CIP Training Series CIP Conductivity Control Cleaning Solution





















- Slides: 25

CIP Training Series CIP Conductivity Control Cleaning Solution Control For Clean-In-Place Systems Used by the Food Processing Industry Training For Sanitation Consultants and Plant Personnel

Conductivity Control Conductivity of Solutions What is conductivity? z Solutions of electrolytes in ionizing solvents (water) conduct current when an electrical potential is applied across electrodes immersed in the solution. z Conductivity is the measured conductance between two opposing cube faces one centimeter on a side. z Conductance is defined as the reciprocal of electrical resistance, as measured between probe leads when the probe is immersed in the solution.

Conductivity Control Conductivity of Solutions Electrical resistance is measured in ohms Ohm’s Law: V = I/R (R is in ohms) Voltage (driving force) = Current (flow of electrons) (how much charge) Resistance (e. g. , wire) z Conductivity of a solution (such as a CIP wash solution) is how well ions move in the solution or conduct electricity y More ions, higher conductivity or conductance z Since conductance is the reciprocal of resistance (C = 1/R), ohm is reversed and is called “mho” y 1 mho = 1, 000 umhos (micromho) z Metric equivalent to the mho is the Siemen. y 1 umhos = 1 u. S (microsiemen) y 1, 000 micromhos = 1 m. S (millisiemen)

Conductivity Control Electrolyte Solutions Solutes are classified, generally, as one of three types based on conductivity in water. z Substances that dissolve as molecules, have relatively non-conducting solutions. y Sugars, fats, and proteins, and other food soils are examples. z Substances that exist in water as as equilibrium mixture of ions and molecules are “weak electrolytes”. y HF exists as H+ and F- in equilibrium with mostly HF. (Although this is a dangerous acid). z Substances that exist almost completely as ions in solution are “strong electrolytes”. Compounds that are ionic in solid state are strong. y Na. OH (caustic soda or sodium hydroxide) and HNO 3 (nitric acid) are examples.

Conductivity Control Effects on Conductivity Electrical Conductivity of a solution is a function of: z The density of the solution z It’s charge z Concentration of the molecule z The size of the molecule z Degree of ionization (strong electrolytes versus weak electrolytes) z Ionic mobility z Any interaction with other species At high concentrations, conductance becomes a complex and non-linear function of concentration.

Conductivity Control What do we control? Conductance measurements are ideally suited for measurement of the concentration of a single strong electrolyte in a dilute solution. For CIP systems in food plants, chemicals often having probe control are: (Note: these provide good linear increase in conductance with low to moderately high concentrations) Alkaline CIP Tank: z Caustic or Alkaline Non-Chlorinated Cleaners or z Chlorinated Alkaline Cleaners (CAC) Acid CIP Tank: z Acid Products

Conductivity Control Ways to Measure it z There are two methods of measuring conductivity, electrode and electrode-less or inductive conductivity. z The electrode method utilizes a probe in the cleaning solution. This is the method commonly used for CIP control. z Electrode-less or Inductive: is the use of a changing magnetic field with the solution using two transformer coils. z Electrode-less conductance: is used for highly corrosive materials y Sulfuric acid, molten ammonium nitrate, hydrofluoric acid, etc.

Conductivity Controllers z Devices start or stop a chemical dispensing pump or an alarm in response to the measured solution conductivity. z Many types, capabilities and prices. z Simplest devices are couple hundred dollars and are most typical in the sanitation industry. z Usually one electrical contact to control a chemical dispensing pump and perhaps an alarm built in. z Can be a “blind” instrument—all that is indicated is if solution is above or below setpoint (and no digital display).

Conductivity Controllers z More complex units have digital display of conductivity and temperature, two or more outputs, 24 volt output signals, and temperature compensation built in. z 8850 Signet Controller can set 2 concentration set points, 2 relays, 2 probes (if want separate probes), 2 outputs, and temperature compensation. z. Can control up to 400, 000 u. S. z Output contacts can each have an adjustable setpoint. z Conductivity control is a reasonable cost for good control. Refer to training section: 8 CIP Chemical Control

Conductivity Control Temperature Compensation z Temperature has a big effect on conductivity (known as percent per °C change from 25°C, or slope of the solution). z Conductivity errors can be as high as 3% per 1°C. z Generally, conductivity increases by 1 -2% per °C. z. Varies by chemical solution. z. Conductivity units have temperature compensation. z(If no temperature compensation, chemical solution made up with ambient water will be too concentrated when heated. )

Conductivity Control Temperature Compensation z Most units are temperature compensated. z The factory default temperature compensation factor for the 8850 Signet Controller is 2%/°C. y The optimum factor can be determined for a certain CIP solution by testing the conductivity of the chemical used over a temperature range (two temperature points) and calculating the “TC slope” according to the controller directions.

Conductivity Control Probe Selection z Probes are selected based on the application, and conductivity controller used. z The probe housing material and sensing elements must be compatible with the chemical used and the temperature range of operation. z Wetted materials (e. g. , o-rings, insulator material) of plastic probes handle generally all CIP conditions. Probes are stainless 316. z Probes are selected based on the concentration range (minimum and maximum) of the solution being controlled. z General probe ranges can be: z 1 -1, 000 u. S (microsiemen) z 10 -10, 000 u. S z 100 -200, 000 u. S z 200 -400, 000 u. S

Conductivity Control Calibrating Solutions An easy method to calibrate a CIP solution (for example: start up a new system) z Fill tank to level control with fresh water and calculate fill volume of CIP tank. z Heat to temperature (not really necessary for temperature compensated devices). z Based on calculation of %product required, add the volume of product to CIP tank manually, measure carefully. z Test concentration with test kit to check. z Adjust and retest as needed. z Measure conductivity and make setpoint adjustment. z Takes into account water chemistry effect on conductivity. z Take into account with the appropriate makeup water or there is an error in solution strength.

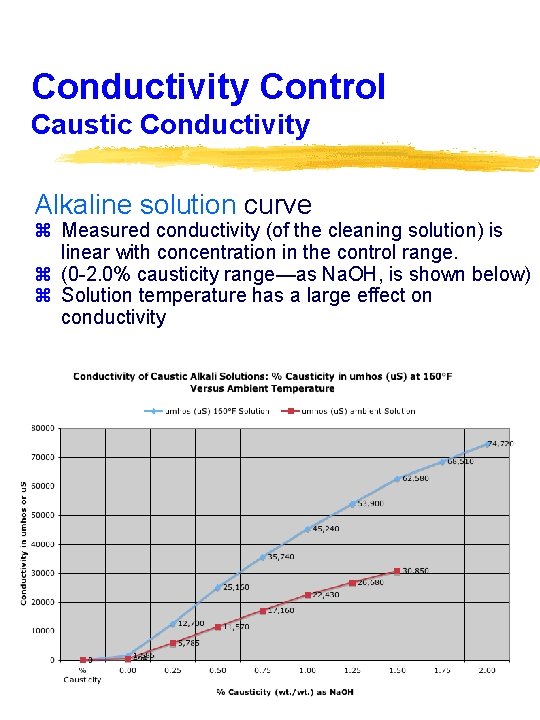
Conductivity Control Caustic Conductivity Alkaline solution curve z Measured conductivity (of the cleaning solution) is linear with concentration in the control range. z (0 -2. 0% causticity range—as Na. OH, is shown below) z Solution temperature has a large effect on conductivity

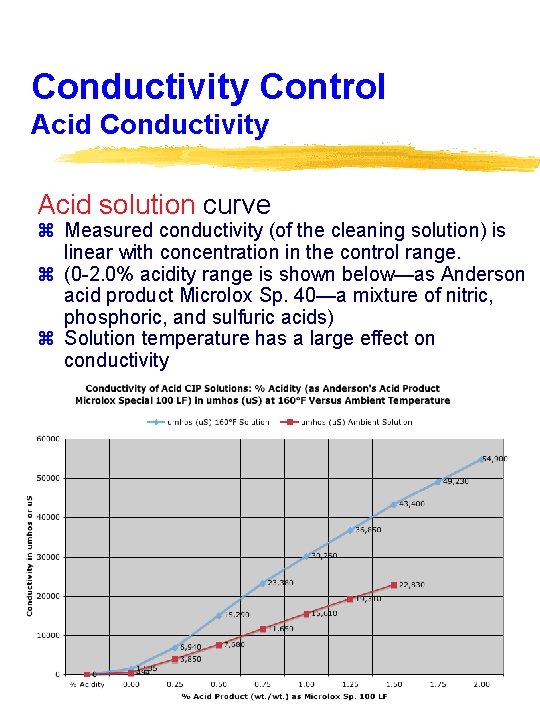
Conductivity Control Acid Conductivity Acid solution curve z Measured conductivity (of the cleaning solution) is linear with concentration in the control range. z (0 -2. 0% acidity range is shown below—as Anderson acid product Microlox Sp. 40—a mixture of nitric, phosphoric, and sulfuric acids) z Solution temperature has a large effect on conductivity

Conductivity Control Wash Solution Conductivity Effect of water hardness and soil on conductivity (of chlorinated alk. sol’n) Data/Conditions: -Water ~300 ppm total hardness, 350 ppm “M” alkalinity -Soil load is 1 oz. /gal. of 2% fat milk -CAC = Bio-Chlor Plus (10% Na. OH active and 3% Na. OCl) -Conductivity measured in umhos (u. S) z Deionized water makeup -Deionized only 1 umho -CAC at 0. 6% 5, 000 umho (u. S) -CAC at 0. 6% with soil 4, 850 umho (u. S) z Hard water makeup -Hard water only -CAC at 0. 6% with soil 530 umho 3, 150 umho 3, 080 umho Milk soil slightly suppresses conductivity 0. 6% CAC

Conductivity Control Wash Solution Conductivities Effect of water hardness and soil on conductivity (of acid solution) Data/Conditions: -Water ~300 ppm total hardness, 350 ppm “M” alkalinity -Soil load is 1 oz. /gal. of 2% milk -Acid = Microlox Sp. 40 LF (phosphoric, nitric, sulfuric) -Conductivities in micromho’s z Deionized water makeup Deionized only Acid at 0. 5% with soil z Hard water makeup Hard water only Acid at 0. 5% with soil 1 umho 10, 340 umho 10, 010 umho 530 umho 6, 700 umho 6, 575 umho Milk soil slightly suppresses conductivity 0. 6% Acid

Conductivity Control Effects on Conductivity Effect of chemistry (CAC vs. acid), water hardness, and soil on conductivity Some Conclusions: z Water chemistry significantly affects conductivity. z Retest the water chemistry used in a CIP system periodically z The conductivity varies based on chemistry of cleaner. z Milk-based soils reduce the solution conductivity. z Soil load affects conductivity. z Badly scaled or soiled probes can lead to significant errors.

Conductivity Control Probe Maintenance z Electrical connections need to be checked occasionally to ensure the terminals are not corroding. z Inspect on a regular basis for scale deposits. z Inspect on regular basis for cracks in the housing of the probe. z Inspect on a regular basis for organic or mineral contamination. z It is recommended to have a PM program to de-scale probes on a regular basis (in-tank probes in the alkaline CIP tank).

Conductivity Control Probe Position: In Tank z Position of the probe depends on the size of the tank, the degree of mixing and the position of the feed point. z If the mixing action in a CIP tank is good, position the probe near the tank discharge, ensuring that the detergent is “seen” throughout the tank. z If mixing is poor, the probe must be located near the dosing point in order to minimize the potential for overshoot. z The feed rate must also be controlled to dose at a slower rate. z The probe must not be located too high in the tank solution or the tank level may fall below the level of the probe.

Conductivity Control Probe Position: In-Line z The most efficient set up has conductivity control occurring on the supply-side of the CIP system. z The ideal place to locate the probe is to mount it in the line on the discharge side of the CIP supply pump and fairly close to the tank outlet. z Dosing of a cleaning chemical is often to the CIP wash tank. y. The sanitizer is typically dosed in-line, to the supply pump suction-side. Sanitizers are normally NOT controlled by probe.

Conductivity Control Other Functions z A seconductivity unit on the return side of the CIP system may monitor the wash solution and can be used to control rinse times. (This probe does not have anything to do with chemical concentration control). z An in-line conductivity probe on the return side can be used to route CIP solutions to the CIP wash tanks (if conductivity is above the setpoint) — or routed to the drain (if the conductivity is below a certain setpoint) z This type of setup allows additional efficiencies and savings of rinse water.

Conductivity Control Other Functions CIP of long line or equipment circuits: z Probes located only on the return side piping (to control CIP detergents) may result in too long of a delay for the control of the wash solution. y This is also discussed in training session 8, chemical control z This can result in the overfeed of cleaning products and waste.

Conductivity Control System Design z The food facility personnel involved in CIP system operation should work closely with their sanitation supplier to achieve good concentration control.

CIP Training Thank you for your time! Questions?