Chemistry Jeopardy Sig Figs Electron Configs Density Formulas






![Electron Configurations for 200 The element that has the abbreviated notation of [Kr] 5 Electron Configurations for 200 The element that has the abbreviated notation of [Kr] 5](https://slidetodoc.com/presentation_image_h2/88abb2d821367d4ae92f0b192bca0e95/image-9.jpg)
![Electron Configurations for 300 The noble gas configuration for Ge What is [Ar]4 s Electron Configurations for 300 The noble gas configuration for Ge What is [Ar]4 s](https://slidetodoc.com/presentation_image_h2/88abb2d821367d4ae92f0b192bca0e95/image-10.jpg)








































- Slides: 57


Chemistry Jeopardy Sig Figs Electron Configs Density Formulas Conversions The Periodic Table 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500 Round 2 FINAL

Sig Figs for 100 The number of significant figures in 0. 0023 What is 2?

Sig Figs for 200 The number of significant figures in 13, 000. 0 What is 6?

Sig Figs for 300 The correct answer to 18. 0/3. 0 What is 6. 0?

Sig Figs for 400 The correct answer to 10. + 22. 11 What is 32?

Sig Figs for 500 The correct answer to 20. 0/5 + 14. 3 What is 18?

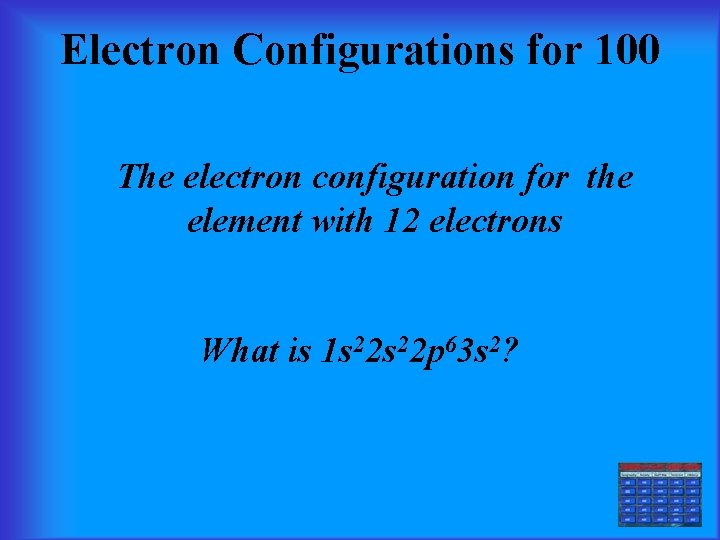
Electron Configurations for 100 The electron configuration for the element with 12 electrons What is 1 s 22 p 63 s 2?
![Electron Configurations for 200 The element that has the abbreviated notation of Kr 5 Electron Configurations for 200 The element that has the abbreviated notation of [Kr] 5](https://slidetodoc.com/presentation_image_h2/88abb2d821367d4ae92f0b192bca0e95/image-9.jpg)
Electron Configurations for 200 The element that has the abbreviated notation of [Kr] 5 s 24 d 4 What is Molybdenum (Mo)?
![Electron Configurations for 300 The noble gas configuration for Ge What is Ar4 s Electron Configurations for 300 The noble gas configuration for Ge What is [Ar]4 s](https://slidetodoc.com/presentation_image_h2/88abb2d821367d4ae92f0b192bca0e95/image-10.jpg)
Electron Configurations for 300 The noble gas configuration for Ge What is [Ar]4 s 23 d 104 p 2?

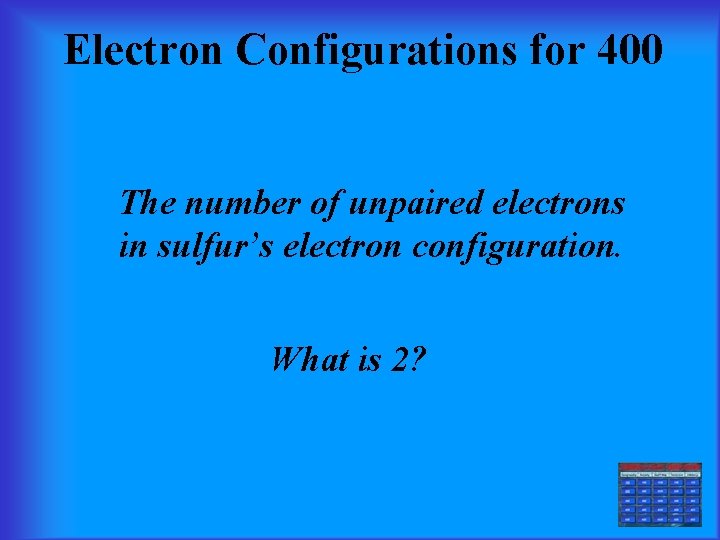
Electron Configurations for 400 The number of unpaired electrons in sulfur’s electron configuration. What is 2?

Electron Configurations for 500 The element that has the following electron configuration: 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 9? What is Silver (Ag)?

Formulas for 100 What Ca. Cl 2 is called. What is calcium chloride?

Formulas for 200 What tin (IV) phosphate is written as (Tin is Sn; Phospate is PO 43 -) What is Sn 3(PO 4)4?

Formulas for 300 The formula for tetracarbon decahydride What is C 4 H 10?

Formulas for 400 What P 3 Cl 6 is called What is triphosphorus hexachloride?

Formulas for 500 In Mn(SO 3)4 , the charge on Mn (SO 3 has a 2 - charge). What is 8+?

Conversions for 100 65 L converted to k. L. What is 0. 065 k. L.

Conversions for 200 82 g converted to dg What is 820 dg?

Conversions for 300 Daily Double! Only the group selecting this can wager

Conversions for 400 8 Ms converted to das. (M: mega, means million; da: deca, means ten) What is 800, 000 das?

Conversions for 500 12 k. L converted to nm. (n: nano, means billionth) What is 12, 000, 000?

The Periodic Table for 100 The name of the vertical columns on the periodic table. What are groups?

The Periodic Table for 200 Energy levels are indicated by these on the periodic table. What are periods?

The Periodic Table for 300 Where the nonmetals are generally located. What is the right side?

The Periodic Table for 400 The location of the halogens. What is group 17/7 A?

The Periodic Table for 500 The meaning of atomic mass. What is the AVERAGE mass of all isotopes of an element.

Chemistry Jeopardy-round 2 Periodic Trends Shapes Density Moles Misc. A Misc. B 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500 FINAL

Periodic Trends for 100 The trend for atomic radius as you go down a group. What is increase? Round 2

Periodic Trends for 200 The trend for electronegativity as you go across a period. What is increase? Round 2

Periodic Trends for 300 The trend for ionization energy as you go down a group. What is decrease? Round 2

Periodic Trends for 400 The trend for electron affinity as you go across a period? What is increase? Round 2

Periodic Trends for 500 The trend for electron shielding as you go across a period. What is no change. Round 2

Misc. A for 100 Burning wood is this type of change. What is a chemical change. Round 2

Misc. A for 200 Compared to red, green light has a larger value of these two things. What are frequency and energy? Round 2

Misc. A for 300 Boiling water is this type of change. What is a physical change? Round 2

Misc. A for 400 The Alkaline Earth Metals are located in this group. What is group 2? Round 2

Misc. A for 500 A mixture that will not settle when you let it sit for a long time. What is a homogeneous mixture? Round 22 Round

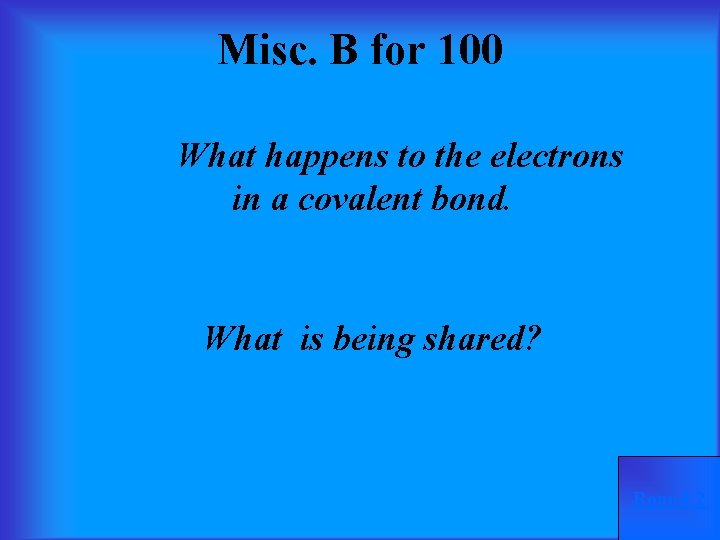
Misc. B for 100 What happens to the electrons in a covalent bond. What is being shared? Round 2

Misc. B for 200 A type of reaction that absorbs heat. Who is an endothermic reaction? Round 2

Misc. B for 300 The number of electrons that the f subshell can hold. What is 14? Round 2

Misc. B for 400 What‘s produced when an excited electron drops to its ground state. What is light? Round 2

Misc. B for 500 The amount of heat required to raise the temperature of 10 g of water by 10 o. C. What is 418 J? Round 22 Round

Moles for 100 The number of candy canes in a mole of candy canes. What is 6. 02 x 1023? Round 2

Moles for 200 Moles of atoms in 1. 806 x 1024 atoms. What is 3 moles? Round 2

Moles for 300 The mass of 2 moles of oxygen. What is 32 g. Round 2

Moles for 400 Moles of aluminum in 135 g of aluminum. What is 5? Round 2

Moles for 500 Number of atoms in 0. 10 grams of hydrogen. What is 6. 02 x 1022? Round 2

Final Jeopardy Write down how many points you will wager on the Final Jeopardy question. What is the octet rule? That every atom (except H and He) wants to have 8 valence electrons to be “full. ”

Conversions for 300 62, 000 ms converted to ks. What is 0. 062 ks?

Sig Figs for 200 Double Jeopardy Only the group selecting this category gets to wager on it.

Shapes for 500 The shape for CO 2 What is linear? Round 2

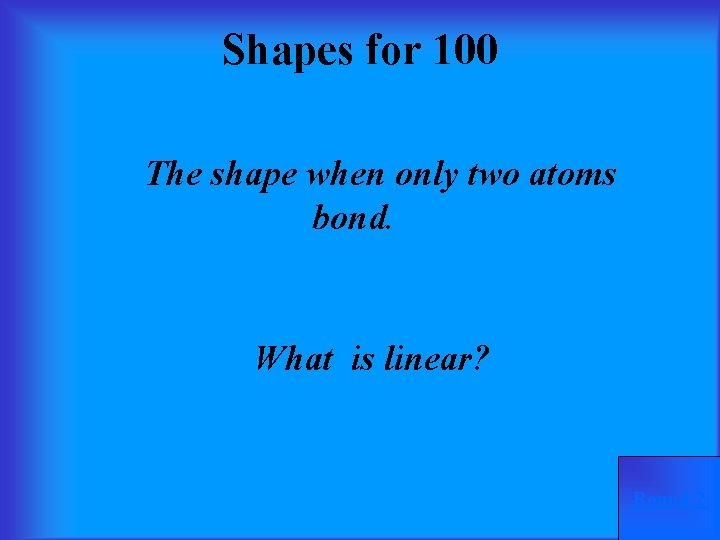
Shapes for 100 The shape when only two atoms bond. What is linear? Round 2

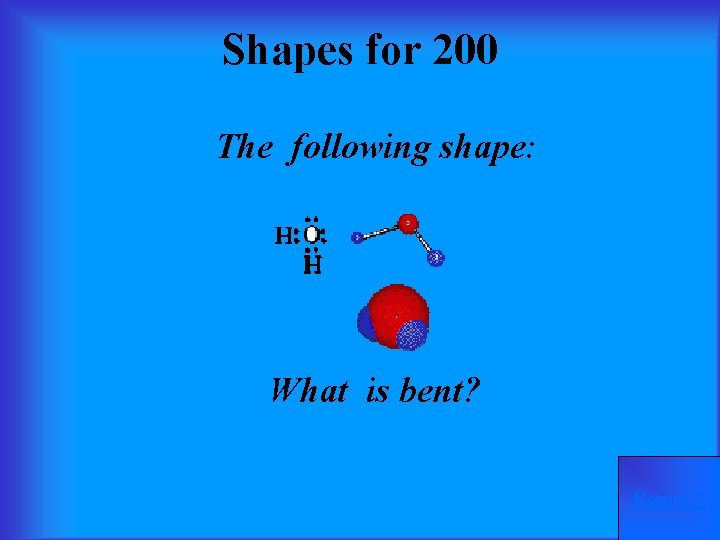
Shapes for 200 The following shape: What is bent? Round 2

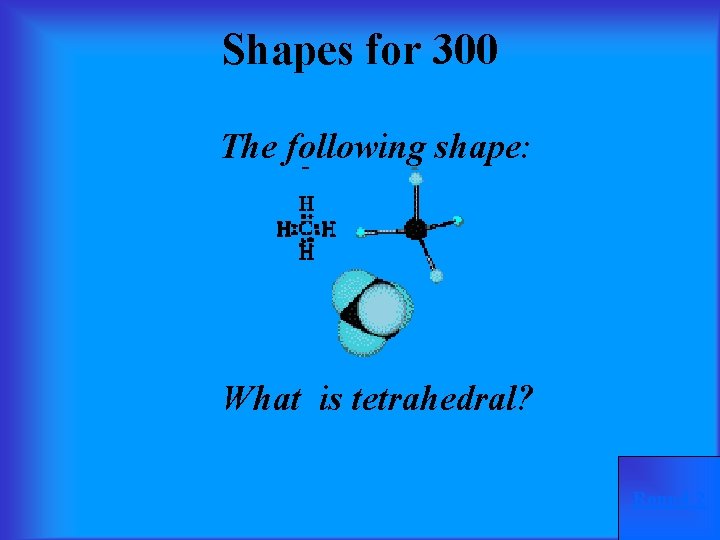
Shapes for 300 The following shape: What is tetrahedral? Round 2

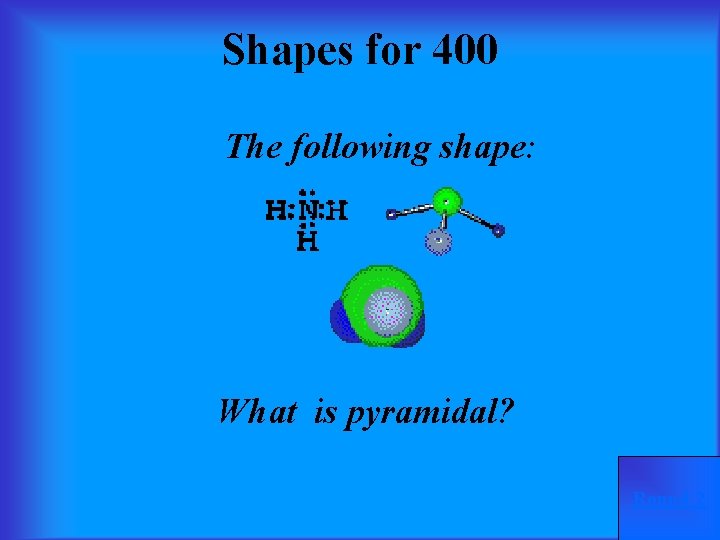
Shapes for 400 The following shape: What is pyramidal? Round 2

Shapes for 500 Double Jeopardy Only the group selecting this category gets to wager on it. Round 2