Chemistry A level topic 007 Transition metals Lesson
![In water – pretty much all transition metals form hexaaqua ions [M(H 2 O)6] In water – pretty much all transition metals form hexaaqua ions [M(H 2 O)6]](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-18.jpg)
![COMPLEX FORMATION Bidentate ligands – form two co-ordinate bonds 1, 2 -diaminoethane (en) [Cr(en)3]3+ COMPLEX FORMATION Bidentate ligands – form two co-ordinate bonds 1, 2 -diaminoethane (en) [Cr(en)3]3+](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-21.jpg)
![SHAPES OF COMPLEX IONS Geometric Isomerism cis e. g. [Pt. Cl 2(NH 3)2] trans SHAPES OF COMPLEX IONS Geometric Isomerism cis e. g. [Pt. Cl 2(NH 3)2] trans](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-26.jpg)
![SHAPES OF COMPLEX IONS Geometric isomerism e. g. [Co. Cl 2(NH 3)4]+ SHAPES OF COMPLEX IONS Geometric isomerism e. g. [Co. Cl 2(NH 3)4]+](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-27.jpg)
![SHAPES OF COMPLEX IONS Optical Isomerism e. g. [Co(en)3]3+ SHAPES OF COMPLEX IONS Optical Isomerism e. g. [Co(en)3]3+](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-28.jpg)
![What about…. Co(H 2 NCH 2 NH 2)2 Cl 2]3+ What about…. Co(H 2 NCH 2 NH 2)2 Cl 2]3+](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-30.jpg)

- Slides: 34

Chemistry A level topic 007 Transition metals Lesson 04 of 08: complex ion shapes

Main topic: Transition metals Sub-topic: Complex ions shapes Paper 1 and 3

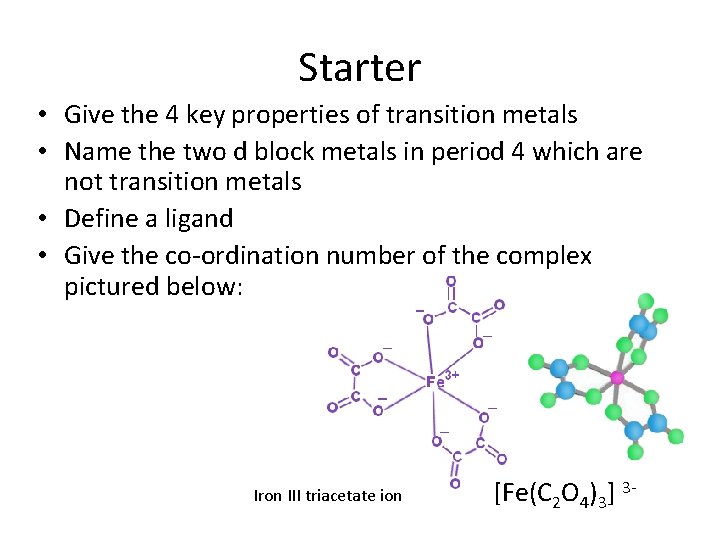
Starter • Give the 4 key properties of transition metals • Name the two d block metals in period 4 which are not transition metals • Define a ligand • Give the co-ordination number of the complex pictured below: Iron III triacetate ion [Fe(C 2 O 4)3] 3 -

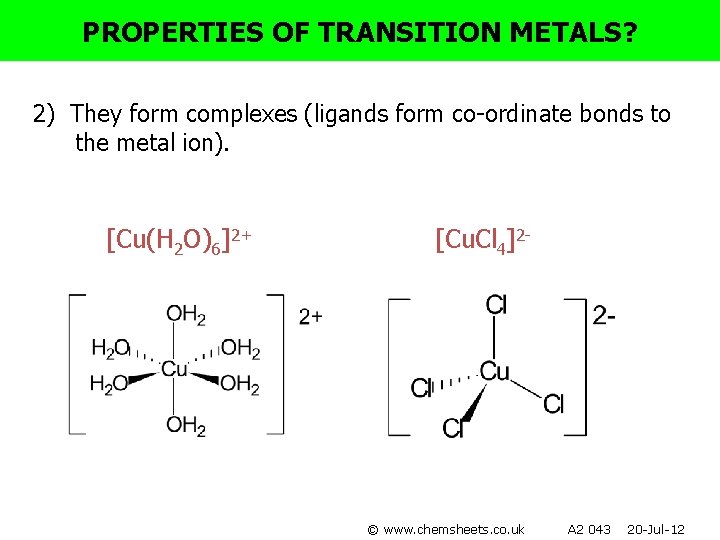
PROPERTIES OF TRANSITION METALS? 2) They form complexes (ligands form co-ordinate bonds to the metal ion). [Cu(H 2 O)6]2+ [Cu. Cl 4]2 - © www. chemsheets. co. uk A 2 043 20 -Jul-12

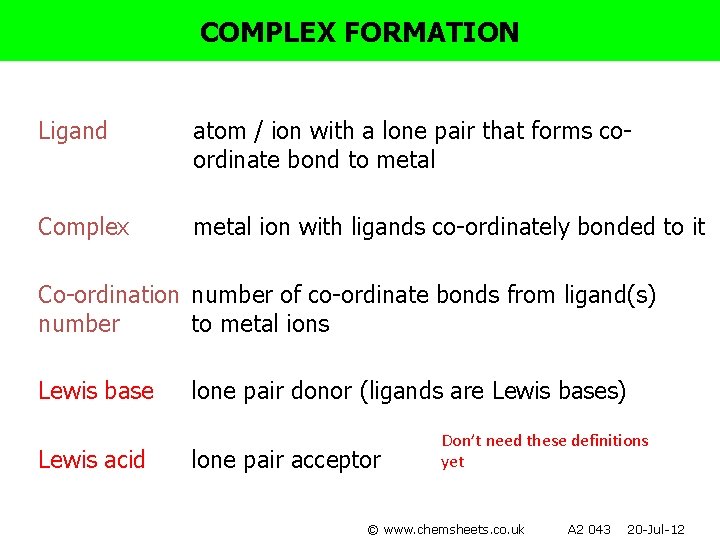
COMPLEX FORMATION Ligand atom / ion with a lone pair that forms coordinate bond to metal Complex metal ion with ligands co-ordinately bonded to it Co-ordination number of co-ordinate bonds from ligand(s) number to metal ions Lewis base Lewis acid lone pair donor (ligands are Lewis bases) lone pair acceptor Don’t need these definitions yet © www. chemsheets. co. uk A 2 043 20 -Jul-12

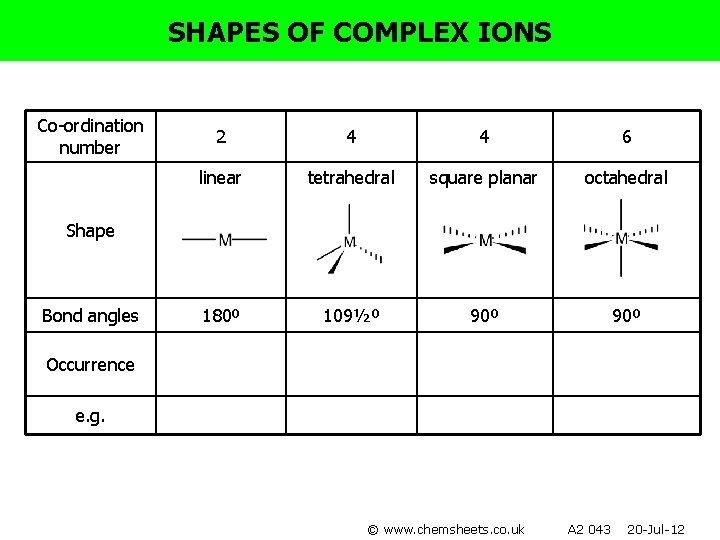
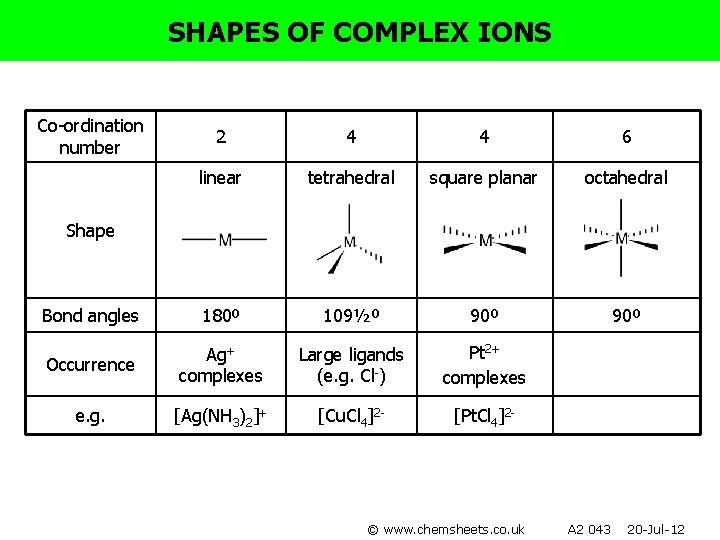
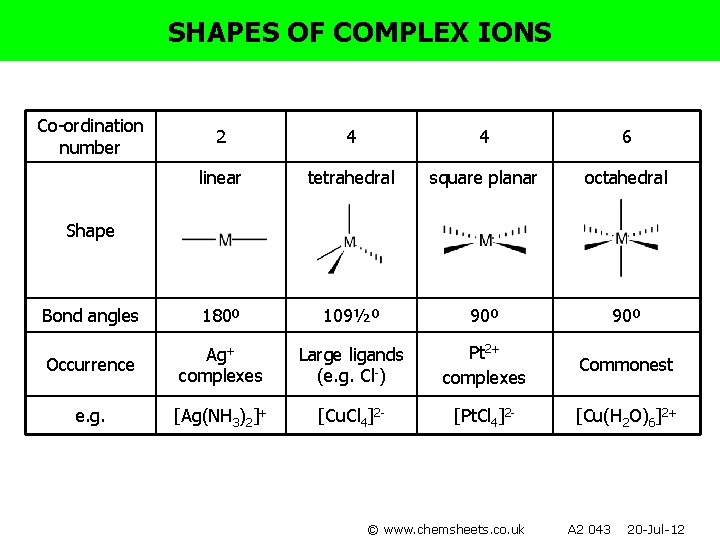
SHAPES OF COMPLEX IONS Co-ordination number 2 4 4 6 linear tetrahedral square planar octahedral 180º 109½º 90º Shape Bond angles Occurrence e. g. © www. chemsheets. co. uk A 2 043 20 -Jul-12

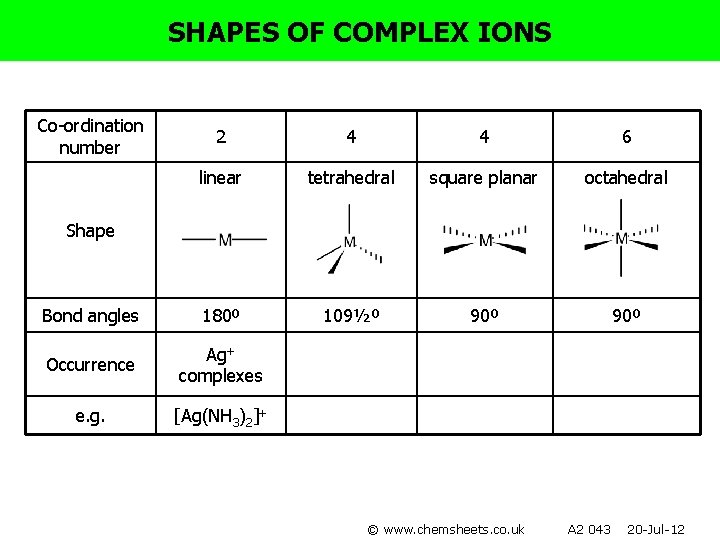
SHAPES OF COMPLEX IONS Co-ordination number 2 4 4 6 linear tetrahedral square planar octahedral Bond angles 180º 109½º 90º Occurrence Ag+ complexes e. g. [Ag(NH 3)2]+ Shape © www. chemsheets. co. uk A 2 043 20 -Jul-12

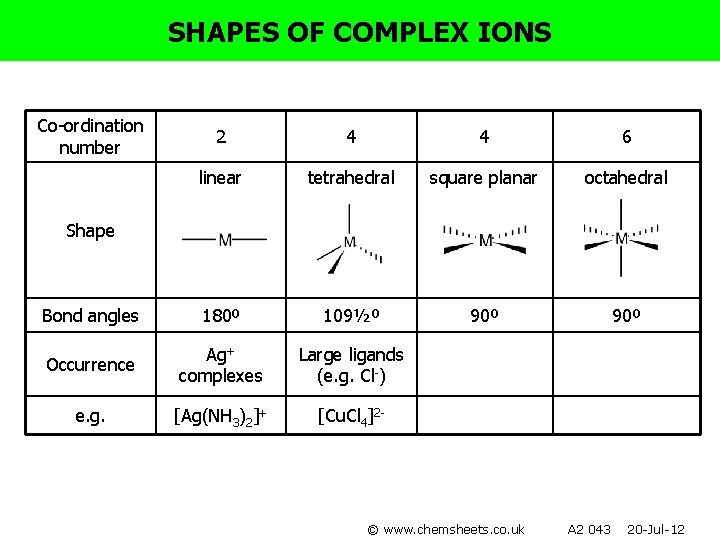
SHAPES OF COMPLEX IONS Co-ordination number 2 4 4 6 linear tetrahedral square planar octahedral Bond angles 180º 109½º 90º Occurrence Ag+ complexes Large ligands (e. g. Cl-) e. g. [Ag(NH 3)2]+ [Cu. Cl 4]2 - Shape © www. chemsheets. co. uk A 2 043 20 -Jul-12

SHAPES OF COMPLEX IONS Co-ordination number 2 4 4 6 linear tetrahedral square planar octahedral Bond angles 180º 109½º 90º Occurrence Ag+ complexes Large ligands (e. g. Cl-) Pt 2+ complexes e. g. [Ag(NH 3)2]+ [Cu. Cl 4]2 - [Pt. Cl 4]2 - Shape © www. chemsheets. co. uk A 2 043 20 -Jul-12

SHAPES OF COMPLEX IONS Co-ordination number 2 4 4 6 linear tetrahedral square planar octahedral Bond angles 180º 109½º 90º Occurrence Ag+ complexes Large ligands (e. g. Cl-) Pt 2+ complexes Commonest e. g. [Ag(NH 3)2]+ [Cu. Cl 4]2 - [Pt. Cl 4]2 - [Cu(H 2 O)6]2+ Shape © www. chemsheets. co. uk A 2 043 20 -Jul-12

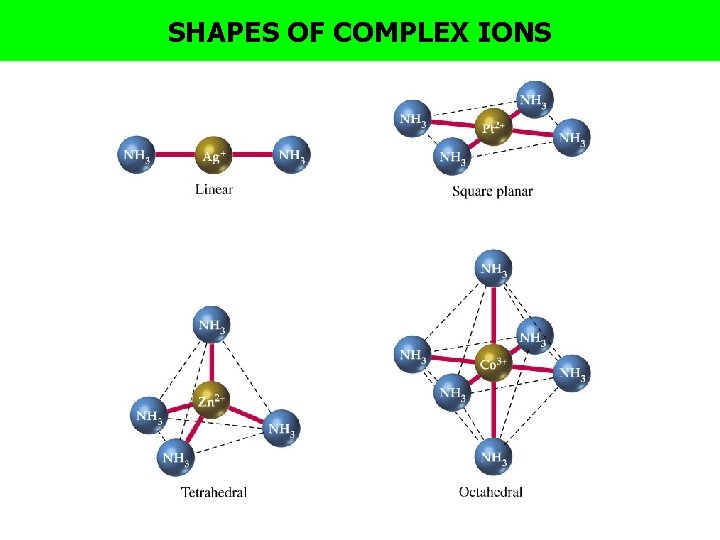
SHAPES OF COMPLEX IONS

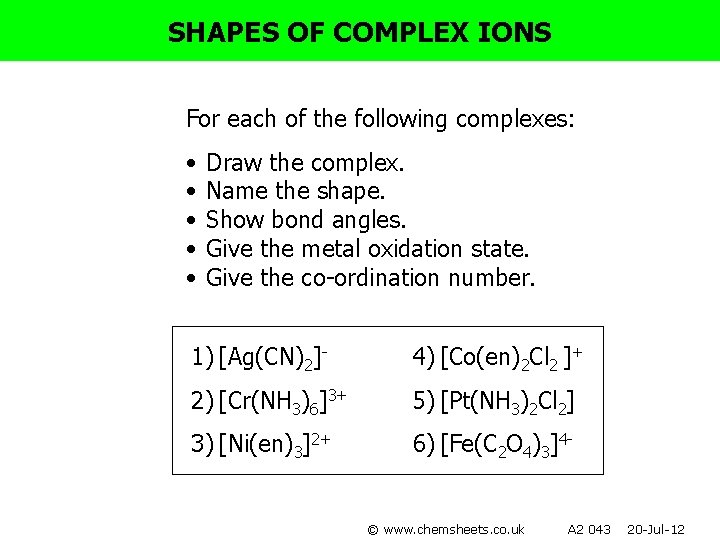
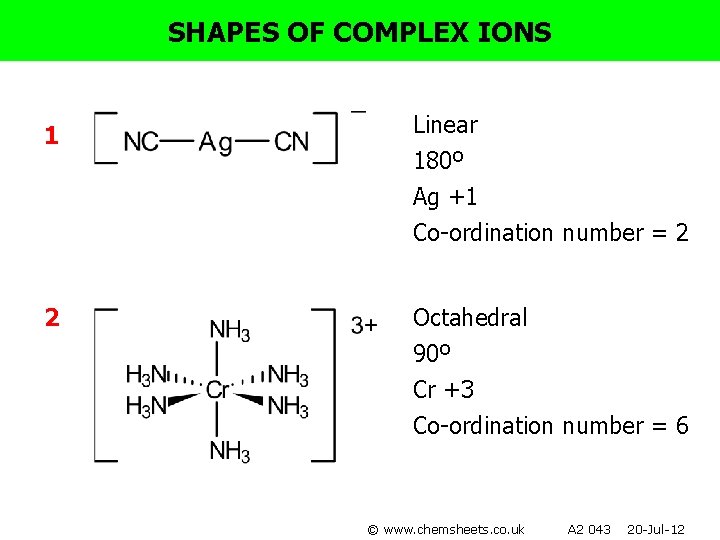
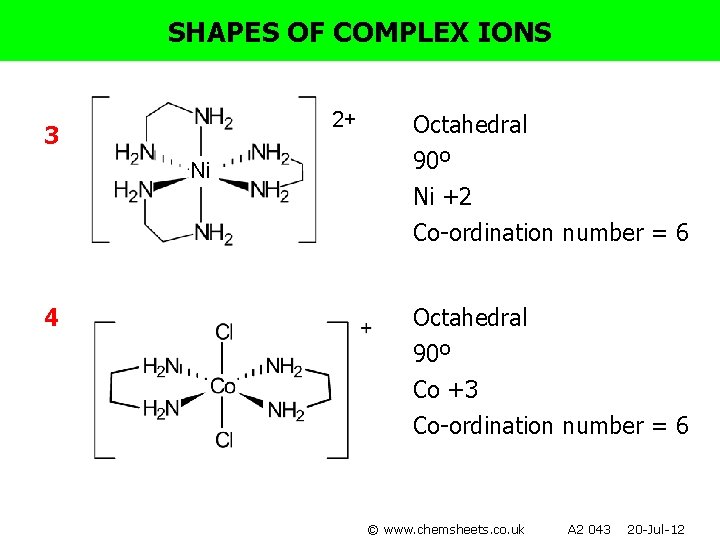
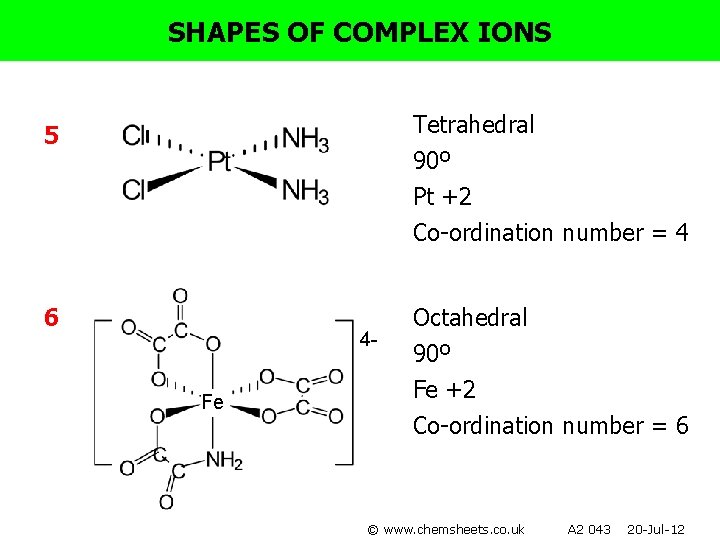
SHAPES OF COMPLEX IONS For each of the following complexes: • • • Draw the complex. Name the shape. Show bond angles. Give the metal oxidation state. Give the co-ordination number. 1) [Ag(CN)2]- 4) [Co(en)2 Cl 2 ]+ 2) [Cr(NH 3)6]3+ 5) [Pt(NH 3)2 Cl 2] 3) [Ni(en)3]2+ 6) [Fe(C 2 O 4)3]4© www. chemsheets. co. uk A 2 043 20 -Jul-12

SHAPES OF COMPLEX IONS 1 Linear 180º Ag +1 Co-ordination number = 2 2 Octahedral 90º Cr +3 Co-ordination number = 6 © www. chemsheets. co. uk A 2 043 20 -Jul-12

SHAPES OF COMPLEX IONS 2+ 3 Ni Octahedral 90º Ni +2 Co-ordination number = 6 4 Octahedral 90º Co +3 Co-ordination number = 6 © www. chemsheets. co. uk A 2 043 20 -Jul-12

SHAPES OF COMPLEX IONS Tetrahedral 90º Pt +2 5 Co-ordination number = 4 6 4 Fe Octahedral 90º Fe +2 Co-ordination number = 6 © www. chemsheets. co. uk A 2 043 20 -Jul-12

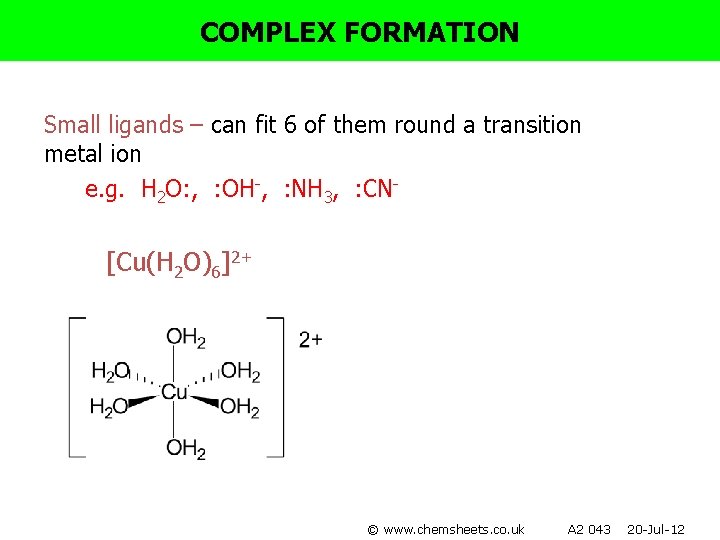
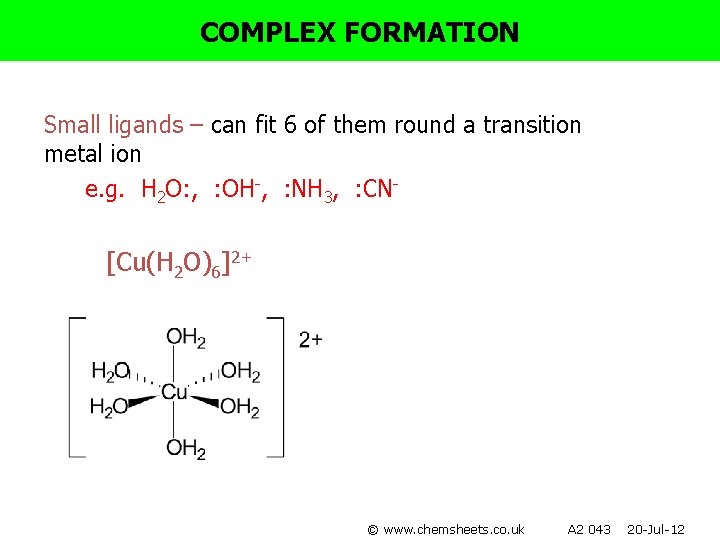
COMPLEX FORMATION Small ligands – can fit 6 of them round a transition metal ion e. g. H 2 O: , : OH-, : NH 3, : CN- [Cu(H 2 O)6]2+ © www. chemsheets. co. uk A 2 043 20 -Jul-12

COMPLEX FORMATION Small ligands – can fit 6 of them round a transition metal ion e. g. H 2 O: , : OH-, : NH 3, : CN- [Cu(H 2 O)6]2+ © www. chemsheets. co. uk A 2 043 20 -Jul-12
![In water pretty much all transition metals form hexaaqua ions MH 2 O6 In water – pretty much all transition metals form hexaaqua ions [M(H 2 O)6]](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-18.jpg)
In water – pretty much all transition metals form hexaaqua ions [M(H 2 O)6] z+ In this case [Co(H 2 O)6] 2+

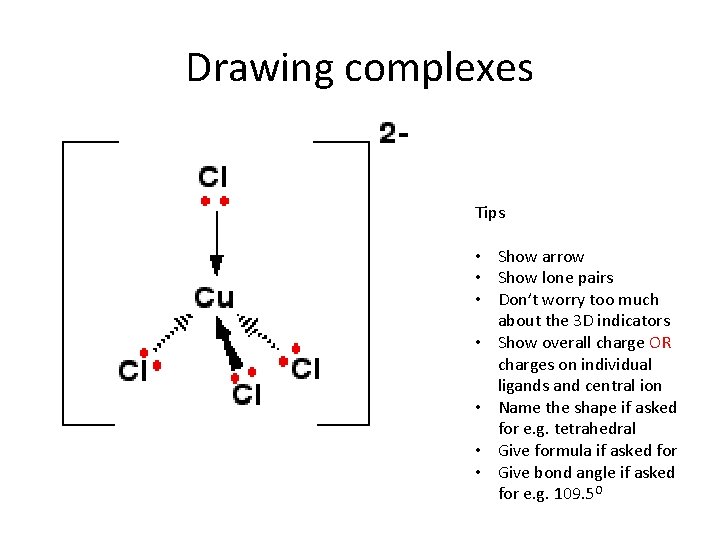
Drawing complexes Tips • Show arrow • Show lone pairs • Don’t worry too much about the 3 D indicators • Show overall charge OR charges on individual ligands and central ion • Name the shape if asked for e. g. tetrahedral • Give formula if asked for • Give bond angle if asked for e. g. 109. 5 O

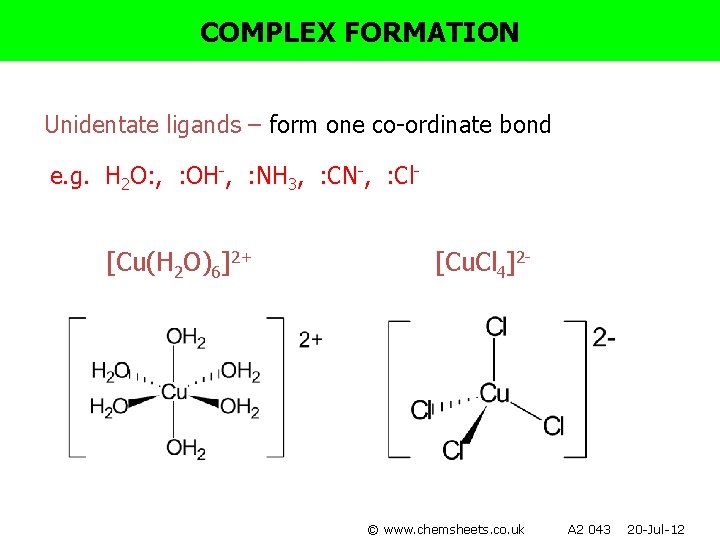
COMPLEX FORMATION Unidentate ligands – form one co-ordinate bond e. g. H 2 O: , : OH-, : NH 3, : CN-, : Cl- [Cu(H 2 O)6]2+ [Cu. Cl 4]2 - © www. chemsheets. co. uk A 2 043 20 -Jul-12
![COMPLEX FORMATION Bidentate ligands form two coordinate bonds 1 2 diaminoethane en Cren33 COMPLEX FORMATION Bidentate ligands – form two co-ordinate bonds 1, 2 -diaminoethane (en) [Cr(en)3]3+](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-21.jpg)
COMPLEX FORMATION Bidentate ligands – form two co-ordinate bonds 1, 2 -diaminoethane (en) [Cr(en)3]3+ ethanedioate (C 2 O 42 -) [Cr(C 2 O 4)3]3 - © www. chemsheets. co. uk A 2 043 20 -Jul-12

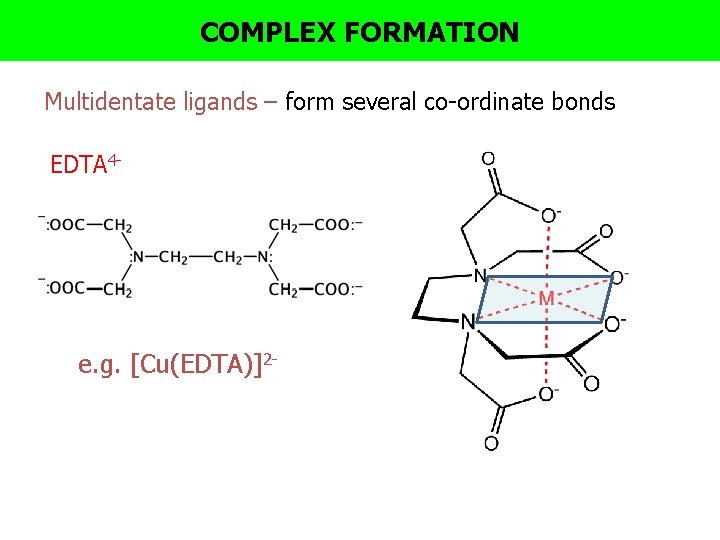
COMPLEX FORMATION Multidentate ligands – form several co-ordinate bonds EDTA 4 - e. g. [Cu(EDTA)]2 -

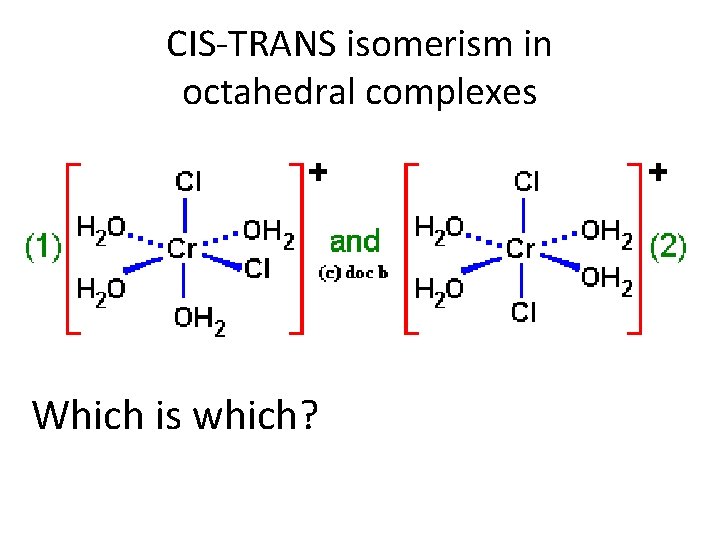
CIS-TRANS isomerism in octahedral complexes Which is which?

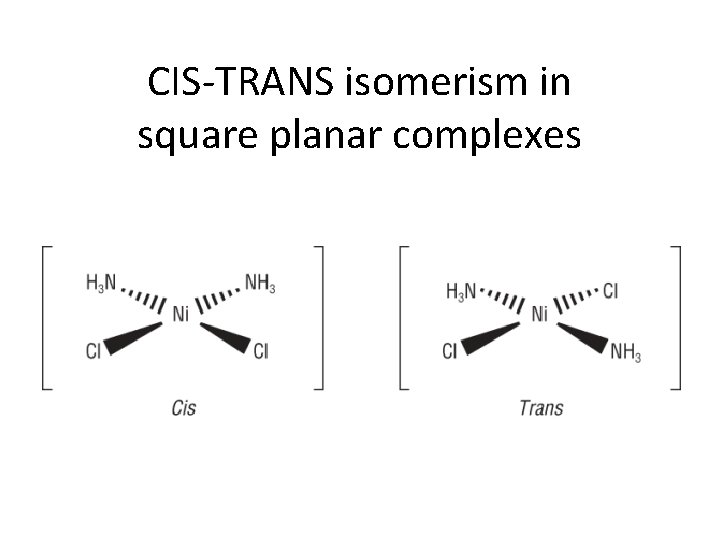
CIS-TRANS isomerism in square planar complexes

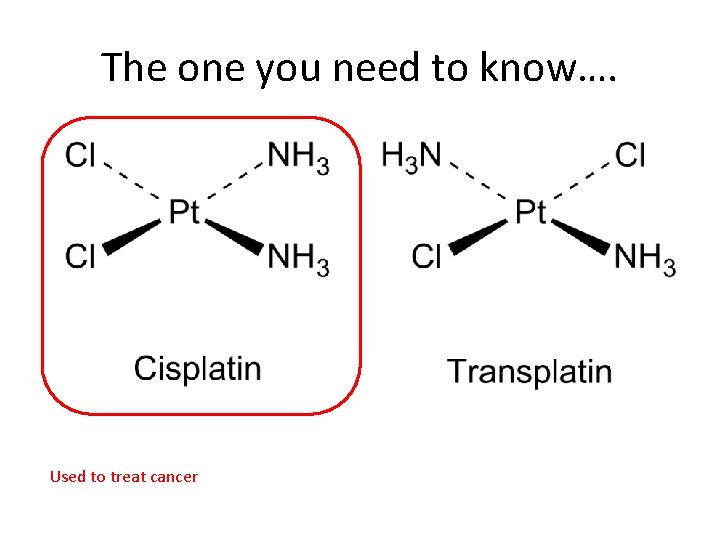
The one you need to know…. Used to treat cancer
![SHAPES OF COMPLEX IONS Geometric Isomerism cis e g Pt Cl 2NH 32 trans SHAPES OF COMPLEX IONS Geometric Isomerism cis e. g. [Pt. Cl 2(NH 3)2] trans](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-26.jpg)
SHAPES OF COMPLEX IONS Geometric Isomerism cis e. g. [Pt. Cl 2(NH 3)2] trans
![SHAPES OF COMPLEX IONS Geometric isomerism e g Co Cl 2NH 34 SHAPES OF COMPLEX IONS Geometric isomerism e. g. [Co. Cl 2(NH 3)4]+](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-27.jpg)
SHAPES OF COMPLEX IONS Geometric isomerism e. g. [Co. Cl 2(NH 3)4]+
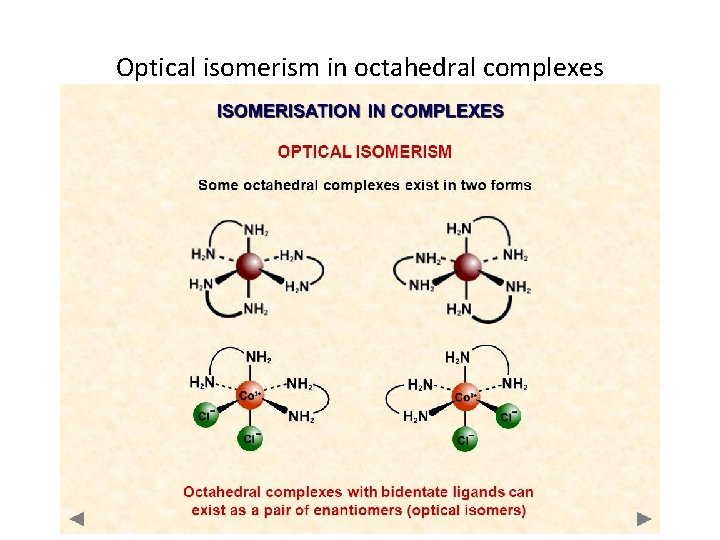
![SHAPES OF COMPLEX IONS Optical Isomerism e g Coen33 SHAPES OF COMPLEX IONS Optical Isomerism e. g. [Co(en)3]3+](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-28.jpg)
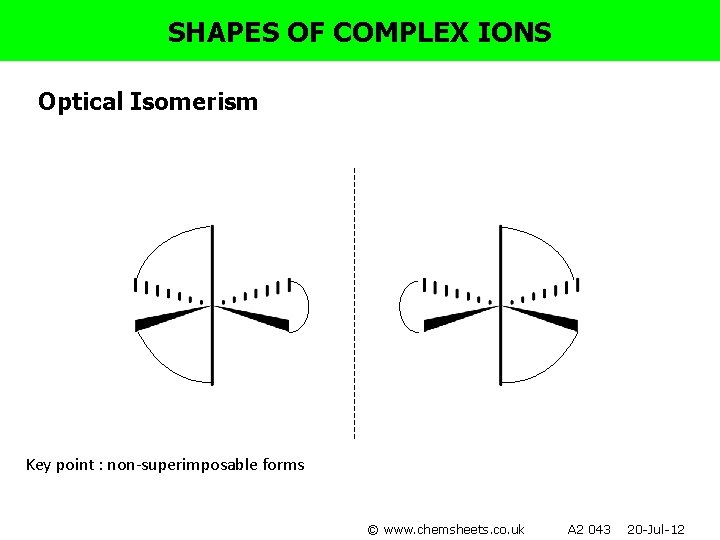
SHAPES OF COMPLEX IONS Optical Isomerism e. g. [Co(en)3]3+

SHAPES OF COMPLEX IONS Optical Isomerism Key point : non-superimposable forms © www. chemsheets. co. uk A 2 043 20 -Jul-12
![What about CoH 2 NCH 2 NH 22 Cl 23 What about…. Co(H 2 NCH 2 NH 2)2 Cl 2]3+](https://slidetodoc.com/presentation_image/4231e4b2af2fb6fb22992cacecdff01c/image-30.jpg)
What about…. Co(H 2 NCH 2 NH 2)2 Cl 2]3+

Optical isomerism in octahedral complexes

Knowledge check… Can you define/explain cis-trans isomerism in transition metal complexes? CIS-TRANS Isomers are complexes that have the same molecular formula, but have a different arrangement of the ligands in space Can you define/explain optical isomerism in transition metal complexes? Optical Isomers are complexes that have the same molecular formula, but rotate plane polarise light in opposite directions as their 3 D arrangements are no-superimposable.

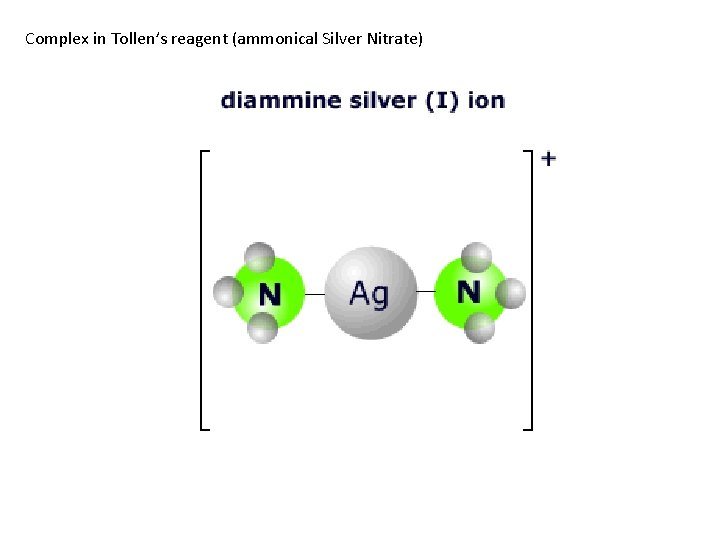
Complex in Tollen’s reagent (ammonical Silver Nitrate)
