Significant Figures and Scientific Notation How to use
















- Slides: 20

Significant Figures and Scientific Notation

How to use your calculator with big and small numbers (Chemistry relates really tiny particles) The most common number used in chemistry is the mole: 6. 02 x 1023 How would you find the value of 6. 02 x 1023 / 54. 2? ( 6. 02 2 nd EE 23) / 54. 2 enter answer = 1. 1086 E 22 (1. 1086 x 1022)

Dimensional Analysis A) If you are going 50 miles per hour, how many feet per second are you traveling? B) Your car's gas tank holds 18. 6 gallons and is one quarter full. Your car gets 16 miles/gal. You see a sign saying, "Next gas 73 miles. " Do you have enough gas? C) Aluminum has a density of 2. 70 g/cm 3. What would be the volume of a piece of aluminum foil that has a mass of 12. 3 g? (formula for density? ? )

Quick Metric Conversion Review Kilo Hecto Deka BASE deci (King Henry Died By Drinking Chocolate Milk) centi milli


Metric Conversion Practice A) 3. 5 Da. L = _____ m. L B) 87 Hm = _____ Km C) 0. 61 c. L = _____ L D) 29. 5 kg = _____ dg

Temperature Conversions Celsius, Fahrenheit, Kelvin o. F = (1. 8 x o. C) + 32 o. C = (o. F - 32) / 1. 8 K = o. C + 273. 15 o. C = K – 273. 15

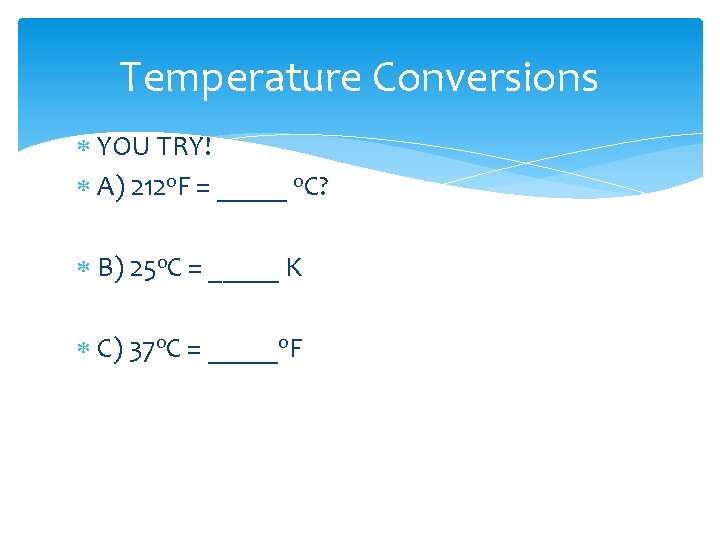
Temperature Conversions YOU TRY! A) 212 o. F = _____ o. C? B) 25 o. C = _____ K C) 37 o. C = _____o. F

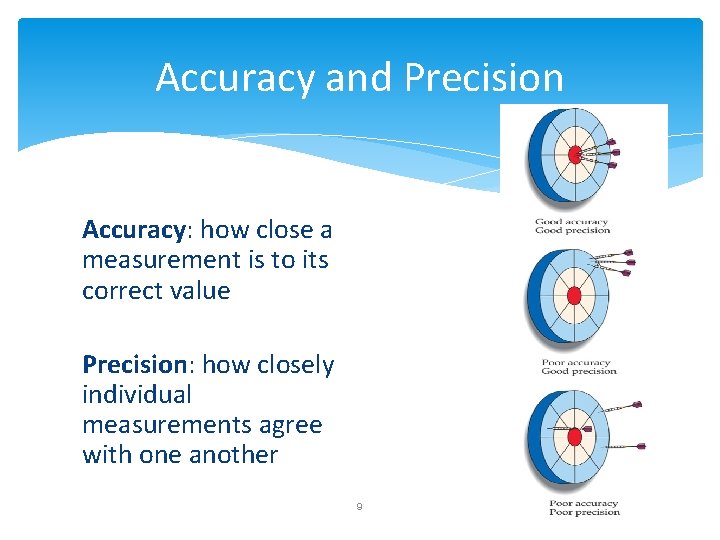
Accuracy and Precision Accuracy: how close a measurement is to its correct value Precision: how closely individual measurements agree with one another 9

Percent Error Percent error is the difference between the measured value and the exact/known value; and given as a percent |accepted value – experimental value| x 100 % accepted value Example: You are given aluminum and you measure the dimensions of the block and its displacement in a container. You calculate the density of the Al to be 2. 67 g/cm 3. You look up the density of the Al and find it to actually be 2. 70 g/cm 3. Calculate the % error of your measurement.

Percent Error YOU TRY! A) Joshua uses his thermometer and finds the boiling point of ethyl alcohol to be 75 o C. He looks in a reference book and finds that the actual boiling point of ethyl alcohol is 80 o. C. What is his percent error?

Scientific Notation Write a very large number in scientific notation by moving the decimal point to the left until only one digit remains to the left. The number of moves of the decimal point gives you the exponent. Examples: 3, 454, 000 = 3. 454 x 106 (your calculator may report 3. 454 E 6) 1, 200 = 1. 2 x 103

Scientific Notation For very small numbers, you move the decimal point to the right until only one digit remains to the left of the decimal point. The number of moves to the right gives you a negative exponent: Example: 0. 000000543 = 5. 43 x 10 -7 0. 0092 = 9. 2 x 10 -3

YOU TRY! Write the following in scientific notation. A) 908, 000, 000 = B) 0. 00502 = C) 0. 000077 = D) 135, 700 =

Sig Fig Rules 1) ALL non-zero numbers (1, 2, 3, 4, 5, 6, 7, 8, 9) are ALWAYS significant. 2) ALL zeroes between non-zero numbers are ALWAYS significant. 3) ALL zeroes which are SIMULTANEOUSLY to the right of the decimal point AND at the end of the number are ALWAYS significant. 4) LEADING zeros are NOT significant

How many sig figs? ? A) 3. 967 B) 900. 06 C) 0. 00040 D) 780 E) 780.

More practice? ? ? F) 501. 703 G) 8. 10 H) 300, 000 I) 0. 0409 J) 24. 07

Math with sig figs In mathematical operations involving significant figures, the answer is reported in such a way that it reflects the reliability of the least precise operation.

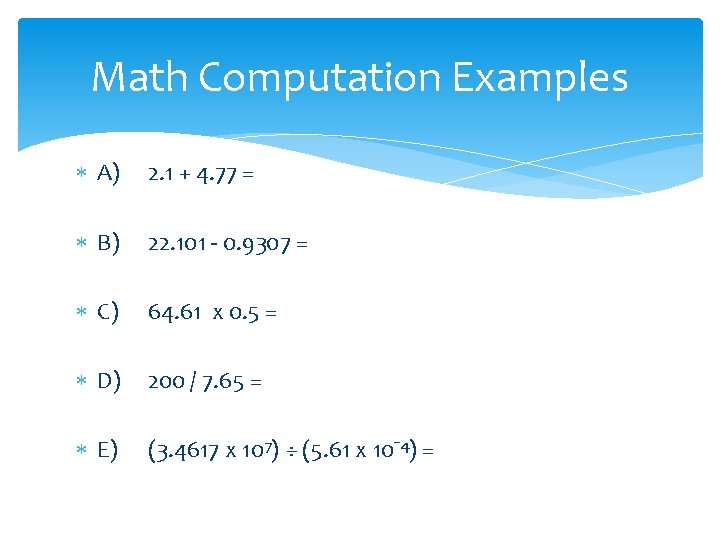
Math Computation Examples A) 2. 1 + 4. 77 = B) 22. 101 - 0. 9307 = C) 64. 61 x 0. 5 = D) 200 / 7. 65 = E) (3. 4617 x 107) ÷ (5. 61 x 10¯ 4) =

Math with sig figs Addition/Subtraction: (look at decimal place) 1) Count the number of digits in the decimal portion of each number in the problem. 2) Add or subtract in the normal fashion. 3) Round the answer to the LEAST number of places in the decimal portion of any number in the problem. Multiplication/Division (look at sig figs) 1) Count the number of sig figs 2) The LEAST number of sig determines the number of sig figs in the answer