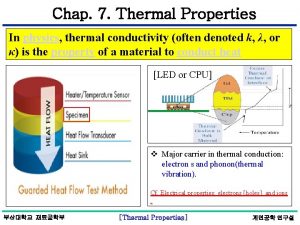
Thermal Properties of Materials Thermal conductivity electrons phonons

![Thermal Expansion Change of mean interatomic distance with temperature: Argon (kfz) Density [g/cm³] Lattice Thermal Expansion Change of mean interatomic distance with temperature: Argon (kfz) Density [g/cm³] Lattice](https://slidetodoc.com/presentation_image_h/7e500c9595b27a25abead2da2ed87607/image-31.jpg)
- Slides: 32

Thermal Properties of Materials Ø Thermal conductivity (electrons, phonons) Ø Heat capacity (specific heat) at constant volume Ø Heat capacity (specific heat) at constant pressure Ø Thermal expansion 1

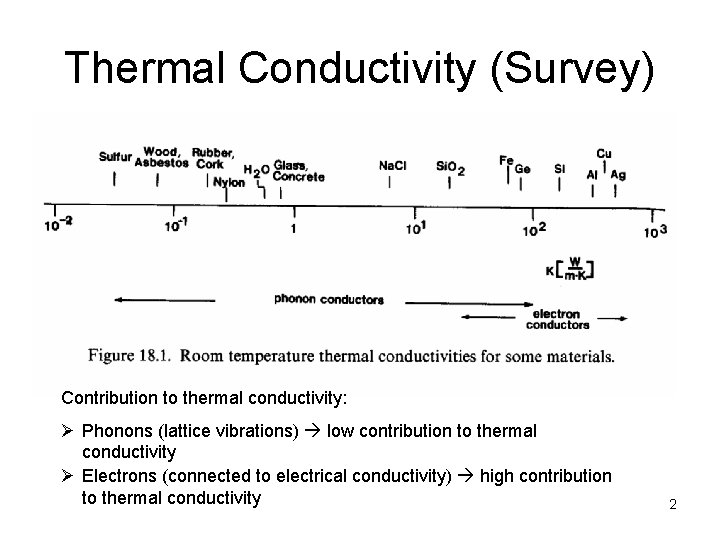
Thermal Conductivity (Survey) Contribution to thermal conductivity: Ø Phonons (lattice vibrations) low contribution to thermal conductivity Ø Electrons (connected to electrical conductivity) high contribution to thermal conductivity 2

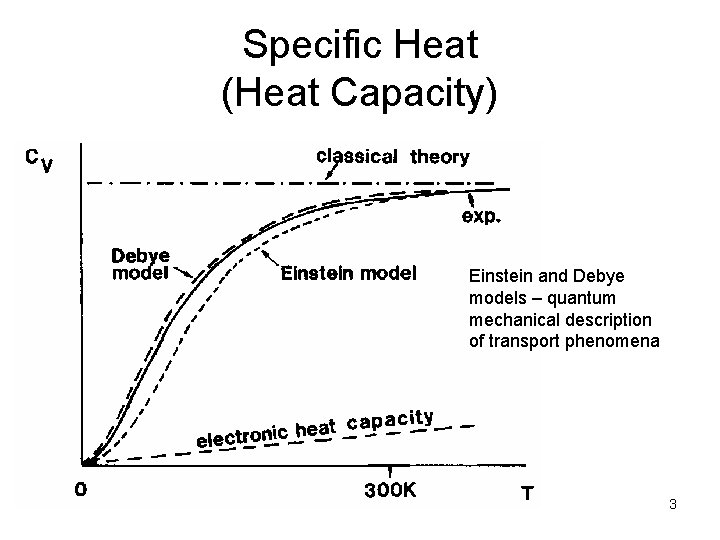
Specific Heat (Heat Capacity) Einstein and Debye models – quantum mechanical description of transport phenomena 3

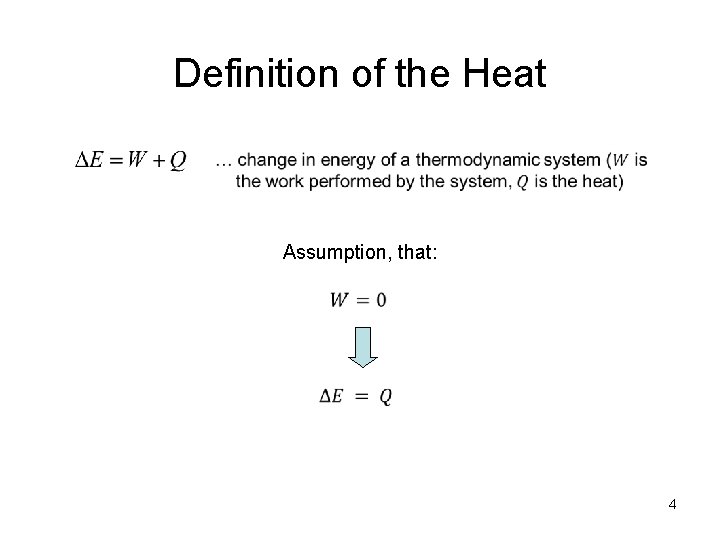
Definition of the Heat Assumption, that: 4

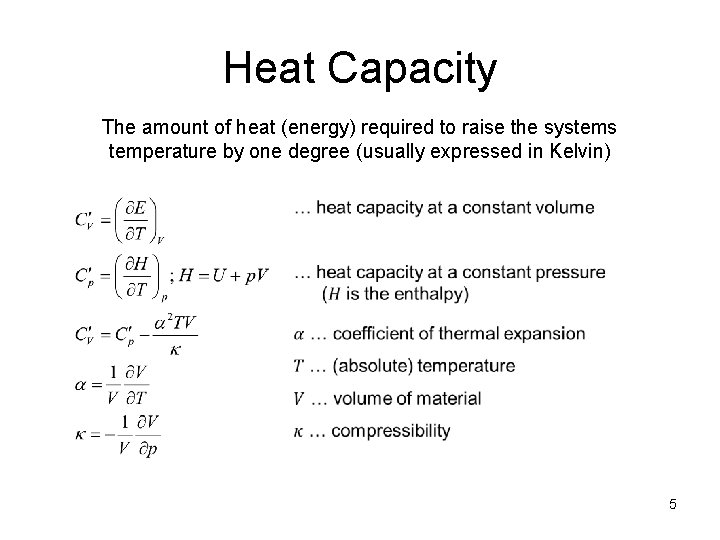
Heat Capacity The amount of heat (energy) required to raise the systems temperature by one degree (usually expressed in Kelvin) 5

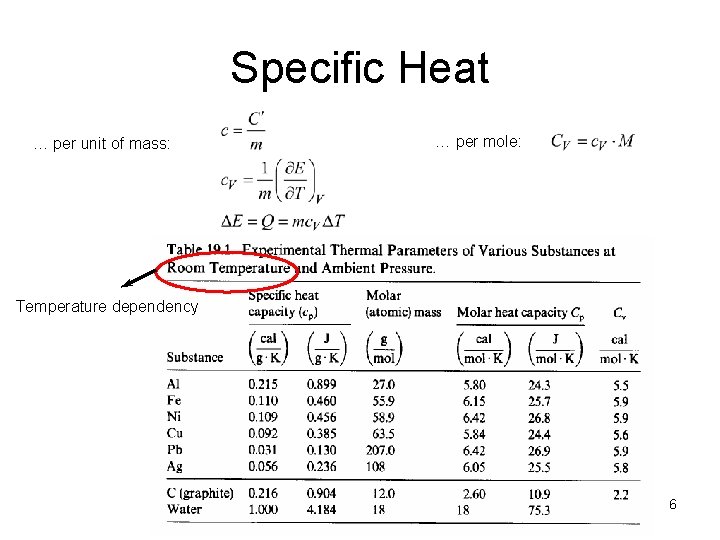
Specific Heat … per unit of mass: … per mole: Temperature dependency 6

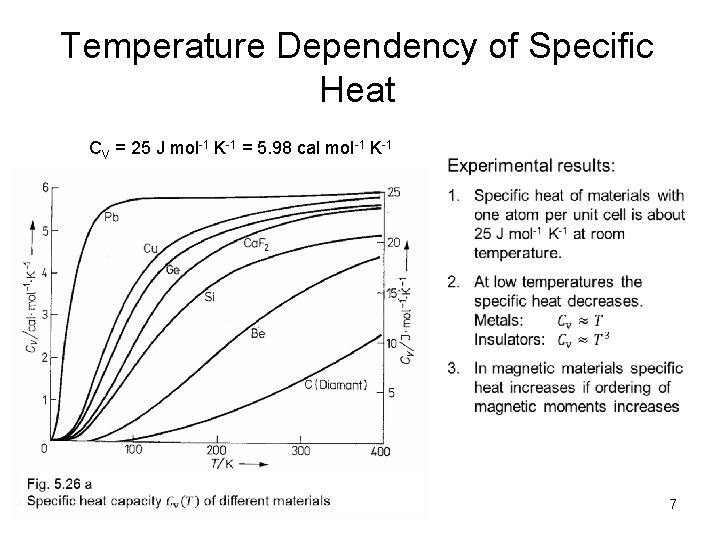
Temperature Dependency of Specific Heat CV = 25 J mol-1 K-1 = 5. 98 cal mol-1 K-1 7

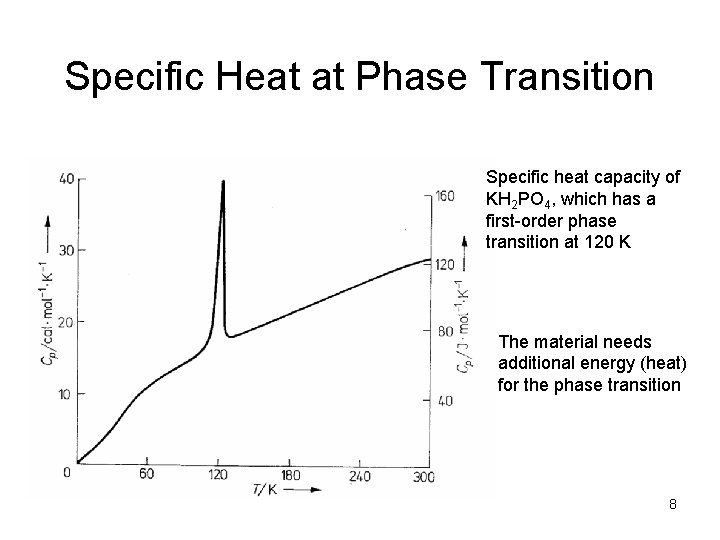
Specific Heat at Phase Transition Specific heat capacity of KH 2 PO 4, which has a first-order phase transition at 120 K The material needs additional energy (heat) for the phase transition 8

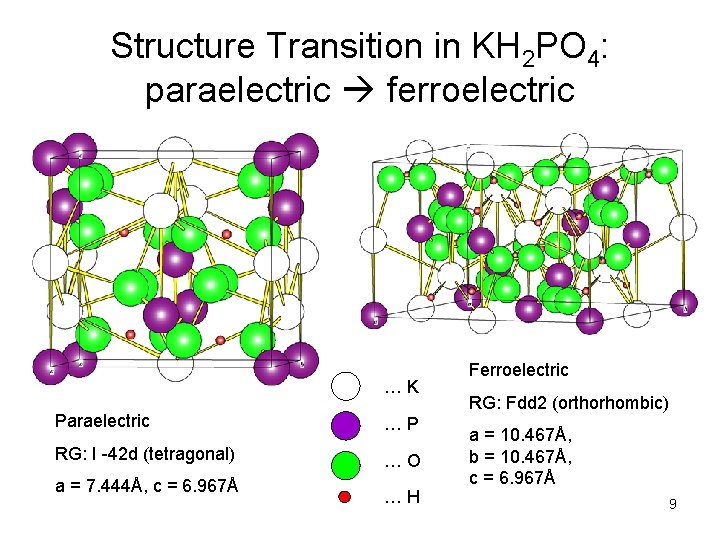
Structure Transition in KH 2 PO 4: paraelectric ferroelectric … K Paraelectric … P RG: I -42 d (tetragonal) … O a = 7. 444Å, c = 6. 967Å … H Ferroelectric RG: Fdd 2 (orthorhombic) a = 10. 467Å, b = 10. 467Å, c = 6. 967Å 9

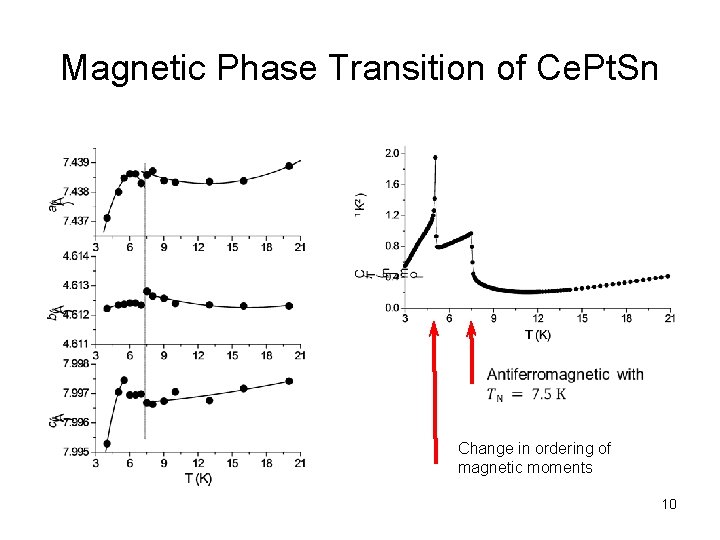
Magnetic Phase Transition of Ce. Pt. Sn Change in ordering of magnetic moments 10

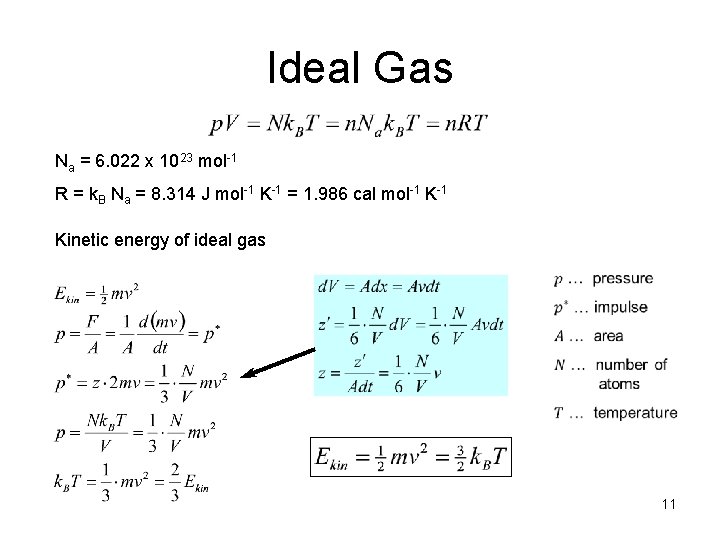
Ideal Gas Na = 6. 022 x 1023 mol-1 R = k. B Na = 8. 314 J mol-1 K-1 = 1. 986 cal mol-1 Kinetic energy of ideal gas 11

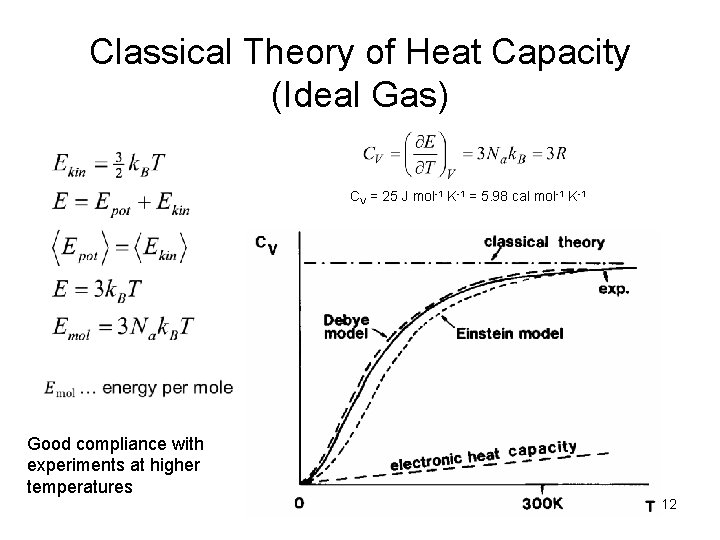
Classical Theory of Heat Capacity (Ideal Gas) CV = 25 J mol-1 K-1 = 5. 98 cal mol-1 K-1 Good compliance with experiments at higher temperatures 12

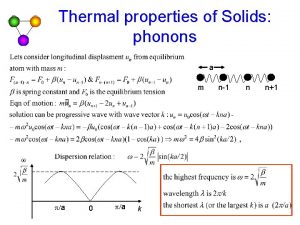
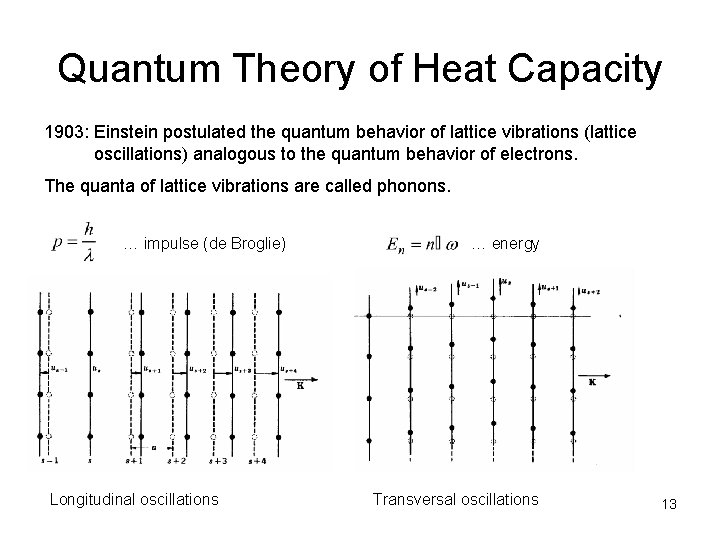
Quantum Theory of Heat Capacity 1903: Einstein postulated the quantum behavior of lattice vibrations (lattice oscillations) analogous to the quantum behavior of electrons. The quanta of lattice vibrations are called phonons. … impulse (de Broglie) Longitudinal oscillations … energy Transversal oscillations 13

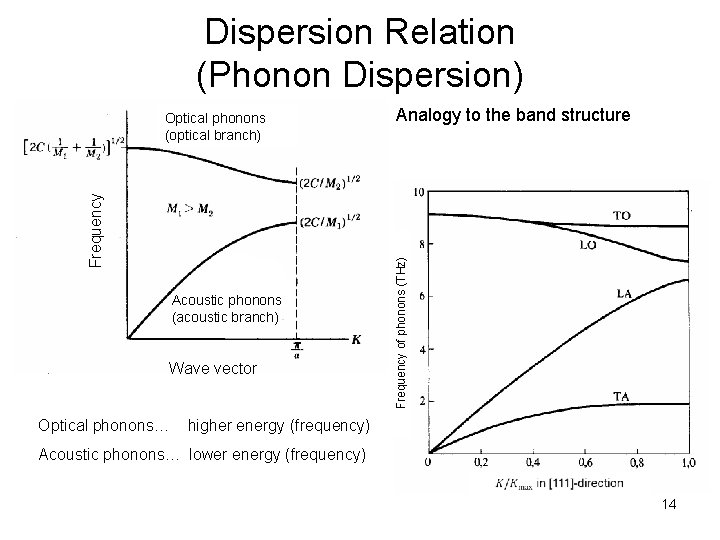
Dispersion Relation (Phonon Dispersion) Acoustic phonons (acoustic branch) Wave vector Analogy to the band structure Frequency of phonons (THz) Frequency Optical phonons (optical branch) Optical phonons… higher energy (frequency) Acoustic phonons… lower energy (frequency) 14

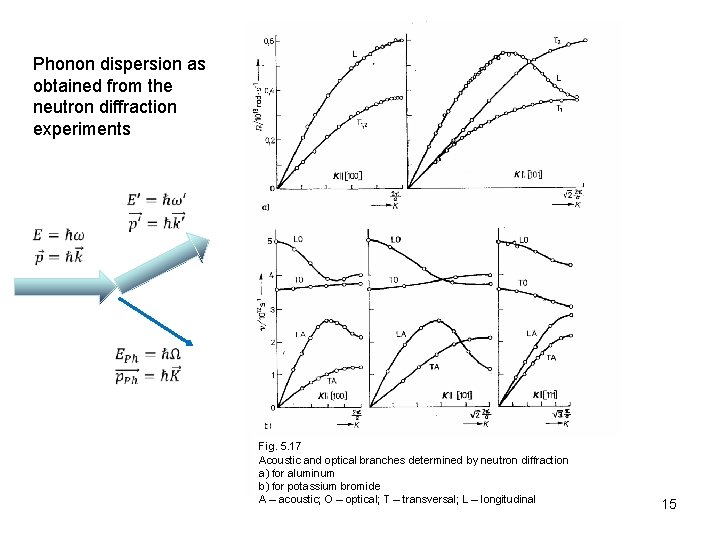
Phonon dispersion as obtained from the neutron diffraction experiments Fig. 5. 17 Acoustic and optical branches determined by neutron diffraction a) for aluminum b) for potassium bromide A – acoustic; O – optical; T – transversal; L – longitudinal 15

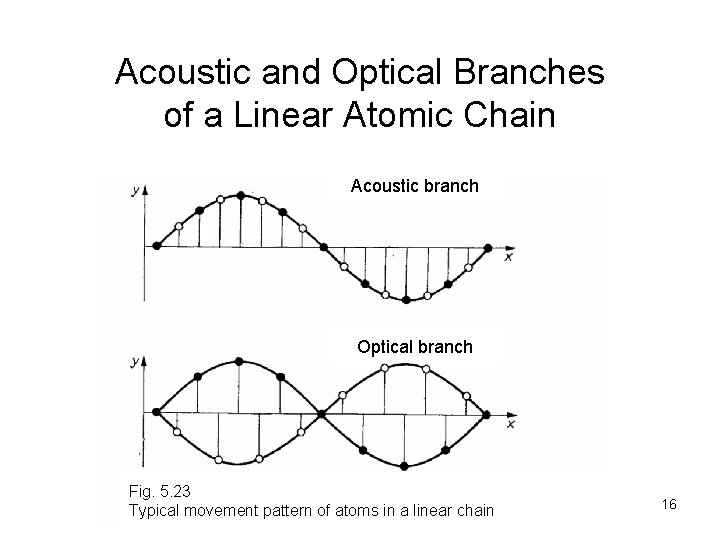
Acoustic and Optical Branches of a Linear Atomic Chain Acoustic branch Optical branch Fig. 5. 23 Typical movement pattern of atoms in a linear chain 16

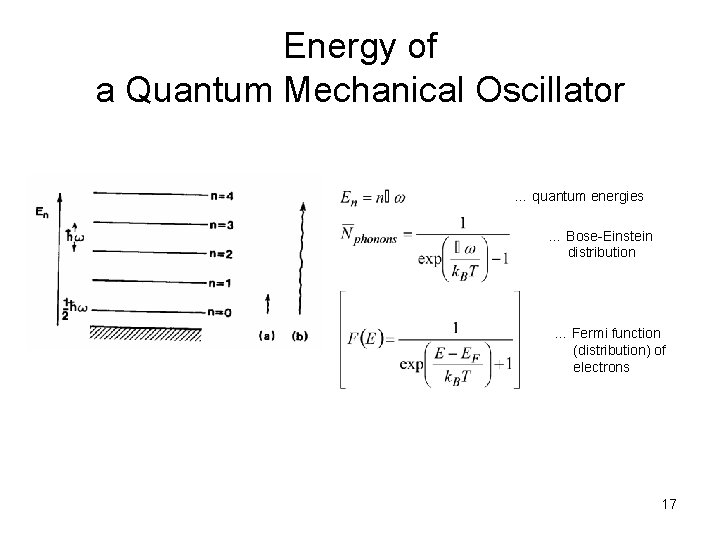
Energy of a Quantum Mechanical Oscillator … quantum energies … Bose-Einstein distribution … Fermi function (distribution) of electrons 17

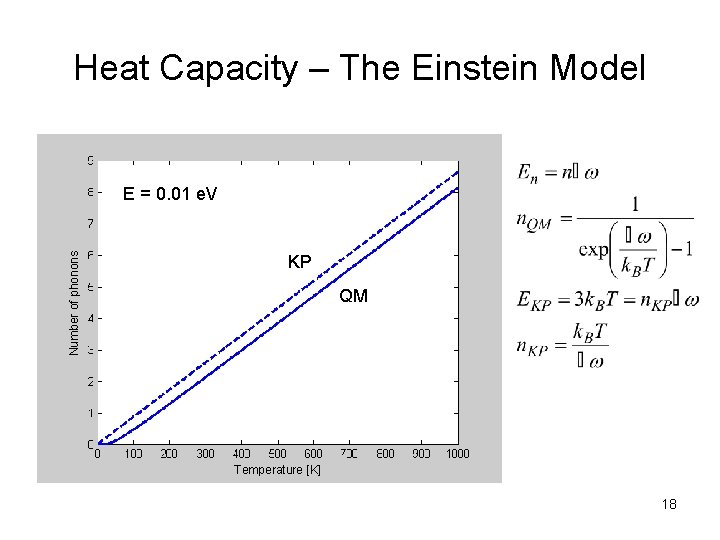
Heat Capacity – The Einstein Model Number of phonons E = 0. 01 e. V KP QM Temperature [K] 18

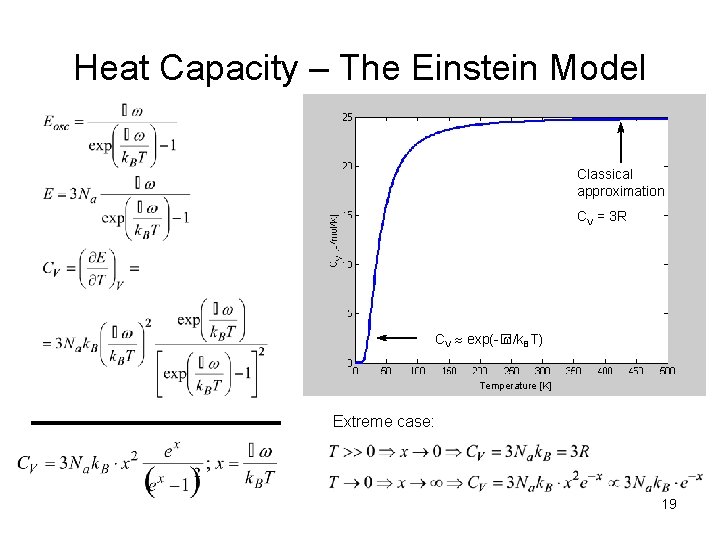
Heat Capacity – The Einstein Model Classical approximation CV = 3 R CV exp(-� /k. BT) Temperature [K] Extreme case: 19

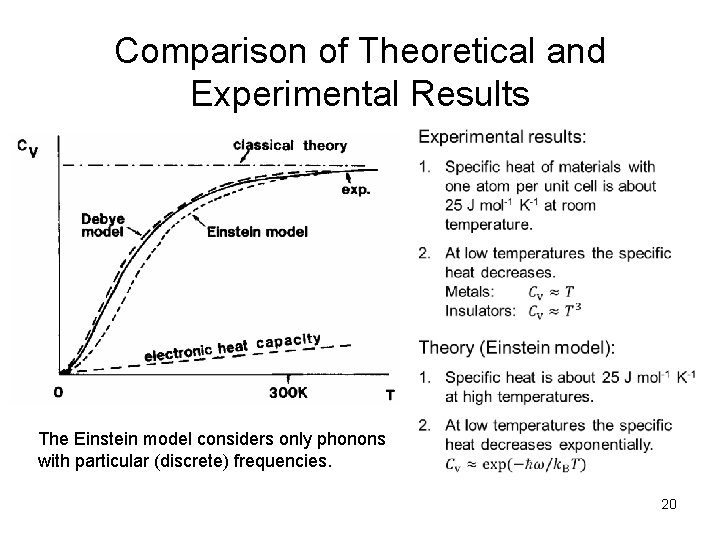
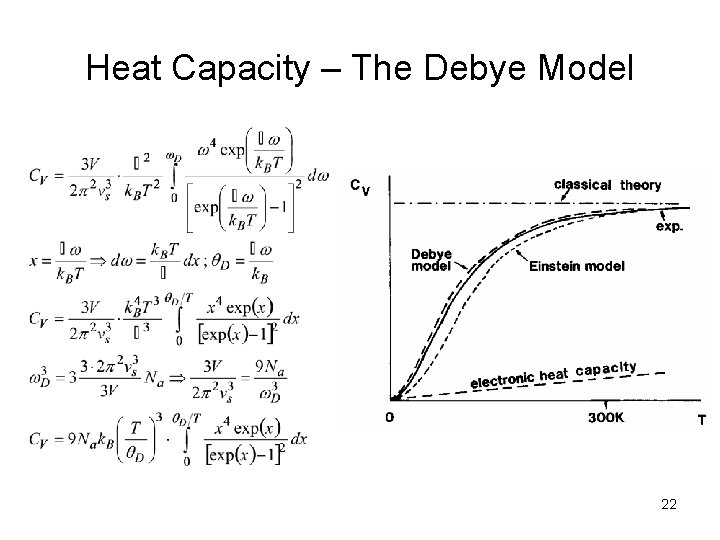
Comparison of Theoretical and Experimental Results The Einstein model considers only phonons with particular (discrete) frequencies. 20

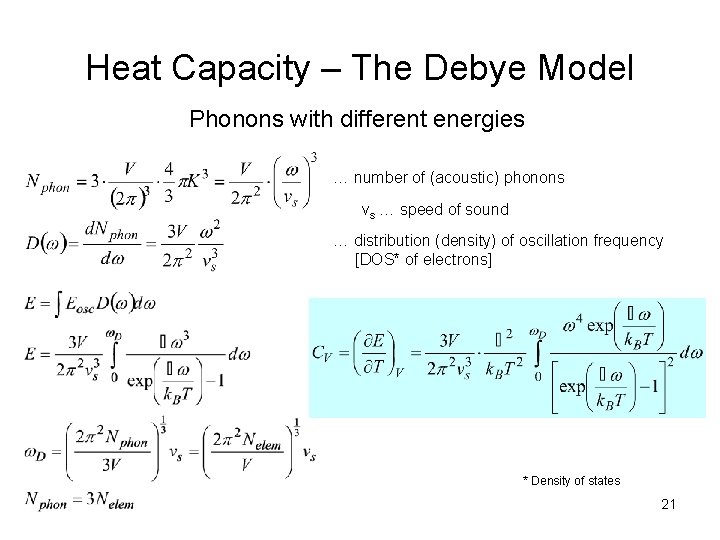
Heat Capacity – The Debye Model Phonons with different energies … number of (acoustic) phonons vs … speed of sound … distribution (density) of oscillation frequency [DOS* of electrons] * Density of states 21

Heat Capacity – The Debye Model 22

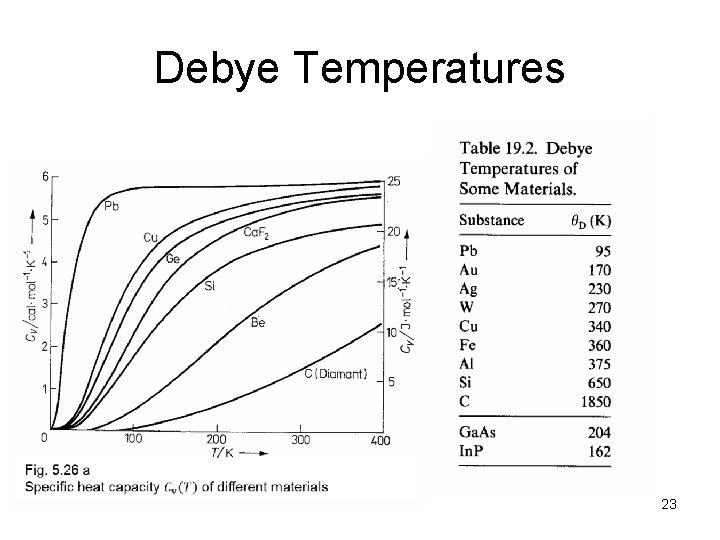
Debye Temperatures 23

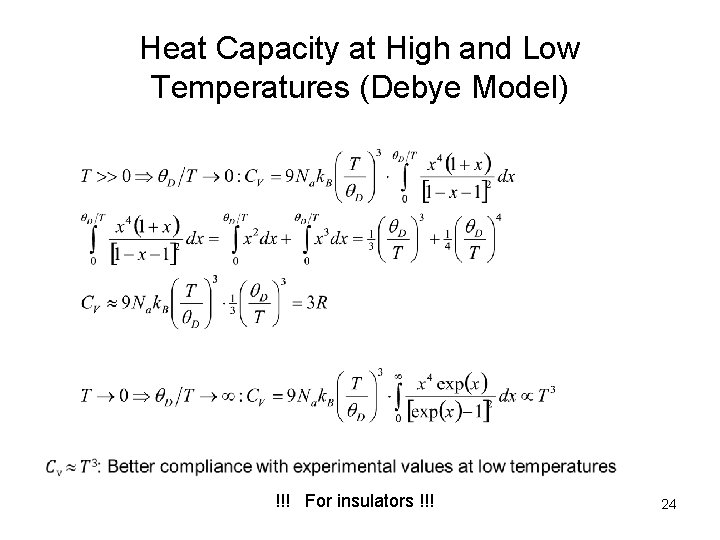
Heat Capacity at High and Low Temperatures (Debye Model) !!! For insulators !!! 24

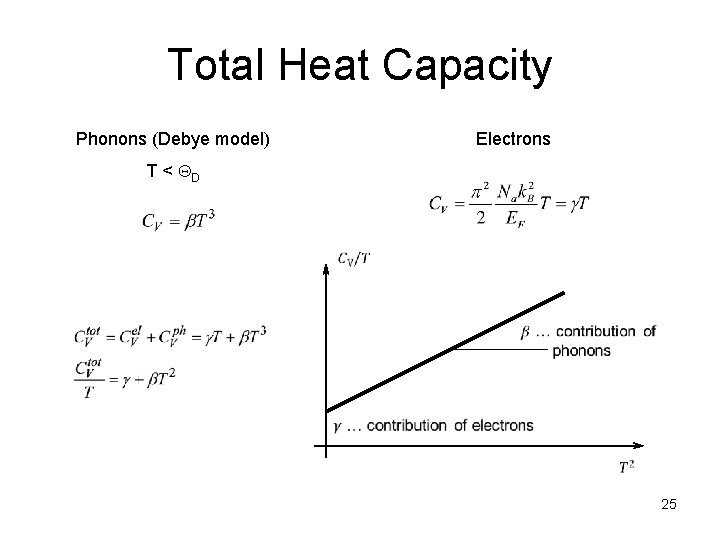
Total Heat Capacity Phonons (Debye model) Electrons T < QD 25

Experimental Methods for the investigation of lattice vibrations X-ray diffraction Neutron diffraction Profile change of electron density (thermal vibrations of electrons) Interaction between low-energy (slow) neutrons and the Phonons Influence on the intensities of diffraction lines 26

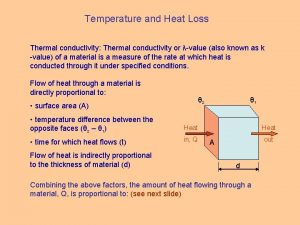
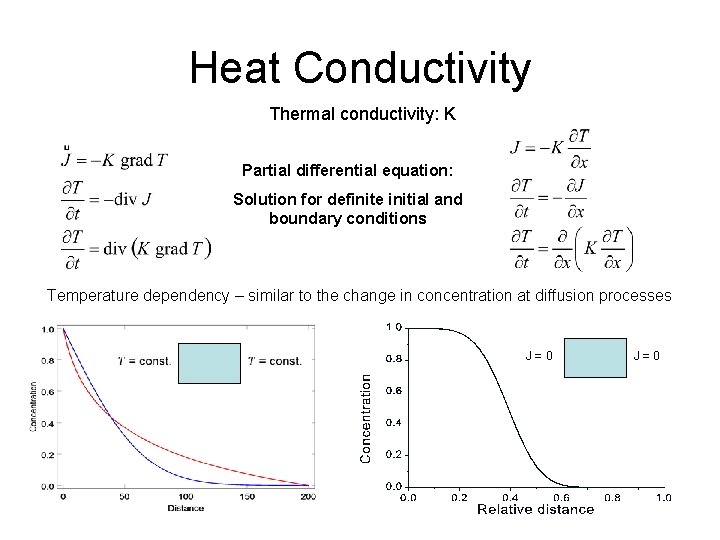
Heat Conductivity Thermal conductivity: K Partial differential equation: Solution for definite initial and boundary conditions Temperature dependency – similar to the change in concentration at diffusion processes J = 0 27

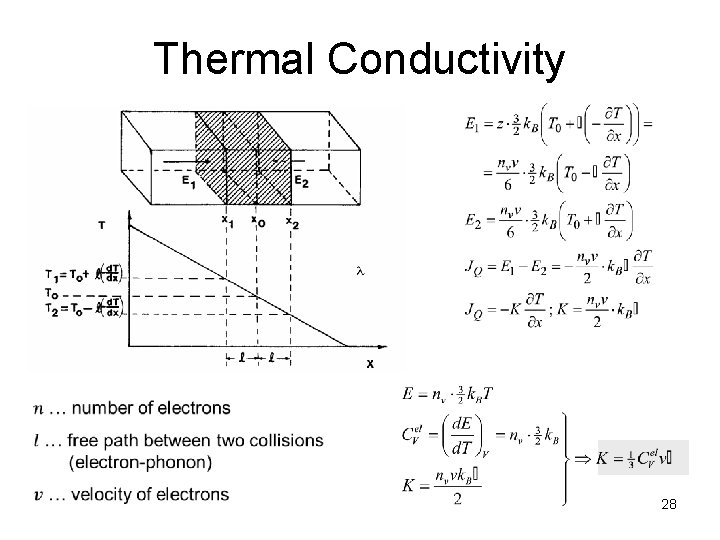
Thermal Conductivity 28

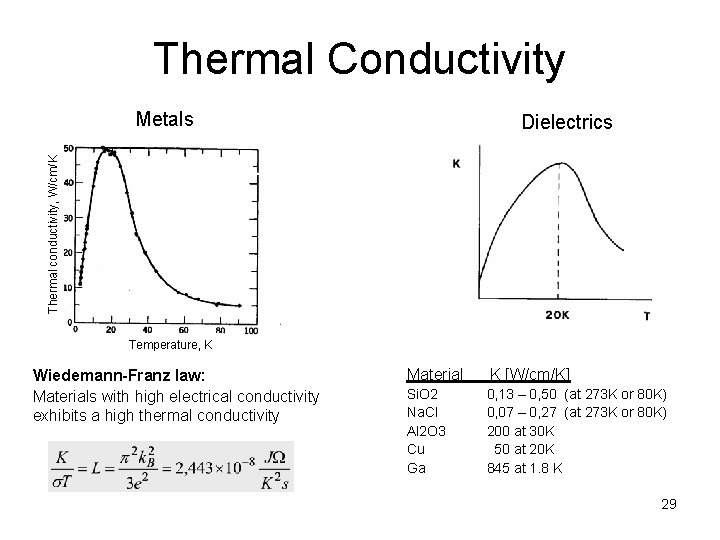
Thermal Conductivity Metals Thermal conductivity, W/cm/K Dielectrics Temperature, K Wiedemann-Franz law: Materials with high electrical conductivity exhibits a high thermal conductivity Material K [W/cm/K] Si. O 2 Na. Cl Al 2 O 3 Cu Ga 0, 13 – 0, 50 (at 273 K or 80 K) 0, 07 – 0, 27 (at 273 K or 80 K) 200 at 30 K 50 at 20 K 845 at 1. 8 K 29

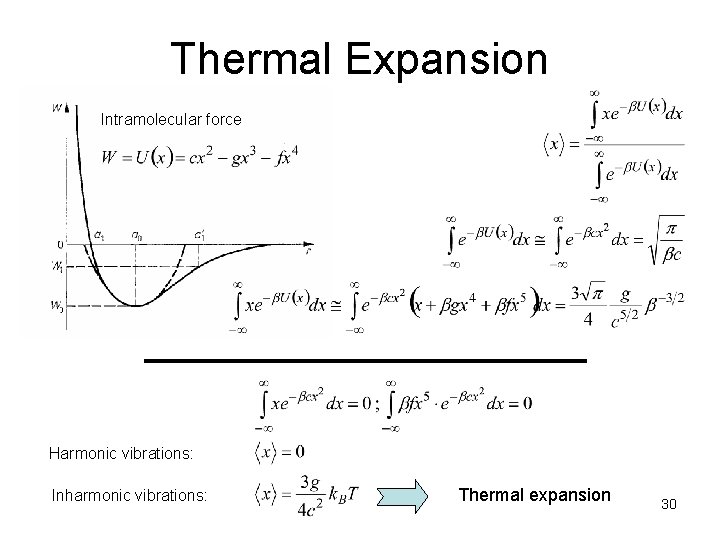
Thermal Expansion Intramolecular force Harmonic vibrations: Inharmonic vibrations: Thermal expansion 30
![Thermal Expansion Change of mean interatomic distance with temperature Argon kfz Density gcm³ Lattice Thermal Expansion Change of mean interatomic distance with temperature: Argon (kfz) Density [g/cm³] Lattice](https://slidetodoc.com/presentation_image_h/7e500c9595b27a25abead2da2ed87607/image-31.jpg)
Thermal Expansion Change of mean interatomic distance with temperature: Argon (kfz) Density [g/cm³] Lattice parameter [Å] Temperature dependency of lattice parameters: Temperature [K] 31

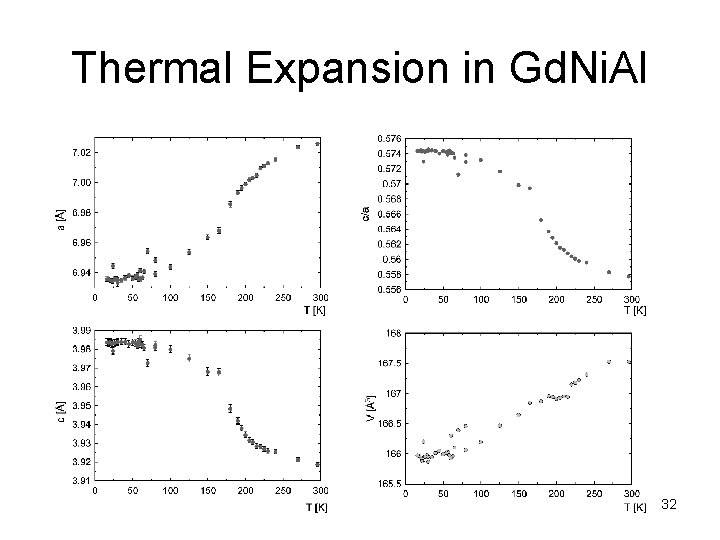
Thermal Expansion in Gd. Ni. Al 32
 Physical and chemical properties of dental materials
Physical and chemical properties of dental materials Optical vs acoustic phonons
Optical vs acoustic phonons Phonons of physical fitness
Phonons of physical fitness Thermal conductivity of styrofoam
Thermal conductivity of styrofoam Are thermal and electrical conductivity related
Are thermal and electrical conductivity related Astm thermal conductivity
Astm thermal conductivity Thermal conductivity detector
Thermal conductivity detector Thermal conductivity detector
Thermal conductivity detector Enhancing thermal conductivity of fluids with nanoparticles
Enhancing thermal conductivity of fluids with nanoparticles Dimensions of thermal conductivity
Dimensions of thermal conductivity Variable thermal conductivity
Variable thermal conductivity Thermal conduction resistance
Thermal conduction resistance Thermal conductivity formula
Thermal conductivity formula Spherical heat equation
Spherical heat equation Fundamentals of thermal-fluidsciences chapter 2 problem 24p
Fundamentals of thermal-fluidsciences chapter 2 problem 24p Section 3 using thermal energy
Section 3 using thermal energy Thermal transfer vs direct thermal printing
Thermal transfer vs direct thermal printing Natural materials
Natural materials Useful materials at home
Useful materials at home Natural man made
Natural man made What is adopting materials
What is adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Viscosity units
Viscosity units Unit 2
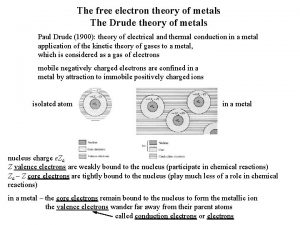
Unit 2 Conductivity k
Conductivity k Drude model
Drude model Electrical conductivity of acids and bases
Electrical conductivity of acids and bases Electrical conductivity soil definition
Electrical conductivity soil definition Ion conductivity
Ion conductivity Hydraulic conductivity equation
Hydraulic conductivity equation Degree of dissociation of electrolyte depends on
Degree of dissociation of electrolyte depends on Resistivity equations
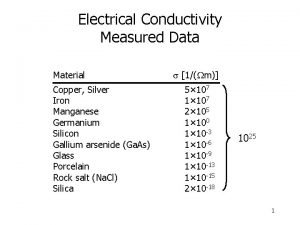
Resistivity equations Electrical conductivity of solids
Electrical conductivity of solids