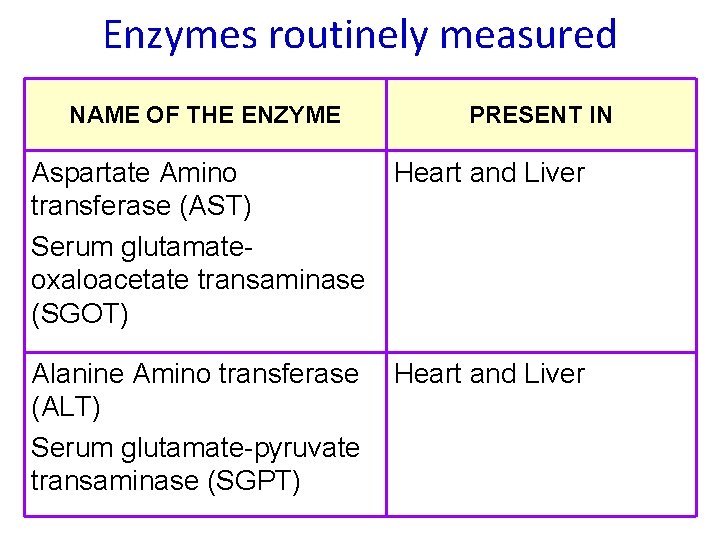
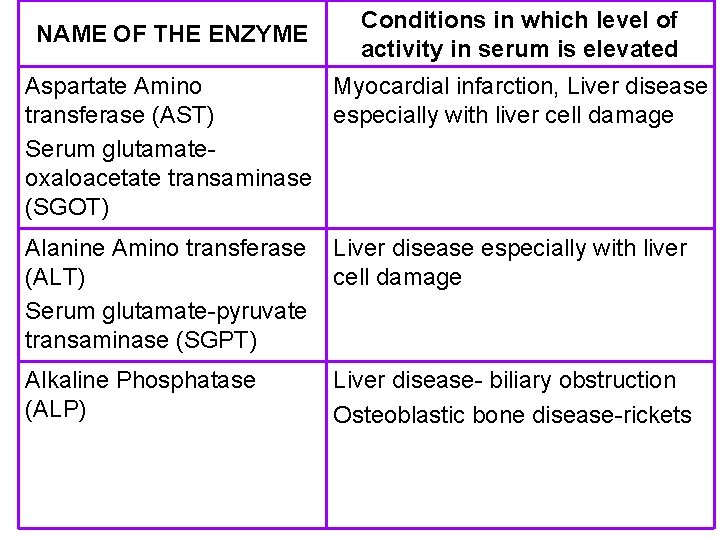
Enzymes routinely measured NAME OF THE ENZYME PRESENT IN Aspartate Amino Heart and Liver transferase (AST) Serum glutamateoxaloacetate transaminase (SGOT) Alanine Amino transferase (ALT) Serum glutamate-pyruvate transaminase (SGPT) Heart and Liver

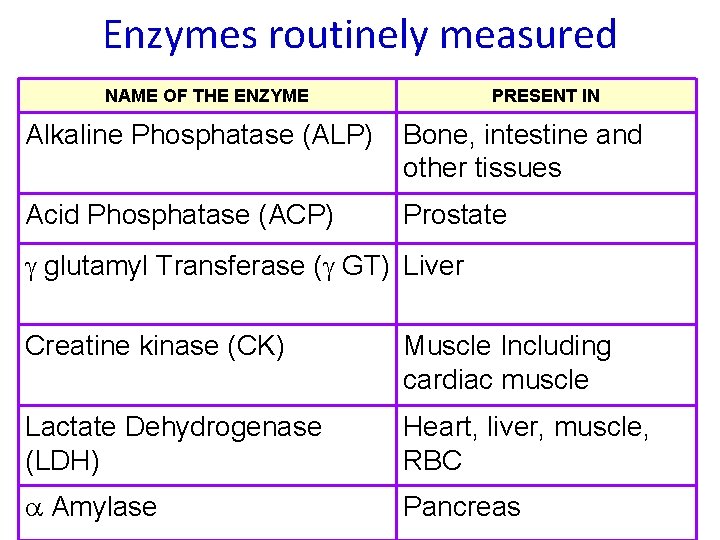
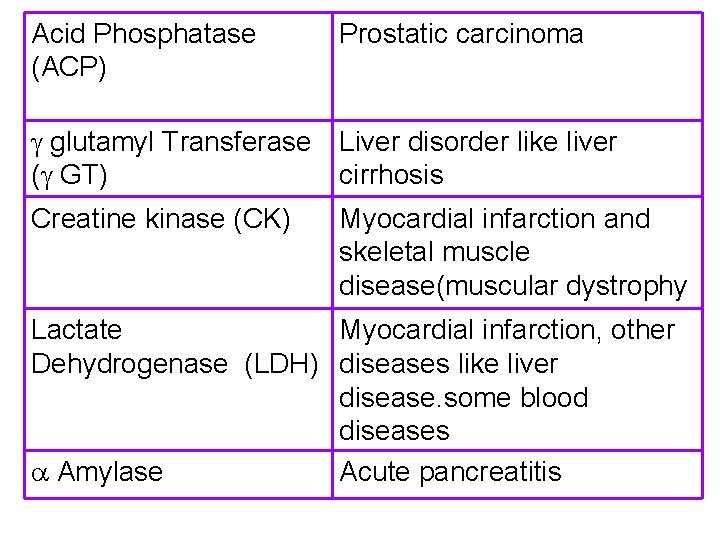
Enzymes routinely measured NAME OF THE ENZYME PRESENT IN Alkaline Phosphatase (ALP) Bone, intestine and other tissues Acid Phosphatase (ACP) Prostate glutamyl Transferase ( GT) Liver Creatine kinase (CK) Muscle Including cardiac muscle Lactate Dehydrogenase (LDH) Heart, liver, muscle, RBC Amylase Pancreas

Myocardial Infarction

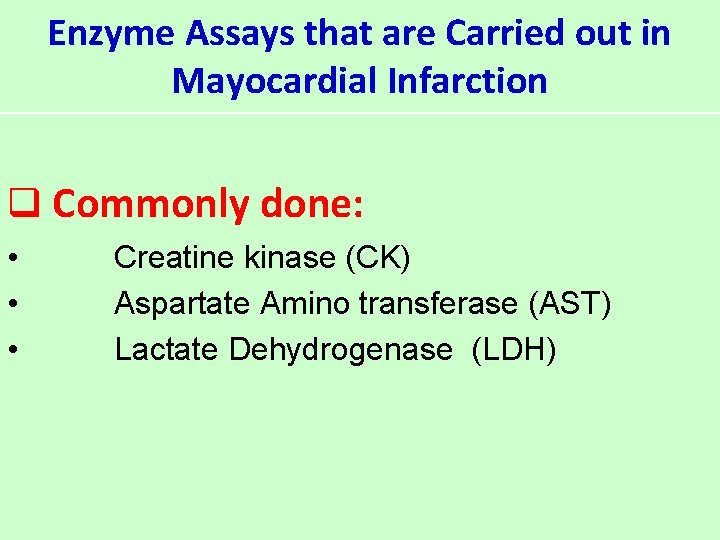
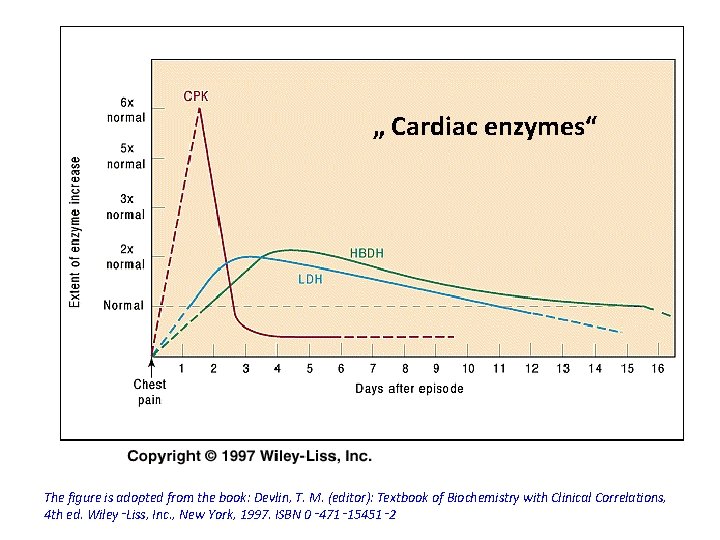
Enzyme Assays that are Carried out in Mayocardial Infarction q Commonly done: • • • Creatine kinase (CK) Aspartate Amino transferase (AST) Lactate Dehydrogenase (LDH)

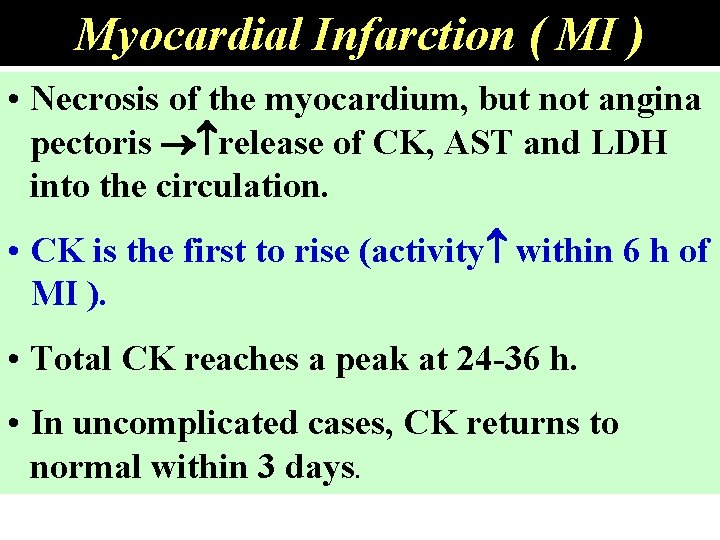
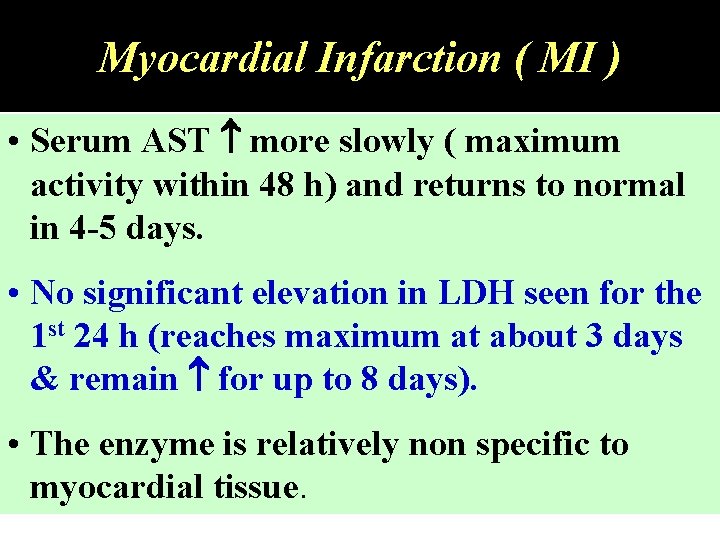
Myocardial Infarction ( MI ) • Necrosis of the myocardium, but not angina pectoris release of CK, AST and LDH into the circulation. • CK is the first to rise (activity within 6 h of MI ). • Total CK reaches a peak at 24 -36 h. • In uncomplicated cases, CK returns to normal within 3 days.

Myocardial Infarction ( MI ) • Serum AST more slowly ( maximum activity within 48 h) and returns to normal in 4 -5 days. • No significant elevation in LDH seen for the 1 st 24 h (reaches maximum at about 3 days & remain for up to 8 days). • The enzyme is relatively non specific to myocardial tissue.

Liver Diseases Hepatic Necrosis Hepatitis Cholestasis Jaundice Hepatocellular Damage

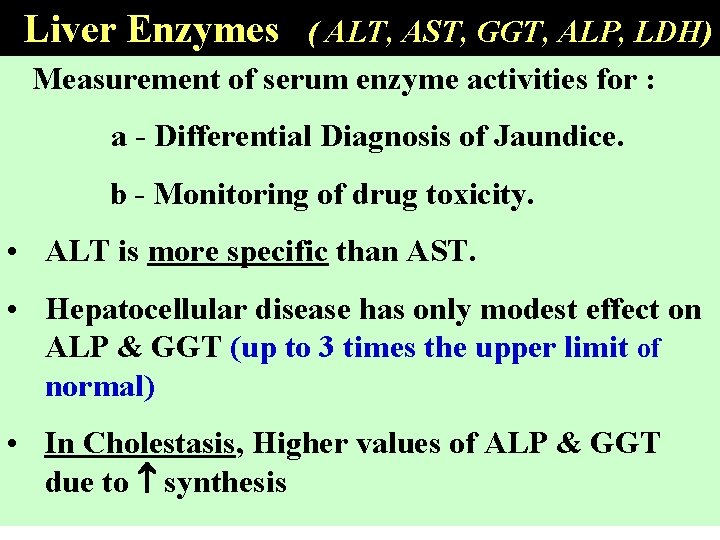
Liver Enzymes ( ALT, AST, GGT, ALP, LDH) Measurement of serum enzyme activities for : a - Differential Diagnosis of Jaundice. b - Monitoring of drug toxicity. • ALT is more specific than AST. • Hepatocellular disease has only modest effect on ALP & GGT (up to 3 times the upper limit of normal) • In Cholestasis, Higher values of ALP & GGT due to synthesis

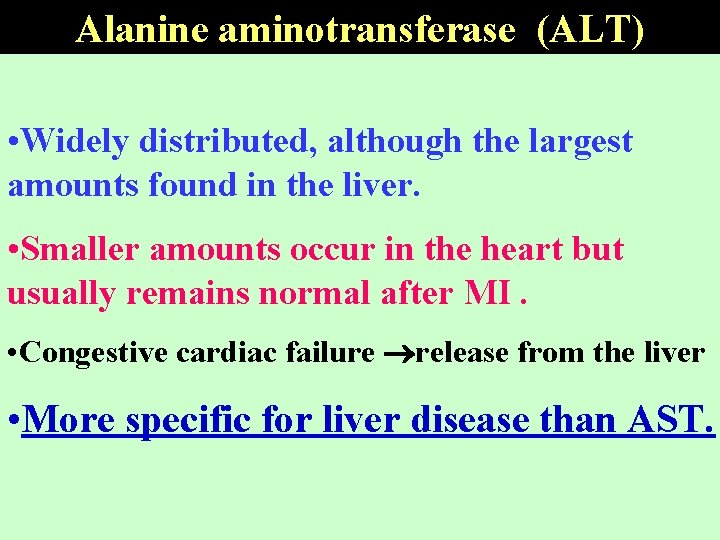
Alanine aminotransferase (ALT) • Widely distributed, although the largest amounts found in the liver. • Smaller amounts occur in the heart but usually remains normal after MI. • Congestive cardiac failure release from the liver • More specific for liver disease than AST.

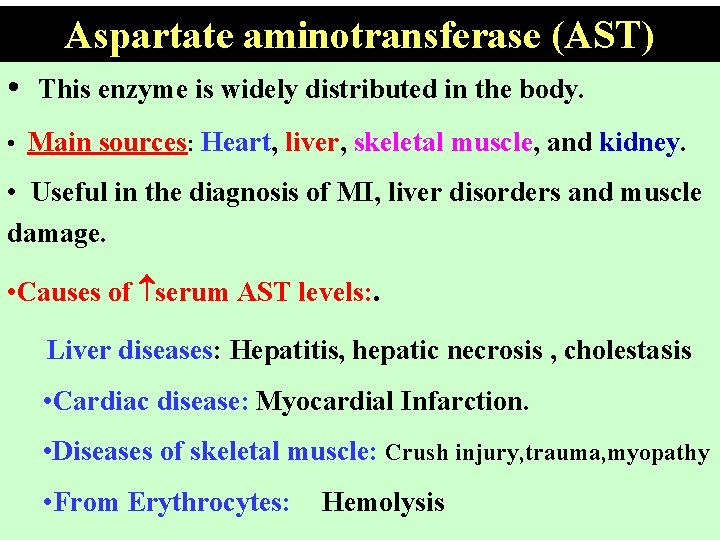
Aspartate aminotransferase (AST) • This enzyme is widely distributed in the body. • Main sources: Heart, liver, skeletal muscle, and kidney. • Useful in the diagnosis of MI, liver disorders and muscle damage. • Causes of serum AST levels: . Liver diseases: Hepatitis, hepatic necrosis , cholestasis • Cardiac disease: Myocardial Infarction. • Diseases of skeletal muscle: Crush injury, trauma, myopathy • From Erythrocytes: Hemolysis

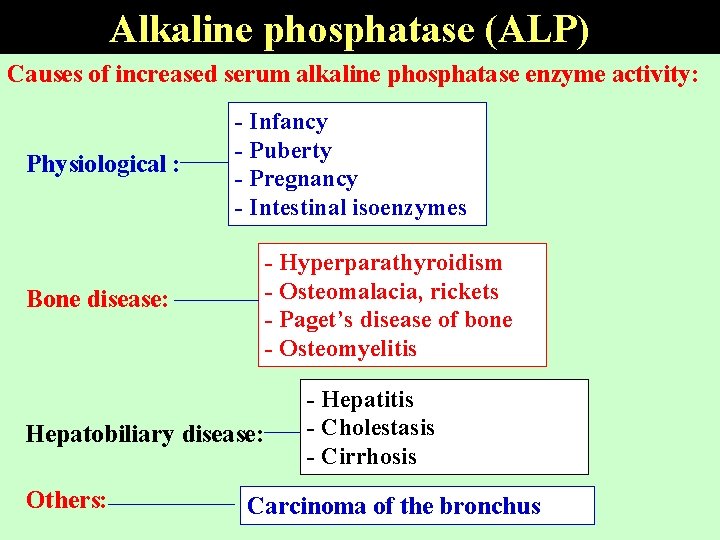
Alkaline phosphatase (ALP) • Widely distributed, high concentrations in intestines, liver, bone, spleen, placenta and kidney. • The main sources of serum ALP are the hepatobiliary tree and bone disorders. • Elevated levels during healing of fractures , active growth and during the 3 rd trimester of pregnancy. • serum ALP activity in liver disease is mainly due to Cholestasis. • Decreased levels are found in the inherited condition

Alkaline phosphatase (ALP) Causes of increased serum alkaline phosphatase enzyme activity: Physiological : Bone disease: - Infancy - Puberty - Pregnancy - Intestinal isoenzymes - Hyperparathyroidism - Osteomalacia, rickets - Paget’s disease of bone - Osteomyelitis Hepatobiliary disease: Others: - Hepatitis - Cholestasis - Cirrhosis Carcinoma of the bronchus

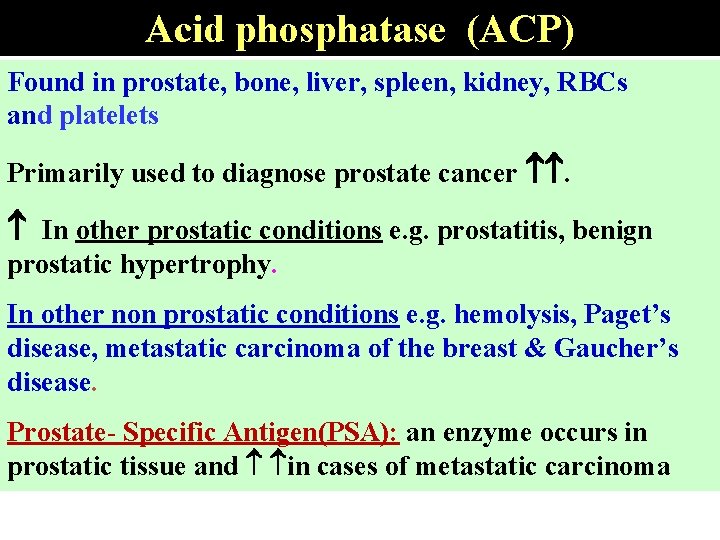
Acid phosphatase (ACP) Found in prostate, bone, liver, spleen, kidney, RBCs and platelets Primarily used to diagnose prostate cancer . In other prostatic conditions e. g. prostatitis, benign prostatic hypertrophy. In other non prostatic conditions e. g. hemolysis, Paget’s disease, metastatic carcinoma of the breast & Gaucher’s disease. Prostate- Specific Antigen(PSA): an enzyme occurs in prostatic tissue and in cases of metastatic carcinoma

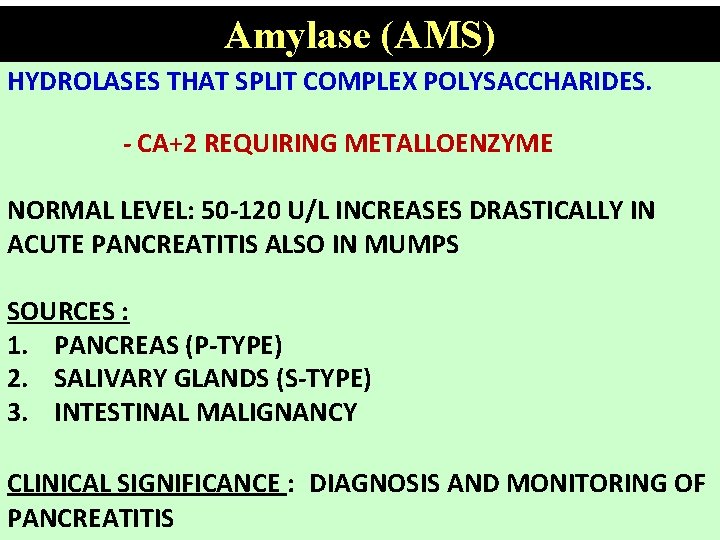
Amylase (AMS) HYDROLASES THAT SPLIT COMPLEX POLYSACCHARIDES. - CA+2 REQUIRING METALLOENZYME NORMAL LEVEL: 50 -120 U/L INCREASES DRASTICALLY IN ACUTE PANCREATITIS ALSO IN MUMPS SOURCES : 1. PANCREAS (P-TYPE) 2. SALIVARY GLANDS (S-TYPE) 3. INTESTINAL MALIGNANCY CLINICAL SIGNIFICANCE : DIAGNOSIS AND MONITORING OF PANCREATITIS

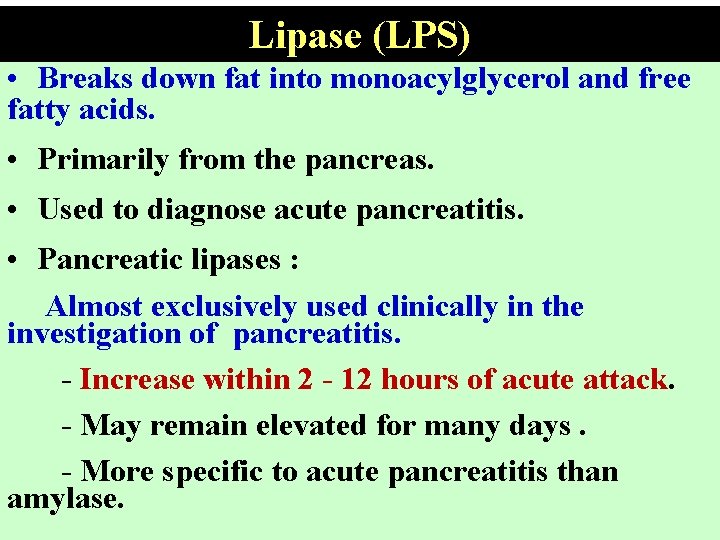
Lipase (LPS) • Breaks down fat into monoacylglycerol and free fatty acids. • Primarily from the pancreas. • Used to diagnose acute pancreatitis. • Pancreatic lipases : Almost exclusively used clinically in the investigation of pancreatitis. - Increase within 2 - 12 hours of acute attack. - May remain elevated for many days. - More specific to acute pancreatitis than amylase.

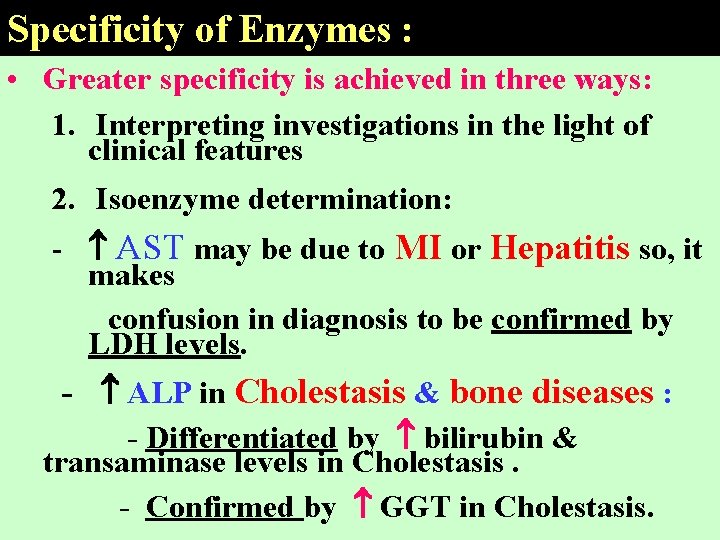
Specificity of Enzymes : • Greater specificity is achieved in three ways: 1. Interpreting investigations in the light of clinical features 2. Isoenzyme determination: - AST may be due to MI or Hepatitis so, it makes confusion in diagnosis to be confirmed by LDH levels. - ALP in Cholestasis & bone diseases : - Differentiated by bilirubin & transaminase levels in Cholestasis. - Confirmed by GGT in Cholestasis.

NAME OF THE ENZYME Conditions in which level of activity in serum is elevated Aspartate Amino Myocardial infarction, Liver disease transferase (AST) especially with liver cell damage Serum glutamateoxaloacetate transaminase (SGOT) Alanine Amino transferase (ALT) Serum glutamate-pyruvate transaminase (SGPT) Liver disease especially with liver cell damage Alkaline Phosphatase (ALP) Liver disease- biliary obstruction Osteoblastic bone disease-rickets

Acid Phosphatase (ACP) Prostatic carcinoma glutamyl Transferase Liver disorder like liver ( GT) cirrhosis Creatine kinase (CK) Myocardial infarction and skeletal muscle disease(muscular dystrophy Lactate Myocardial infarction, other Dehydrogenase (LDH) diseases like liver disease. some blood diseases Amylase Acute pancreatitis

ISOENZYMES • Catalyze the same reaction • Two or more polypeptide chains • Different polypeptide chains are products of different genes • Differ in AA sequence and physical properties • May be separable on the basis of charge • Are tissue specific

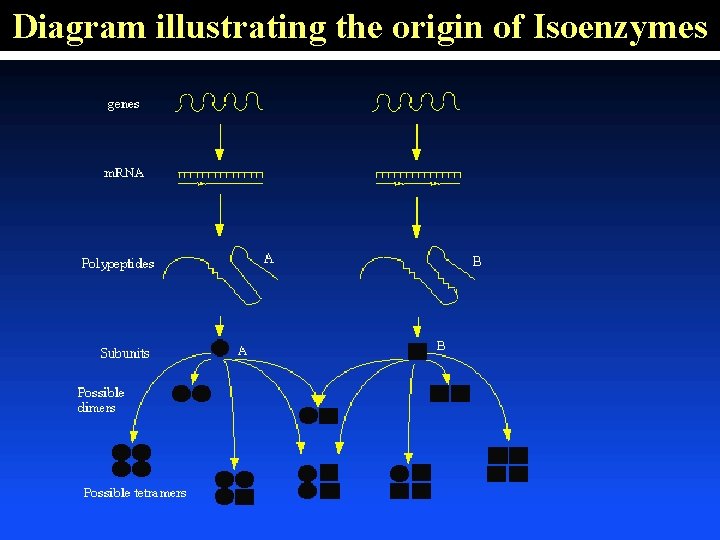
Diagram illustrating the origin of Isoenzymes

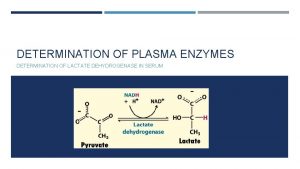
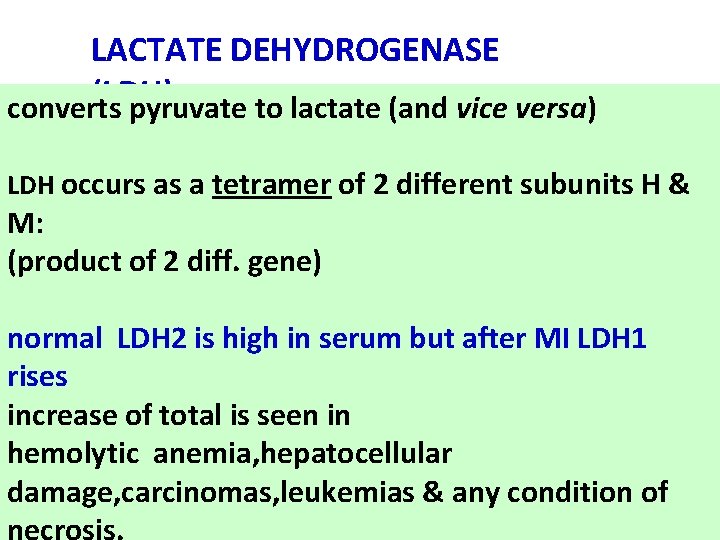
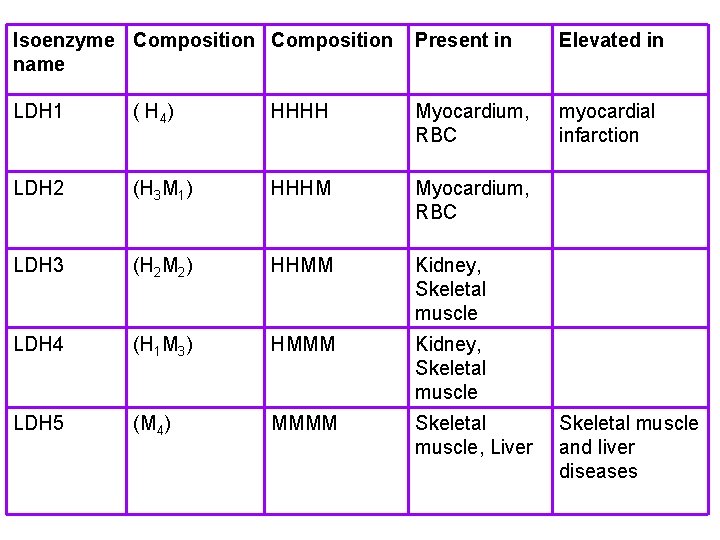
LACTATE DEHYDROGENASE (LDH) converts pyruvate to lactate (and vice versa) LDH occurs as a tetramer of 2 different subunits H & M: (product of 2 diff. gene) normal LDH 2 is high in serum but after MI LDH 1 rises increase of total is seen in hemolytic anemia, hepatocellular damage, carcinomas, leukemias & any condition of necrosis.

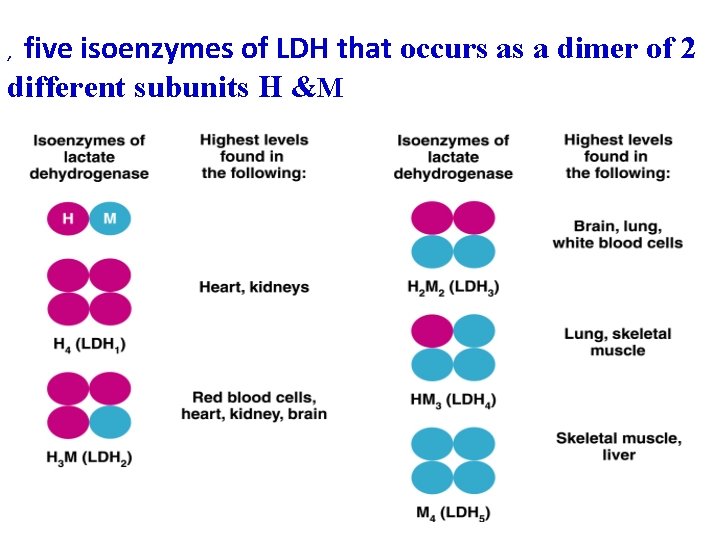
five isoenzymes of LDH that occurs as a dimer of 2 different subunits H &M ,

Isoenzyme Composition name Present in Elevated in LDH 1 ( H 4 ) HHHH Myocardium, RBC myocardial infarction LDH 2 (H 3 M 1) HHHM Myocardium, RBC LDH 3 (H 2 M 2) HHMM Kidney, Skeletal muscle LDH 4 (H 1 M 3) HMMM Kidney, Skeletal muscle LDH 5 (M 4) MMMM Skeletal muscle, Liver Skeletal muscle and liver diseases

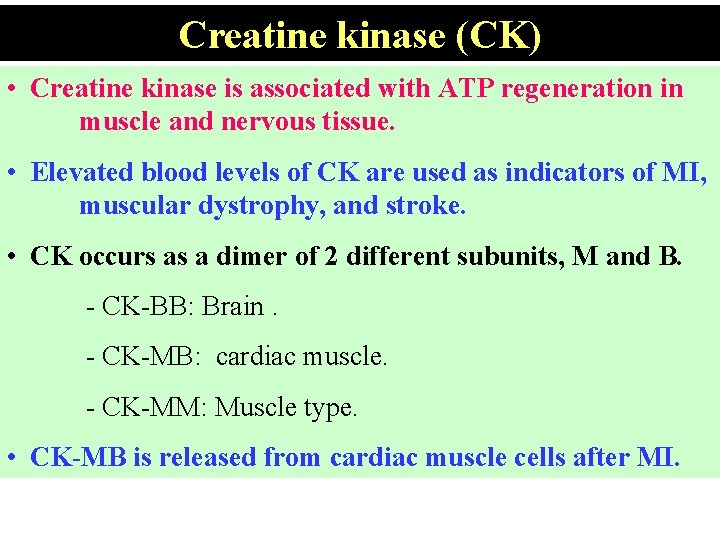
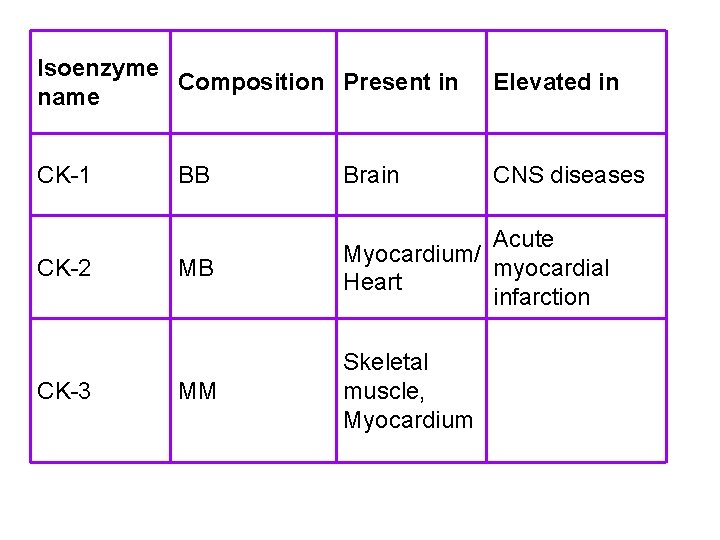
Creatine kinase (CK) • Creatine kinase is associated with ATP regeneration in muscle and nervous tissue. • Elevated blood levels of CK are used as indicators of MI, muscular dystrophy, and stroke. • CK occurs as a dimer of 2 different subunits, M and B. - CK-BB: Brain. - CK-MB: cardiac muscle. - CK-MM: Muscle type. • CK-MB is released from cardiac muscle cells after MI.

Isoenzyme Composition Present in name Elevated in CK-1 CNS diseases CK-2 CK-3 BB Brain MB Acute Myocardium/ myocardial Heart infarction MM Skeletal muscle, Myocardium

„ Cardiac enzymes“ The figure is adopted from the book: Devlin, T. M. (editor): Textbook of Biochemistry with Clinical Correlations, 4 th ed. Wiley‑Liss, Inc. , New York, 1997. ISBN 0‑ 471‑ 15451‑ 2

ENZYMES IN THERAPY • Substitution of missing production of digestive enzymes – pepsin trypsin… • Removal of deposits of death tissue or fibrin (e. g. in lungs, eyes), treatment of skin defects – proteinases, nucleases, collagenase • Acceleration of fibrinolysis in lungs embolization (activation of plasmin and plasminogen) – • streptokinase, urokinase

T h a n k y ou

Quiz 1. An activated enzyme consisting of polypeptide chain and a cofactor is called: • A. Apoenzyme B. Holoenzyme 2. Which one forms the raw material for coenzymes? • A. Viitamins B. Carbohydrates 3. A cofactor made of inorganic ion which is detachable is called • A. Prosthetic group B. Coenzyme

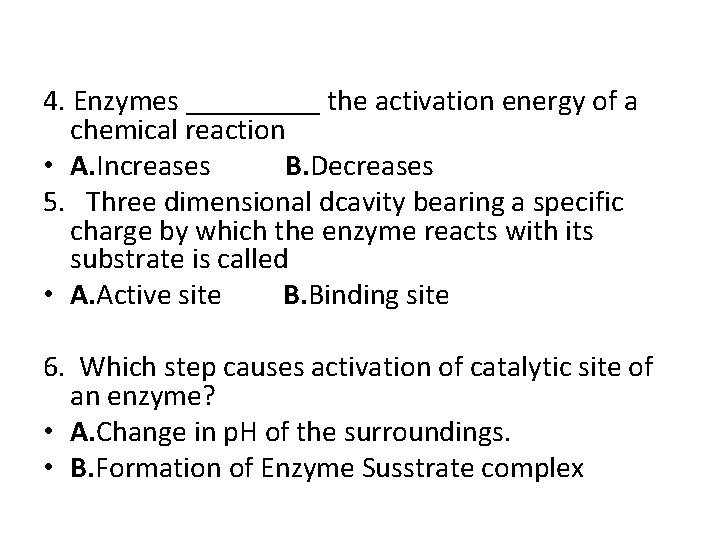
4. Enzymes _____ the activation energy of a chemical reaction • A. Increases B. Decreases 5. Three dimensional dcavity bearing a specific charge by which the enzyme reacts with its substrate is called • A. Active site B. Binding site 6. Which step causes activation of catalytic site of an enzyme? • A. Change in p. H of the surroundings. • B. Formation of Enzyme Susstrate complex

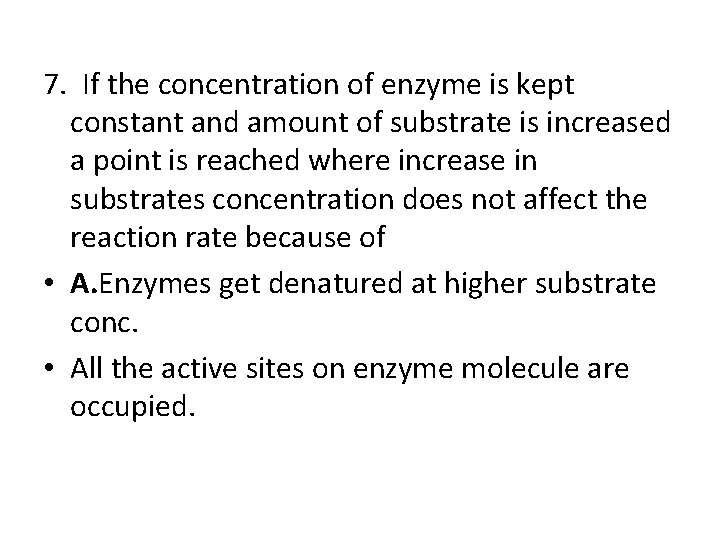
7. If the concentration of enzyme is kept constant and amount of substrate is increased a point is reached where increase in substrates concentration does not affect the reaction rate because of • A. Enzymes get denatured at higher substrate conc. • All the active sites on enzyme molecule are occupied.

7. f more substrate to already occurring enzymatic reaction is added and there is no effect on the rate of the reaction what is the form given to this situation: • A. Saturation B. Denaturation 8. Optimal temperature of enzymes present in human body is • A. 27 C B. 37 C

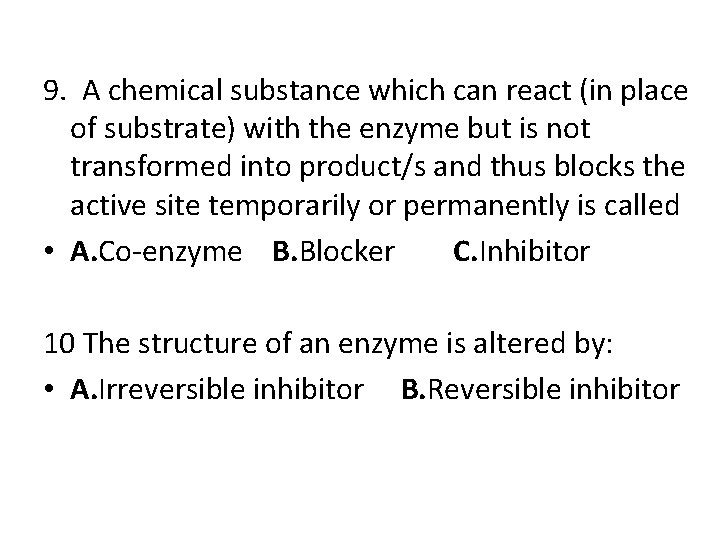
9. A chemical substance which can react (in place of substrate) with the enzyme but is not transformed into product/s and thus blocks the active site temporarily or permanently is called • A. Co-enzyme B. Blocker C. Inhibitor 10 The structure of an enzyme is altered by: • A. Irreversible inhibitor B. Reversible inhibitor
 Diagnostic importance of isoenzymes
Diagnostic importance of isoenzymes Macrohepatic isoenzymes
Macrohepatic isoenzymes Ivd clinical trial design
Ivd clinical trial design Diagnostic clinical landscape
Diagnostic clinical landscape Othlil theory of enzymes with examples
Othlil theory of enzymes with examples Therapeutic milieu
Therapeutic milieu 7 rights of medication administration in order
7 rights of medication administration in order Association for applied and therapeutic humor
Association for applied and therapeutic humor Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Galactosemia
Galactosemia Functional and non functional plasma enzymes
Functional and non functional plasma enzymes Enzymes in plasma
Enzymes in plasma Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes List of enzymes and their functions
List of enzymes and their functions Enzymes and lactose intolerance lab answers
Enzymes and lactose intolerance lab answers Suicide inhibitor
Suicide inhibitor List of enzymes and their functions
List of enzymes and their functions What are 3 characteristics of enzymes
What are 3 characteristics of enzymes Buprenorphine and elevated liver enzymes
Buprenorphine and elevated liver enzymes Increased aerobic and anaerobic enzymes
Increased aerobic and anaerobic enzymes Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Therapeutic orientations
Therapeutic orientations Therapeutic feeding center adalah
Therapeutic feeding center adalah Therapeutic exercise chapter 1 mcqs
Therapeutic exercise chapter 1 mcqs Microwave diathermy block diagram
Microwave diathermy block diagram Therapeutic drift
Therapeutic drift Introduction of therapeutic communication
Introduction of therapeutic communication What is the triangle of care
What is the triangle of care Therapeutic story writing starters
Therapeutic story writing starters Therapeutic communication elements
Therapeutic communication elements Principles of therapeutic exercises
Principles of therapeutic exercises Potency vs efficacy
Potency vs efficacy High therapeutic index
High therapeutic index High therapeutic index
High therapeutic index Classification of therapeutic exercise
Classification of therapeutic exercise Taylor's intentional relationship model
Taylor's intentional relationship model 5 health care career pathways
5 health care career pathways Reproductive cloning process
Reproductive cloning process Multicultural therapeutic communication skills
Multicultural therapeutic communication skills