Chemical Reactor Design YounWoo Lee School of Chemical
















- Slides: 95

Chemical Reactor Design Youn-Woo Lee School of Chemical and Biological Engineering Seoul National University 155 -741, 599 Gwanangro, Gwanak-gu, Seoul, Korea ywlee@snu. ac. kr http: //sfpl. snu. ac. kr

Chapter 8 Chemical Reaction Engineering Multiple Reactions Seoul National University


Introduction Seldom is the reaction of interest the only one that occurs in a chemical reactor. Typically, multiple reactions will occur, some desired and some undesired. One of the key factors in the economic success of a chemical plant is minimization of undesired side reactions that occur along with the desired reaction. In this chapter, we discuss reactor selection and general mole balances for multiple reactions. First, we describe the four basic types of multiple reactions: series, parallel, independent, and complex. Next, we define the selectivity parameter and discuss how it can be used to minimize unwanted side reactions by proper choice of operating conditions and reactor selection. Seoul National University

Objectives Define different types of selectivity and yield Choose a reaction system that would maximize the selectivity of the desired product given the rate laws for all the reactions occurring in the system. Size reactors to maximize the selectivity and to determine the species concentrations in a batch reactor, semi-batch reactor, CSTR, PFR, and PBR, systems. Seoul National University

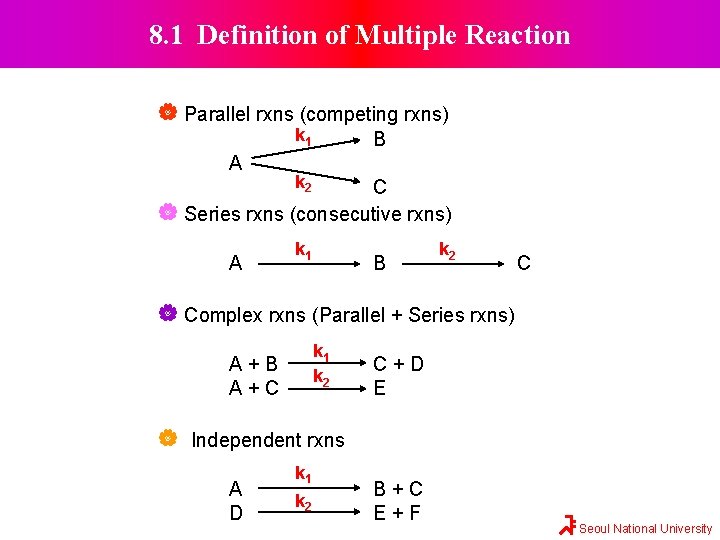
8. 1 Definition of Multiple Reaction Parallel rxns (competing rxns) k 1 A B k 2 C Series rxns (consecutive rxns) A k 1 B k 2 C Complex rxns (Parallel + Series rxns) k 1 k 2 A+B A+C C+D E Independent rxns A D k 1 k 2 B+C E+F Seoul National University

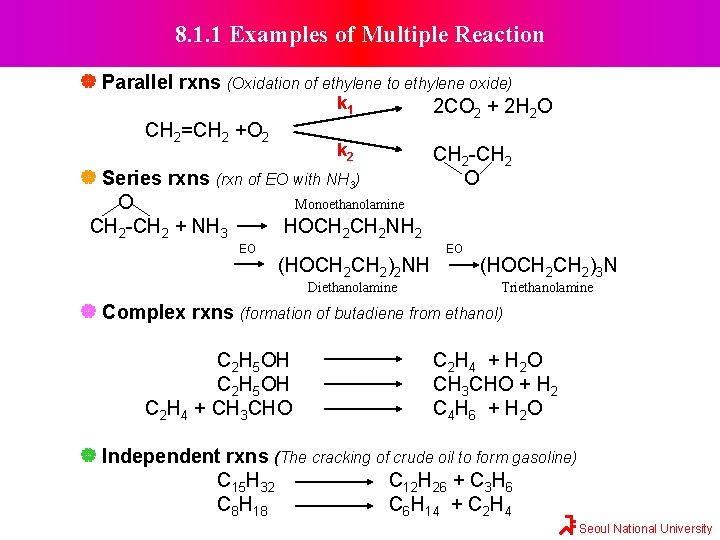
8. 1. 1 Examples of Multiple Reaction Parallel rxns (Oxidation of ethylene to ethylene oxide) k 1 2 CO 2 + 2 H 2 O CH 2=CH 2 +O 2 k 2 CH 2 -CH 2 Series rxns (rxn of EO with NH 3) O O Monoethanolamine CH 2 -CH 2 + NH 3 HOCH 2 NH 2 EO (HOCH 2)2 NH Diethanolamine EO (HOCH 2)3 N Triethanolamine Complex rxns (formation of butadiene from ethanol) C 2 H 5 OH C 2 H 4 + CH 3 CHO C 2 H 4 + H 2 O CH 3 CHO + H 2 C 4 H 6 + H 2 O Independent rxns (The cracking of crude oil to form gasoline) C 15 H 32 C 12 H 26 + C 3 H 6 C 8 H 18 C 6 H 14 + C 2 H 4 Seoul National University

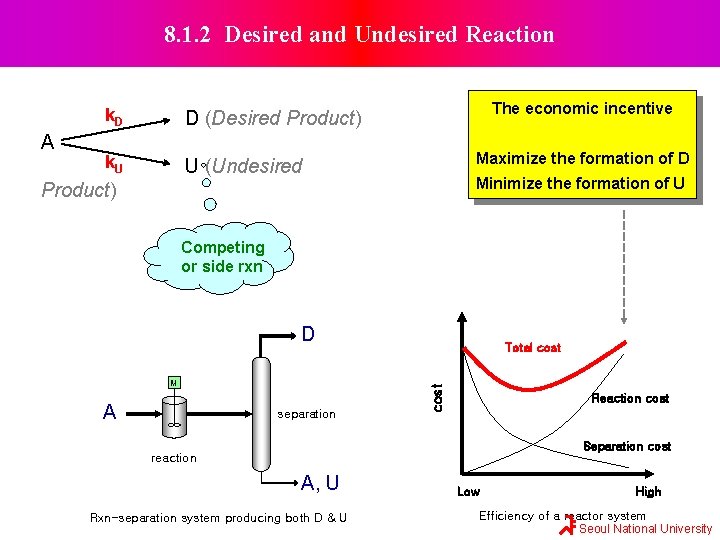
8. 1. 2 Desired and Undesired Reaction A k. D D (Desired Product) k. U U (Undesired The economic incentive Maximize the formation of D Minimize the formation of U Product) Competing or side rxn D A separation cost M Total cost Reaction cost Separation cost reaction A, U Rxn-separation system producing both D & U Low High Efficiency of a reactor system Seoul National University

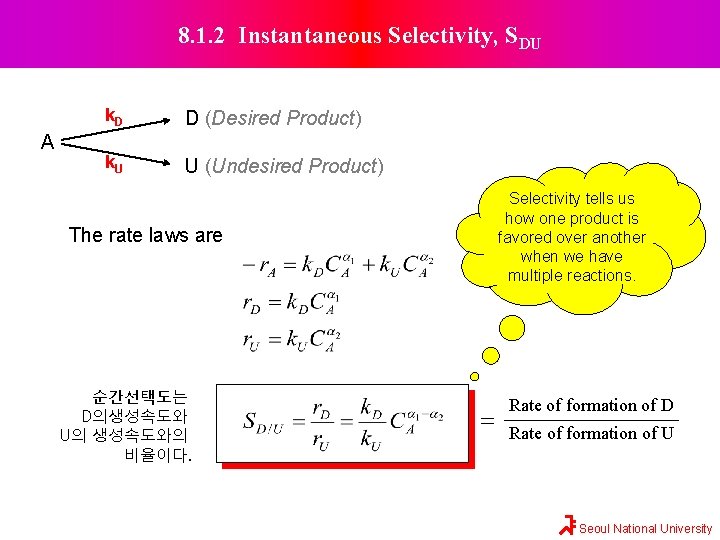
8. 1. 2 Instantaneous Selectivity, SDU A k. D D (Desired Product) k. U U (Undesired Product) Selectivity tells us how one product is favored over another when we have multiple reactions. The rate laws are 순간선택도는 D의생성속도와 U의 생성속도와의 비율이다. = Rate of formation of D Rate of formation of U Seoul National University

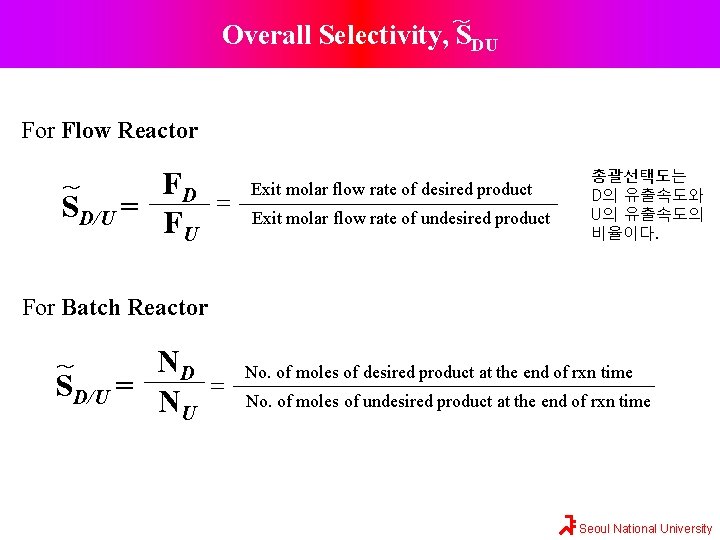
~ Overall Selectivity, SDU For Flow Reactor FD ~ SD/U = FU = Exit molar flow rate of desired product Exit molar flow rate of undesired product 총괄선택도는 D의 유출속도와 U의 유출속도의 비율이다. For Batch Reactor ND SD/U = NU ~ = No. of moles of desired product at the end of rxn time No. of moles of undesired product at the end of rxn time Seoul National University

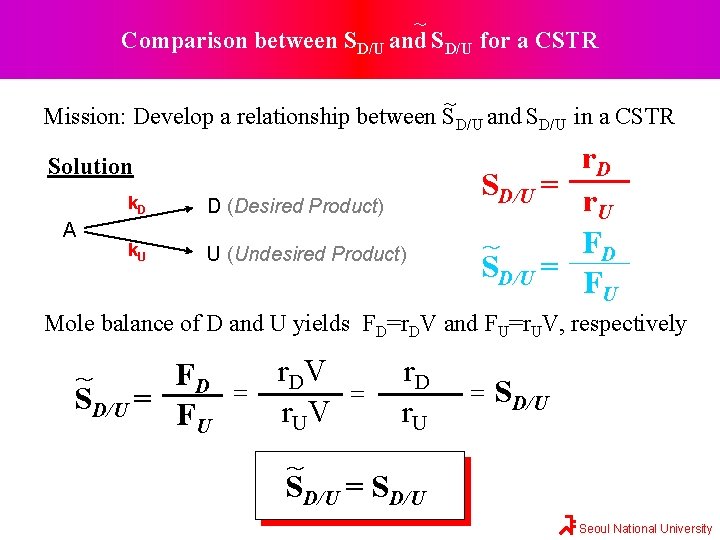
~ Comparison between SD/U and SD/U for a CSTR ~ Mission: Develop a relationship between SD/U and SD/U in a CSTR Solution A k. D D (Desired Product) k. U U (Undesired Product) r. D SD/U = r. U FD ~ SD/U = FU Mole balance of D and U yields FD=r. DV and FU=r. UV, respectively FD SD/U = FU ~ = r. D V r. U V = r. D r. U = SD/U ~ SD/U = SD/U Seoul National University

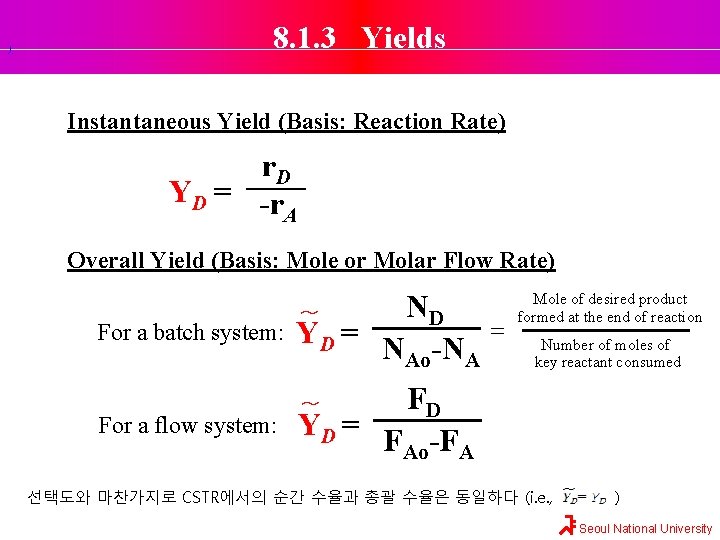
). 8. 1. 3 Yields Instantaneous Yield (Basis: Reaction Rate) r. D YD = -r A Overall Yield (Basis: Mole or Molar Flow Rate) For a batch system: ND ~ YD = N -N Ao A For a flow system: FD YD = F -F Ao A = Mole of desired product formed at the end of reaction Number of moles of key reactant consumed ~ 선택도와 마찬가지로 CSTR에서의 순간 수율과 총괄 수율은 동일하다 (i. e. , ) Seoul National University

Note Different definitions for selectivity and yield Check carefully to ascertain the definition intended by the author From an economic standpoint, overall selectivities and yields are important in determining profits The instantaneous selectivities give insights in choosing reactors and reaction schemes that will help maximize the profit Seoul National University


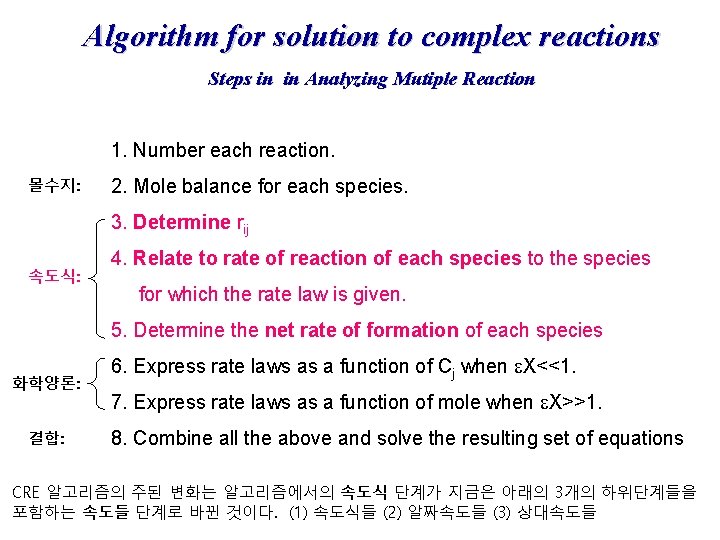
Algorithm for solution to complex reactions Steps in in Analyzing Mutiple Reaction 1. Number each reaction. 몰수지: 2. Mole balance for each species. 3. Determine rij 속도식: 4. Relate to rate of reaction of each species to the species for which the rate law is given. 5. Determine the net rate of formation of each species 화학양론: 결합: 6. Express rate laws as a function of Cj when e. X<<1. 7. Express rate laws as a function of mole when e. X>>1. 8. Combine all the above and solve the resulting set of equations CRE 알고리즘의 주된 변화는 알고리즘에서의 속도식 단계가 지금은 아래의 3개의 하위단계들을 포함하는 속도들 단계로 바뀐 것이다. (1) 속도식들 (2) 알짜속도들 (3) 상대속도들


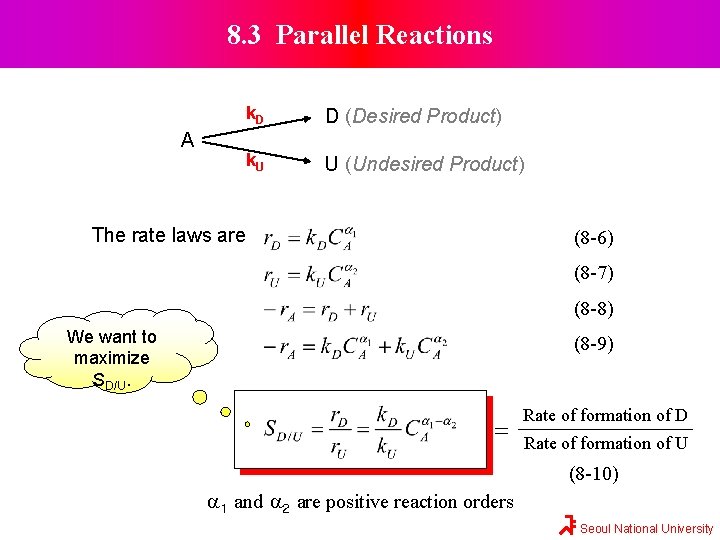
8. 3 Parallel Reactions A k. D D (Desired Product) k. U U (Undesired Product) The rate laws are (8 -6) (8 -7) (8 -8) We want to maximize SD/U. (8 -9) = Rate of formation of D Rate of formation of U (8 -10) a 1 and a 2 are positive reaction orders Seoul National University

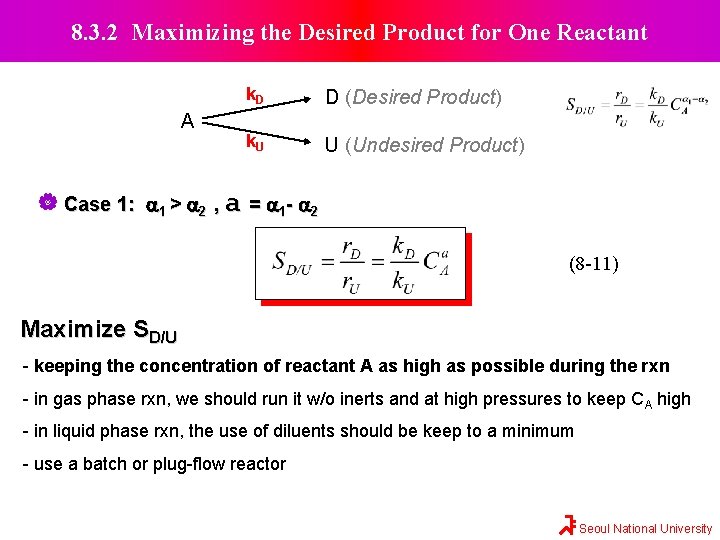
8. 3. 2 Maximizing the Desired Product for One Reactant A k. D D (Desired Product) k. U U (Undesired Product) Case 1: a 1 > a 2 , a = a 1 - a 2 (8 -11) Maximize SD/U - keeping the concentration of reactant A as high as possible during the rxn - in gas phase rxn, we should run it w/o inerts and at high pressures to keep CA high - in liquid phase rxn, the use of diluents should be keep to a minimum - use a batch or plug-flow reactor Seoul National University

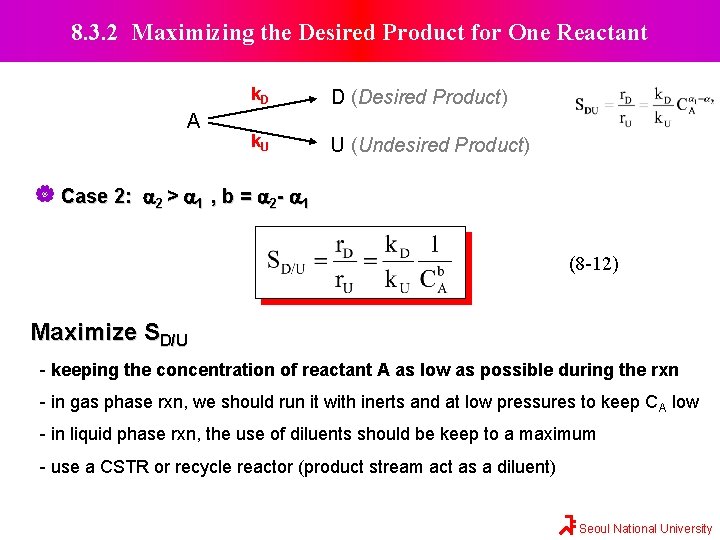
8. 3. 2 Maximizing the Desired Product for One Reactant A k. D D (Desired Product) k. U U (Undesired Product) Case 2: a 2 > a 1 , b = a 2 - a 1 (8 -12) Maximize SD/U - keeping the concentration of reactant A as low as possible during the rxn - in gas phase rxn, we should run it with inerts and at low pressures to keep CA low - in liquid phase rxn, the use of diluents should be keep to a maximum - use a CSTR or recycle reactor (product stream act as a diluent) Seoul National University

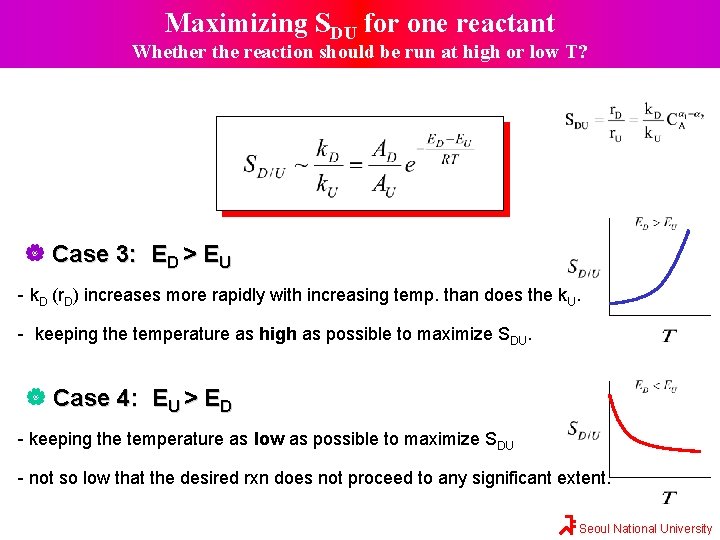
Maximizing SDU for one reactant Whether the reaction should be run at high or low T? Case 3: ED > EU - k. D (r. D) increases more rapidly with increasing temp. than does the k. U. - keeping the temperature as high as possible to maximize SDU. Case 4: EU > ED - keeping the temperature as low as possible to maximize SDU - not so low that the desired rxn does not proceed to any significant extent. Seoul National University

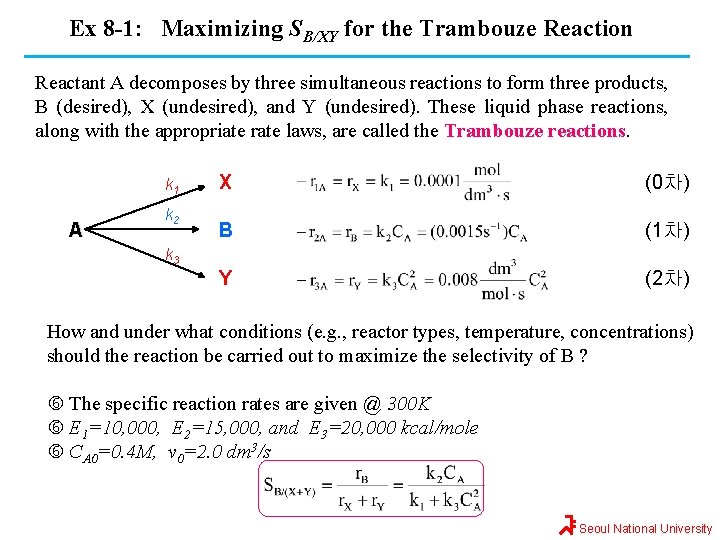
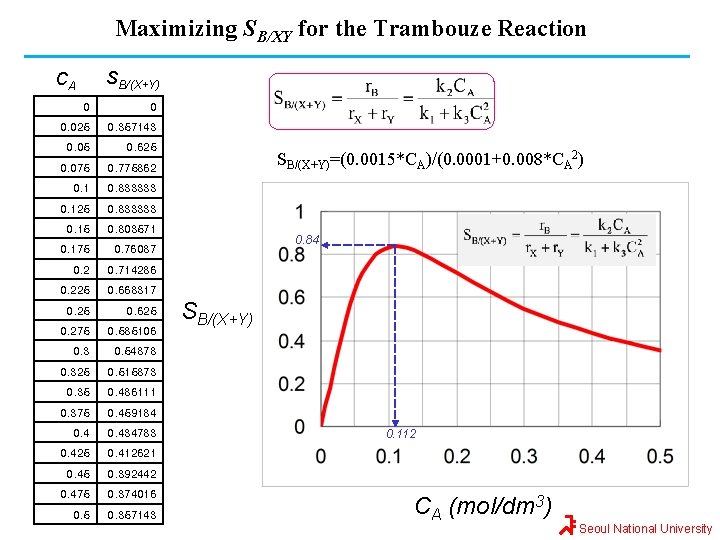
Ex 8 -1: Maximizing SB/XY for the Trambouze Reaction Reactant A decomposes by three simultaneous reactions to form three products, B (desired), X (undesired), and Y (undesired). These liquid phase reactions, along with the appropriate rate laws, are called the Trambouze reactions. k 1 A k 2 X (0차) B (1차) Y (2차) k 3 How and under what conditions (e. g. , reactor types, temperature, concentrations) should the reaction be carried out to maximize the selectivity of B ? The specific reaction rates are given @ 300 K E 1=10, 000, E 2=15, 000, and E 3=20, 000 kcal/mole CA 0=0. 4 M, v 0=2. 0 dm 3/s Seoul National University

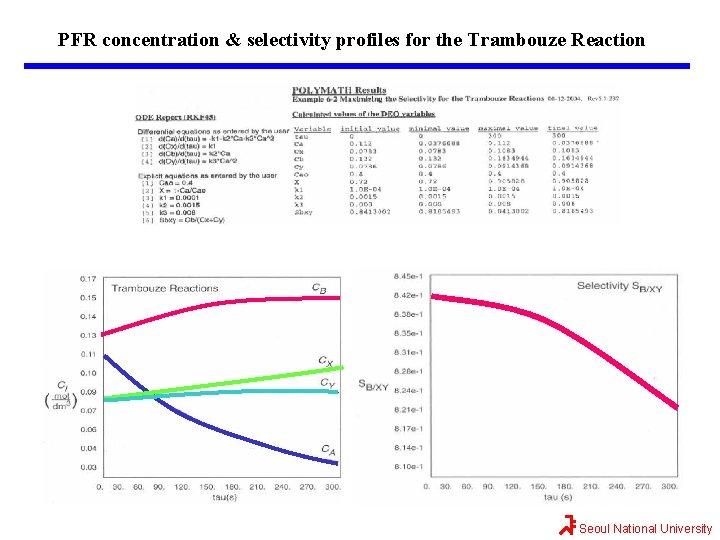
Maximizing SB/XY for the Trambouze Reaction SB/(X+Y) CA 0 0 0. 025 0. 357143 0. 05 0. 625 0. 075 0. 775862 0. 1 0. 833333 0. 125 0. 833333 0. 15 0. 803571 0. 175 0. 76087 0. 2 0. 714286 0. 225 0. 668317 0. 25 0. 625 0. 275 0. 585106 0. 3 0. 54878 0. 325 0. 515873 0. 35 0. 486111 0. 375 0. 459184 0. 434783 0. 425 0. 412621 0. 45 0. 392442 0. 475 0. 374016 0. 5 0. 357143 SB/(X+Y)=(0. 0015*CA)/(0. 0001+0. 008*CA 2) 0. 84 SB/(X+Y) 0. 112 CA (mol/dm 3) Seoul National University

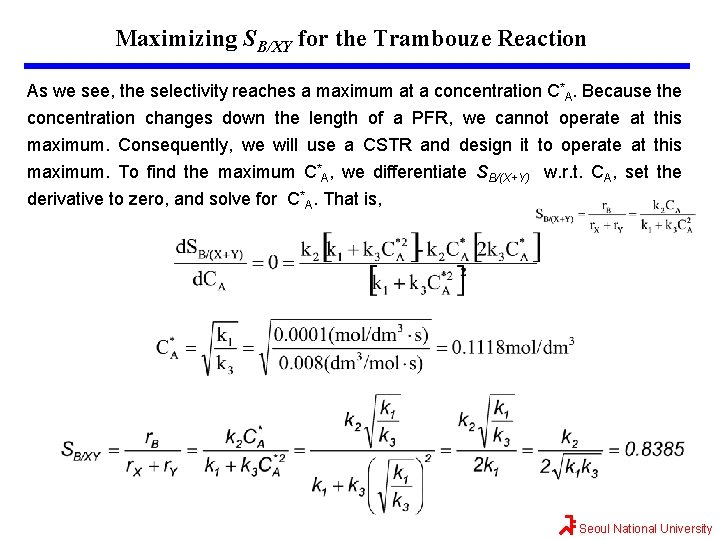
Maximizing SB/XY for the Trambouze Reaction As we see, the selectivity reaches a maximum at a concentration C*A. Because the concentration changes down the length of a PFR, we cannot operate at this maximum. Consequently, we will use a CSTR and design it to operate at this maximum. To find the maximum C*A, we differentiate SB/(X+Y) w. r. t. CA, set the derivative to zero, and solve for C*A. That is, Seoul National University

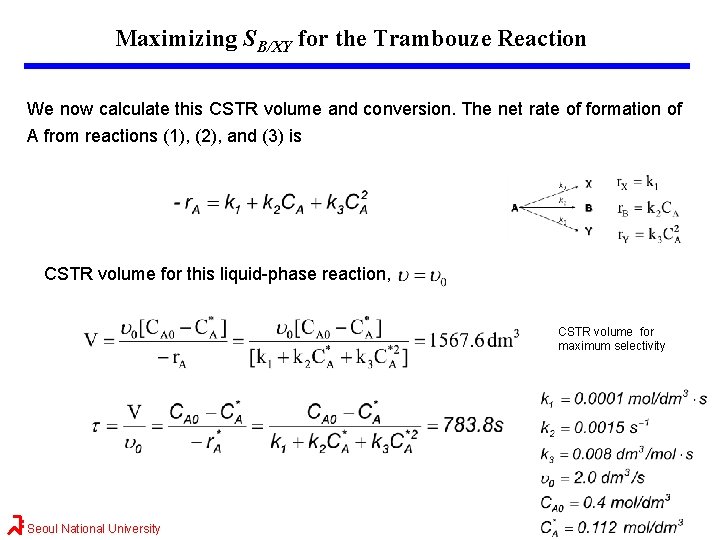
Maximizing SB/XY for the Trambouze Reaction We now calculate this CSTR volume and conversion. The net rate of formation of A from reactions (1), (2), and (3) is CSTR volume for this liquid-phase reaction, CSTR volume for maximum selectivity Seoul National University

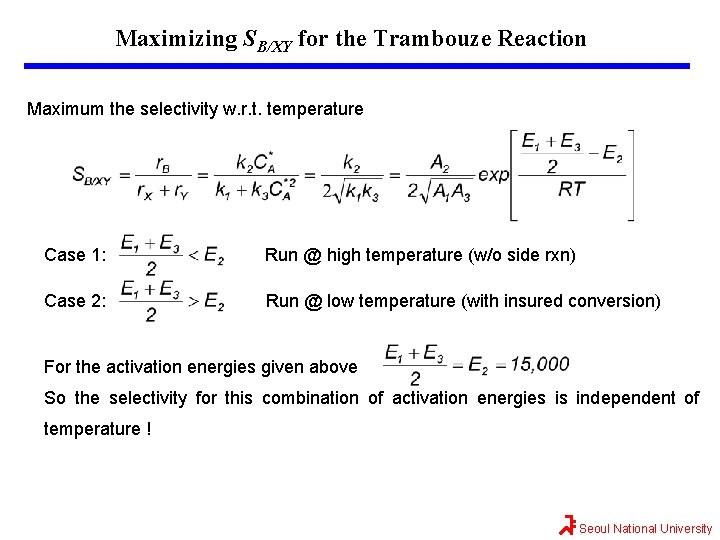
Maximizing SB/XY for the Trambouze Reaction Maximum the selectivity w. r. t. temperature Case 1: Run @ high temperature (w/o side rxn) Case 2: Run @ low temperature (with insured conversion) For the activation energies given above So the selectivity for this combination of activation energies is independent of temperature ! Seoul National University

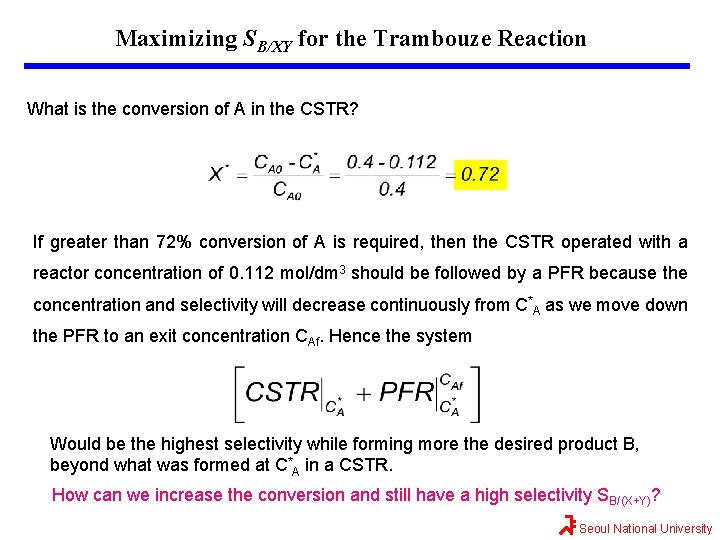
Maximizing SB/XY for the Trambouze Reaction What is the conversion of A in the CSTR? If greater than 72% conversion of A is required, then the CSTR operated with a reactor concentration of 0. 112 mol/dm 3 should be followed by a PFR because the concentration and selectivity will decrease continuously from C*A as we move down the PFR to an exit concentration CAf. Hence the system Would be the highest selectivity while forming more the desired product B, beyond what was formed at C*A in a CSTR. How can we increase the conversion and still have a high selectivity SB/(X+Y)? Seoul National University

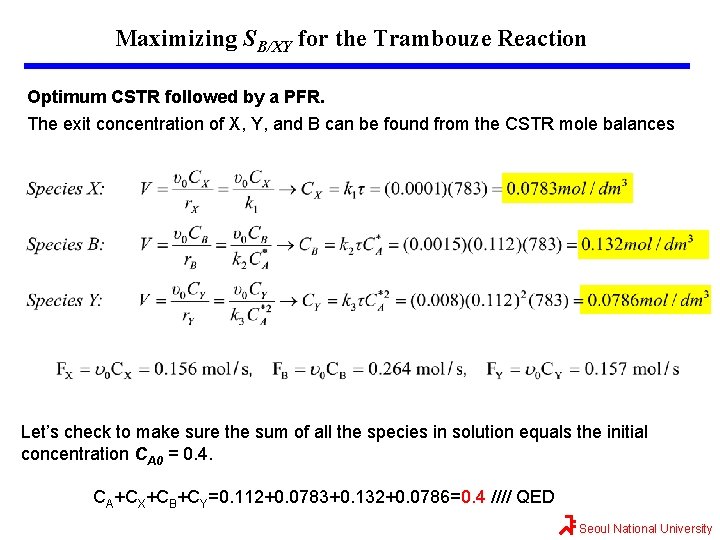
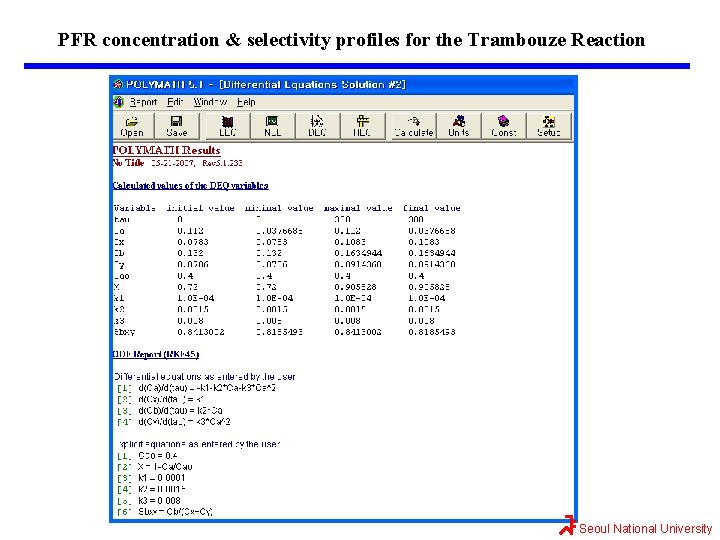
Maximizing SB/XY for the Trambouze Reaction Optimum CSTR followed by a PFR. The exit concentration of X, Y, and B can be found from the CSTR mole balances Let’s check to make sure the sum of all the species in solution equals the initial concentration CA 0 = 0. 4. CA+CX+CB+CY=0. 112+0. 0783+0. 132+0. 0786=0. 4 //// QED Seoul National University

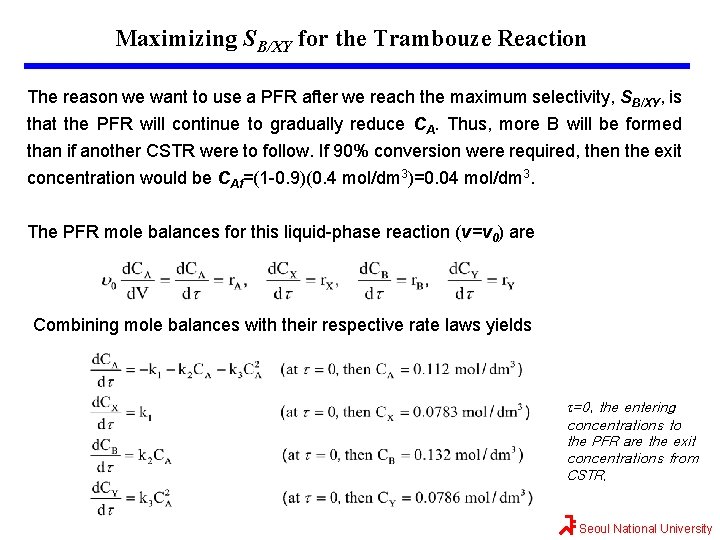
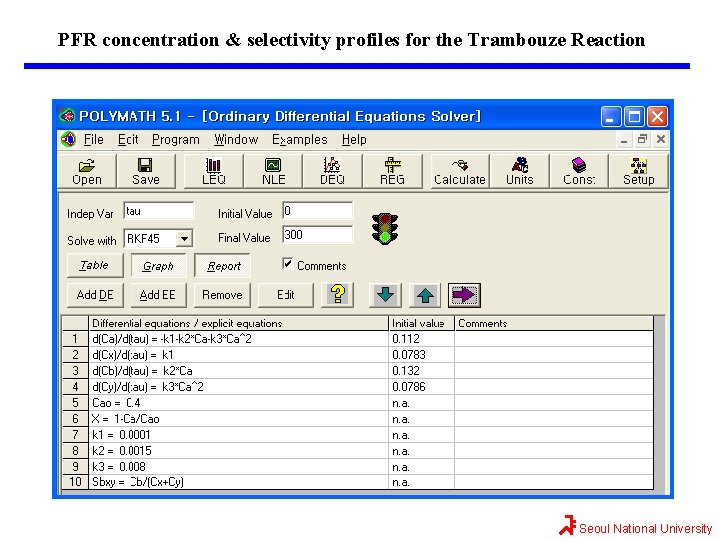
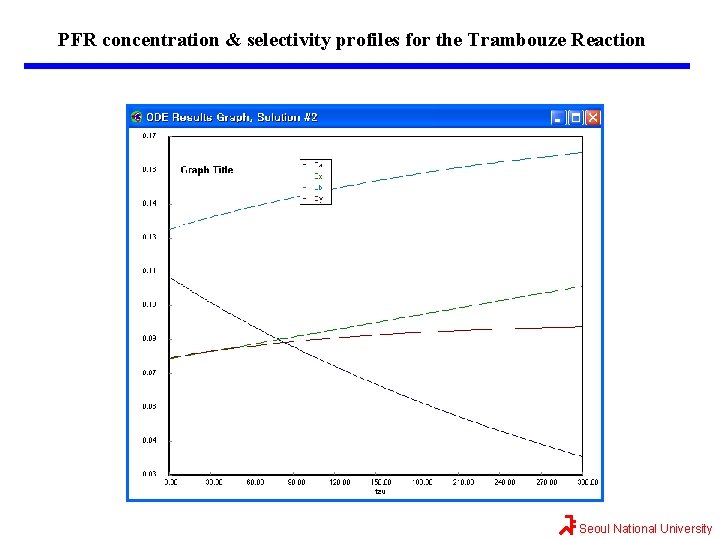
Maximizing SB/XY for the Trambouze Reaction The reason we want to use a PFR after we reach the maximum selectivity, SB/XY, is that the PFR will continue to gradually reduce CA. Thus, more B will be formed than if another CSTR were to follow. If 90% conversion were required, then the exit concentration would be CAf=(1 -0. 9)(0. 4 mol/dm 3)=0. 04 mol/dm 3. The PFR mole balances for this liquid-phase reaction (v=v 0) are Combining mole balances with their respective rate laws yields t=0, the entering concentrations to the PFR are the exit concentrations from CSTR. Seoul National University

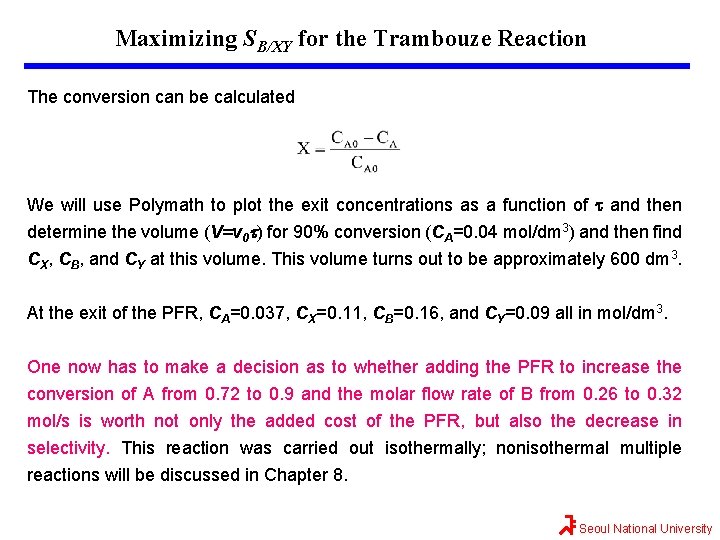
Maximizing SB/XY for the Trambouze Reaction The conversion can be calculated We will use Polymath to plot the exit concentrations as a function of t and then determine the volume (V=v 0 t) for 90% conversion (CA=0. 04 mol/dm 3) and then find CX, CB, and CY at this volume. This volume turns out to be approximately 600 dm 3. At the exit of the PFR, CA=0. 037, CX=0. 11, CB=0. 16, and CY=0. 09 all in mol/dm 3. One now has to make a decision as to whether adding the PFR to increase the conversion of A from 0. 72 to 0. 9 and the molar flow rate of B from 0. 26 to 0. 32 mol/s is worth not only the added cost of the PFR, but also the decrease in selectivity. This reaction was carried out isothermally; nonisothermal multiple reactions will be discussed in Chapter 8. Seoul National University


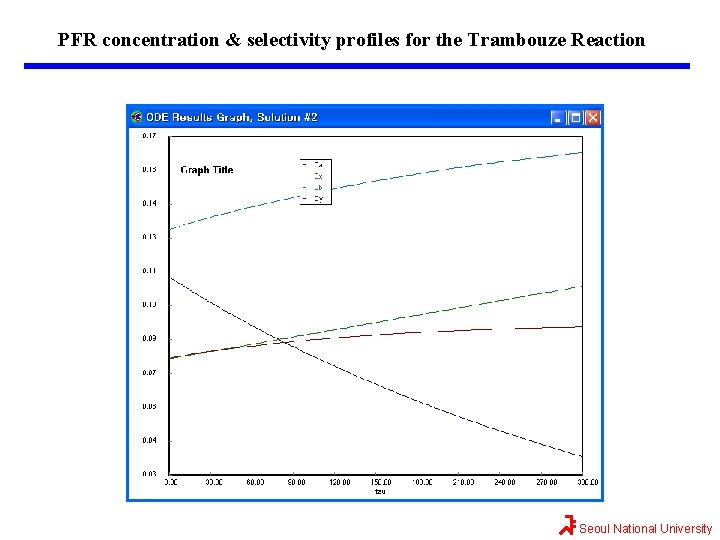
PFR concentration & selectivity profiles for the Trambouze Reaction Seoul National University

PFR concentration & selectivity profiles for the Trambouze Reaction Seoul National University

PFR concentration & selectivity profiles for the Trambouze Reaction Seoul National University

PFR concentration & selectivity profiles for the Trambouze Reaction Seoul National University

PFR concentration & selectivity profiles for the Trambouze Reaction Seoul National University

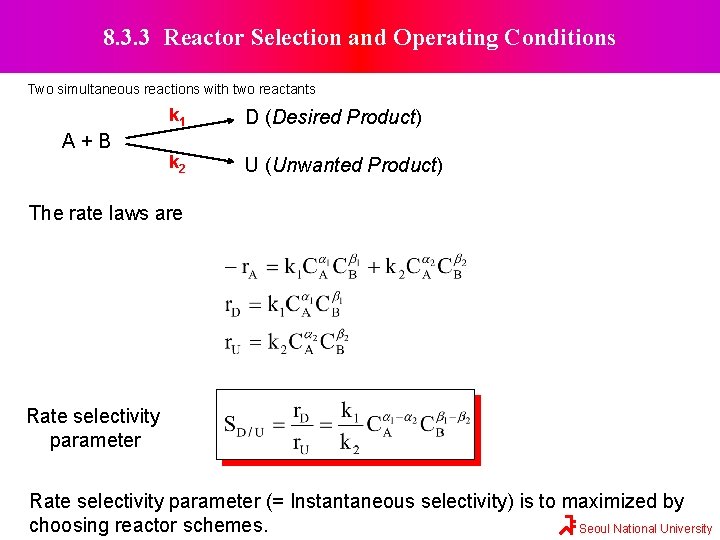
8. 3. 3 Reactor Selection and Operating Conditions Two simultaneous reactions with two reactants A+B k 1 D (Desired Product) k 2 U (Unwanted Product) The rate laws are Rate selectivity parameter (= Instantaneous selectivity) is to maximized by choosing reactor schemes. Seoul National University

Reactor Selection Criteria Selectivity Yield Temperature control Safety Cost Seoul National University

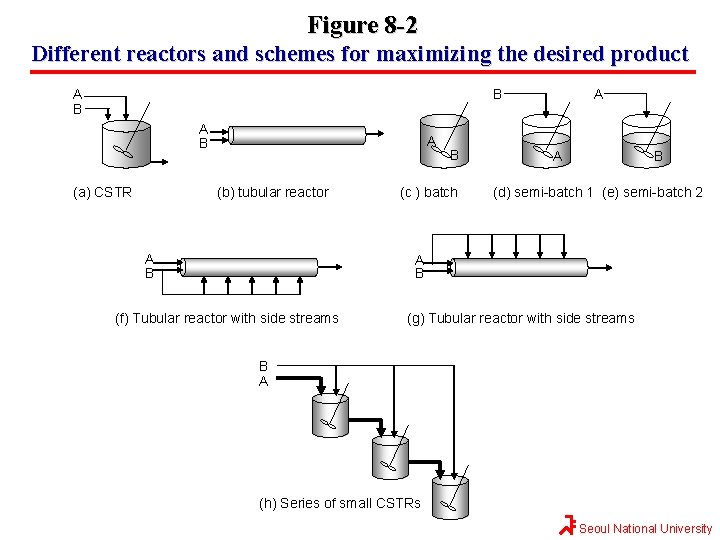
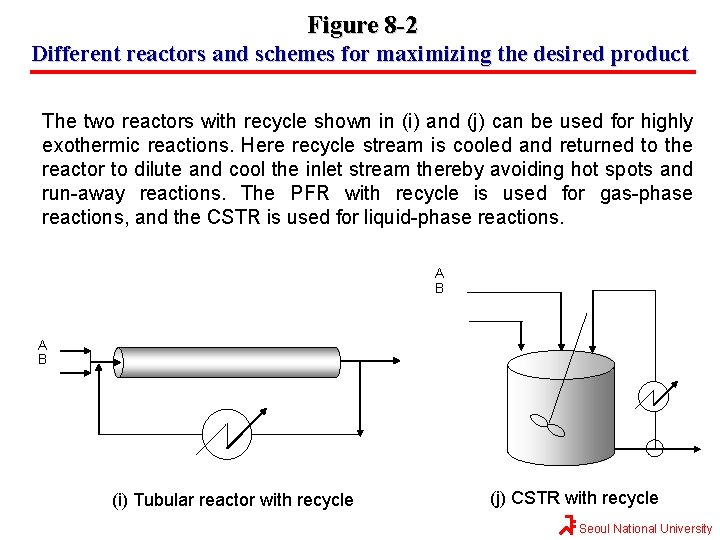
Figure 8 -2 Different reactors and schemes for maximizing the desired product B A B (a) CSTR A (b) tubular reactor A B B (c ) batch A A B (d) semi-batch 1 (e) semi-batch 2 A B (f) Tubular reactor with side streams (g) Tubular reactor with side streams B A (h) Series of small CSTRs Seoul National University

Figure 8 -2 Different reactors and schemes for maximizing the desired product The two reactors with recycle shown in (i) and (j) can be used for highly exothermic reactions. Here recycle stream is cooled and returned to the reactor to dilute and cool the inlet stream thereby avoiding hot spots and run-away reactions. The PFR with recycle is used for gas-phase reactions, and the CSTR is used for liquid-phase reactions. A B (i) Tubular reactor with recycle (j) CSTR with recycle Seoul National University

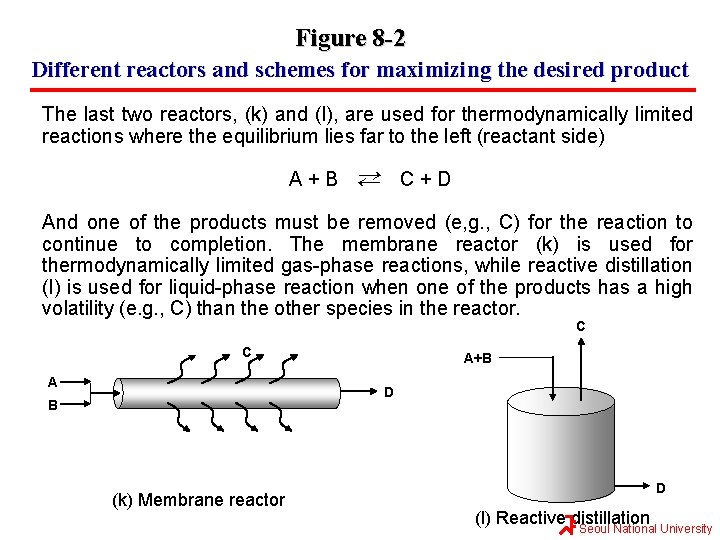
Figure 8 -2 Different reactors and schemes for maximizing the desired product The last two reactors, (k) and (l), are used for thermodynamically limited reactions where the equilibrium lies far to the left (reactant side) ← A+B ← C+D And one of the products must be removed (e, g. , C) for the reaction to continue to completion. The membrane reactor (k) is used for thermodynamically limited gas-phase reactions, while reactive distillation (l) is used for liquid-phase reaction when one of the products has a high volatility (e. g. , C) than the other species in the reactor. C C A A+B D B (k) Membrane reactor D (l) Reactive distillation Seoul National University

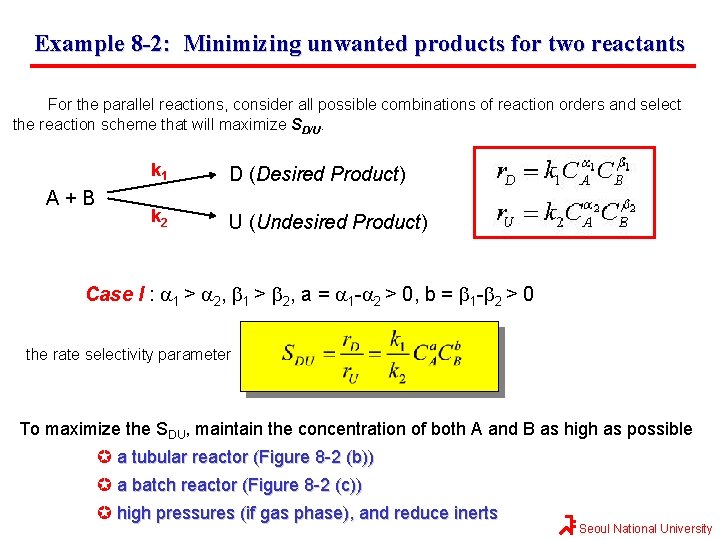
Example 8 -2: Minimizing unwanted products for two reactants For the parallel reactions, consider all possible combinations of reaction orders and select the reaction scheme that will maximize SD/U. A+B k 1 D (Desired Product) k 2 U (Undesired Product) Case I : a 1 > a 2, b 1 > b 2, a = a 1 -a 2 > 0, b = b 1 -b 2 > 0 the rate selectivity parameter To maximize the SDU, maintain the concentration of both A and B as high as possible a tubular reactor (Figure 8 -2 (b)) a batch reactor (Figure 8 -2 (c)) high pressures (if gas phase), and reduce inerts Seoul National University

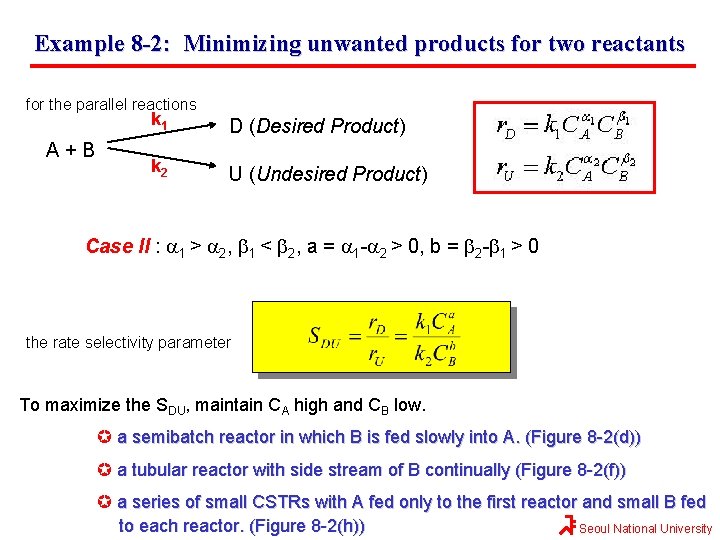
Example 8 -2: Minimizing unwanted products for two reactants for the parallel reactions A+B k 1 D (Desired Product) k 2 U (Undesired Product) Case II : a 1 > a 2, b 1 < b 2, a = a 1 -a 2 > 0, b = b 2 -b 1 > 0 the rate selectivity parameter To maximize the SDU, maintain CA high and CB low. a semibatch reactor in which B is fed slowly into A. (Figure 8 -2(d)) a tubular reactor with side stream of B continually (Figure 8 -2(f)) a series of small CSTRs with A fed only to the first reactor and small B fed to each reactor. (Figure 8 -2(h)) Seoul National University

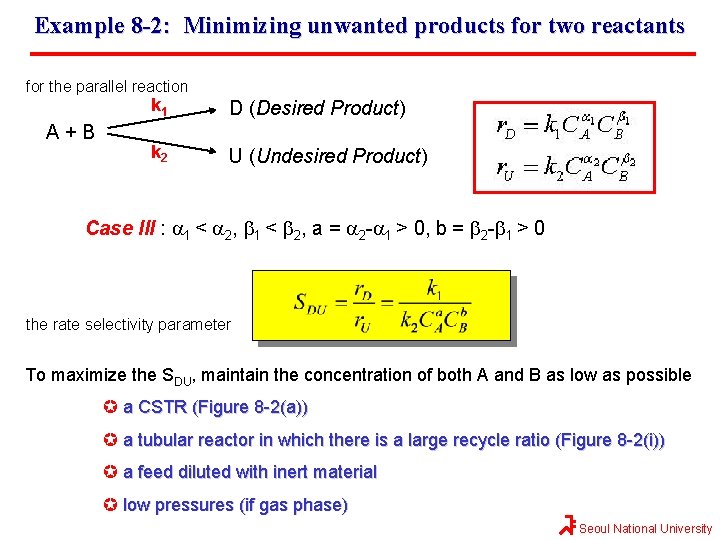
Example 8 -2: Minimizing unwanted products for two reactants for the parallel reaction A+B k 1 D (Desired Product) k 2 U (Undesired Product) Case III : a 1 < a 2, b 1 < b 2, a = a 2 -a 1 > 0, b = b 2 -b 1 > 0 the rate selectivity parameter To maximize the SDU, maintain the concentration of both A and B as low as possible a CSTR (Figure 8 -2(a)) a tubular reactor in which there is a large recycle ratio (Figure 8 -2(i)) a feed diluted with inert material low pressures (if gas phase) Seoul National University

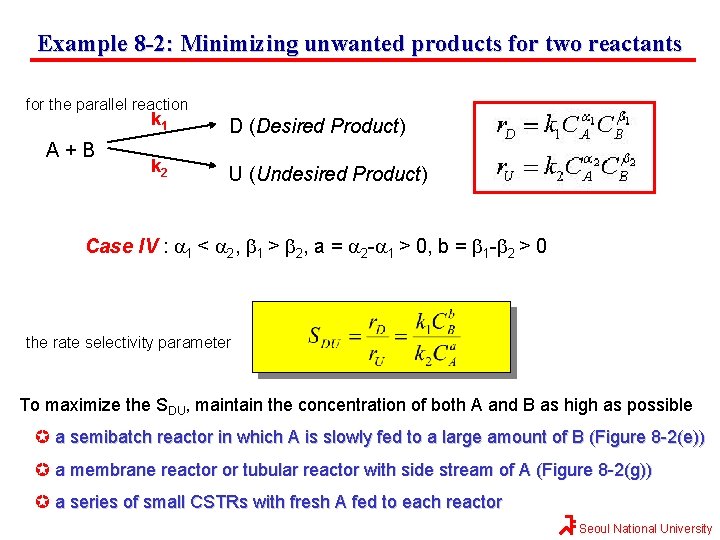
Example 8 -2: Minimizing unwanted products for two reactants for the parallel reaction A+B k 1 D (Desired Product) k 2 U (Undesired Product) Case IV : a 1 < a 2, b 1 > b 2, a = a 2 -a 1 > 0, b = b 1 -b 2 > 0 the rate selectivity parameter To maximize the SDU, maintain the concentration of both A and B as high as possible a semibatch reactor in which A is slowly fed to a large amount of B (Figure 8 -2(e)) a membrane reactor or tubular reactor with side stream of A (Figure 8 -2(g)) a series of small CSTRs with fresh A fed to each reactor Seoul National University

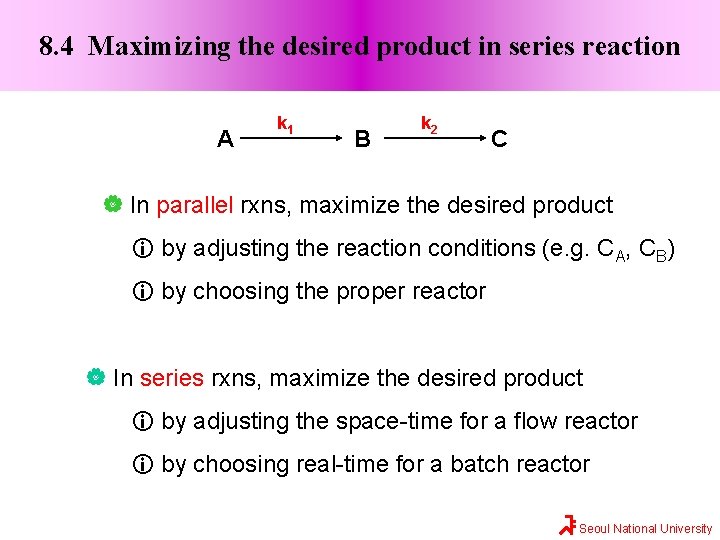
8. 4 Maximizing the desired product in series reaction A k 1 B k 2 C In parallel rxns, maximize the desired product by adjusting the reaction conditions (e. g. CA, CB) by choosing the proper reactor In series rxns, maximize the desired product by adjusting the space-time for a flow reactor by choosing real-time for a batch reactor Seoul National University

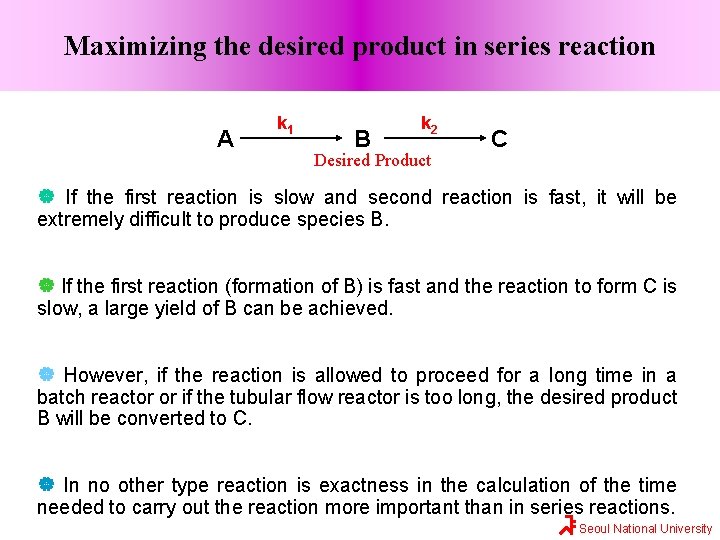
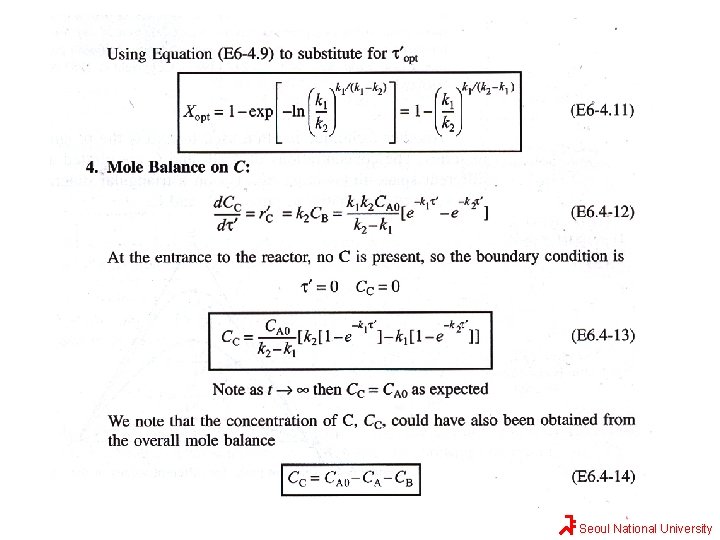
Maximizing the desired product in series reaction A k 1 B k 2 Desired Product C If the first reaction is slow and second reaction is fast, it will be extremely difficult to produce species B. If the first reaction (formation of B) is fast and the reaction to form C is slow, a large yield of B can be achieved. However, if the reaction is allowed to proceed for a long time in a batch reactor or if the tubular flow reactor is too long, the desired product B will be converted to C. In no other type reaction is exactness in the calculation of the time needed to carry out the reaction more important than in series reactions. Seoul National University



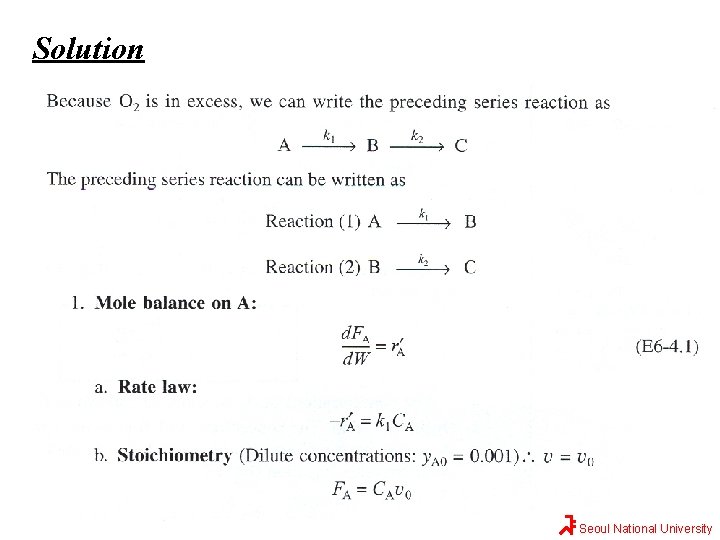
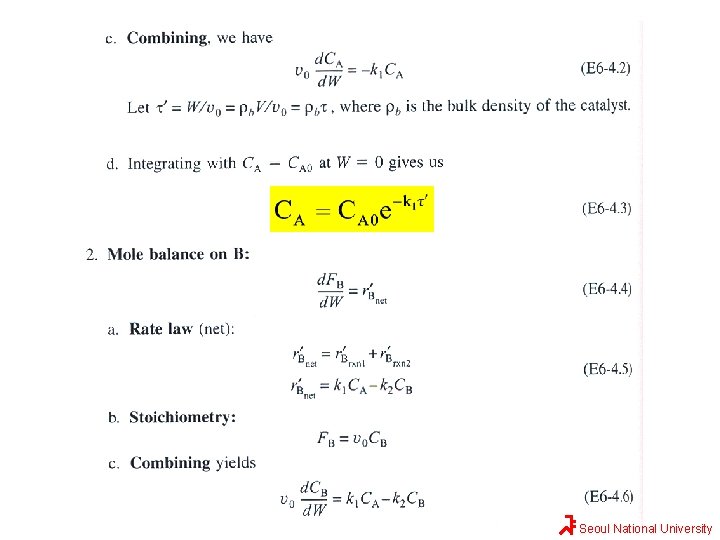
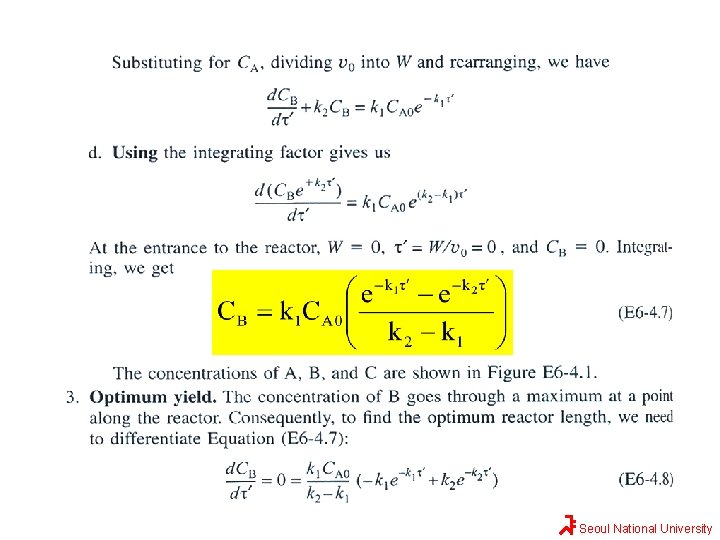
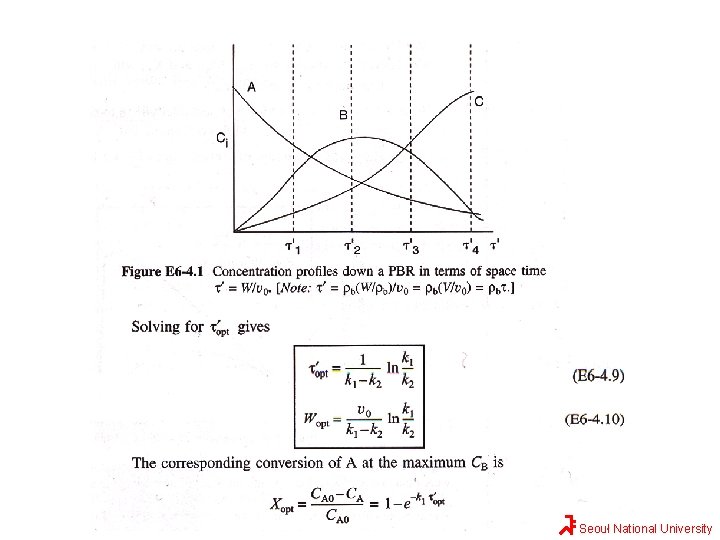
Solution Seoul National University

Seoul National University

Seoul National University

Seoul National University

Seoul National University

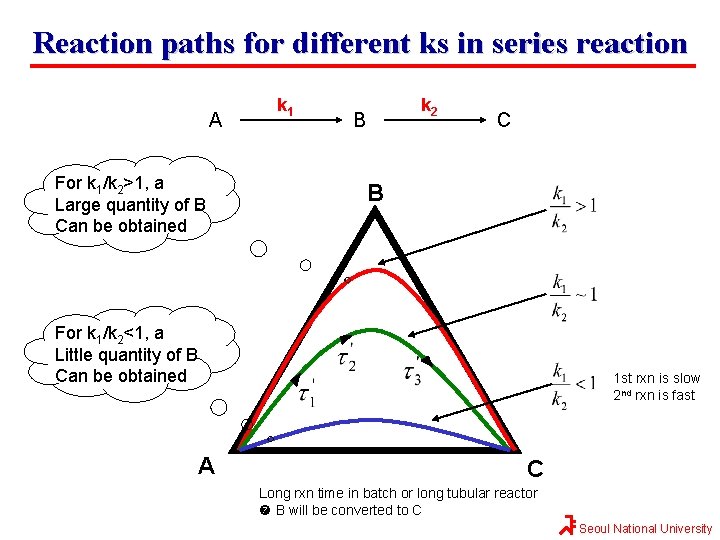
Reaction paths for different ks in series reaction A For k 1/k 2>1, a Large quantity of B Can be obtained k 1 k 2 B C B For k 1/k 2<1, a Little quantity of B Can be obtained A 1 st rxn is slow 2 nd rxn is fast C Long rxn time in batch or long tubular reactor B will be converted to C Seoul National University








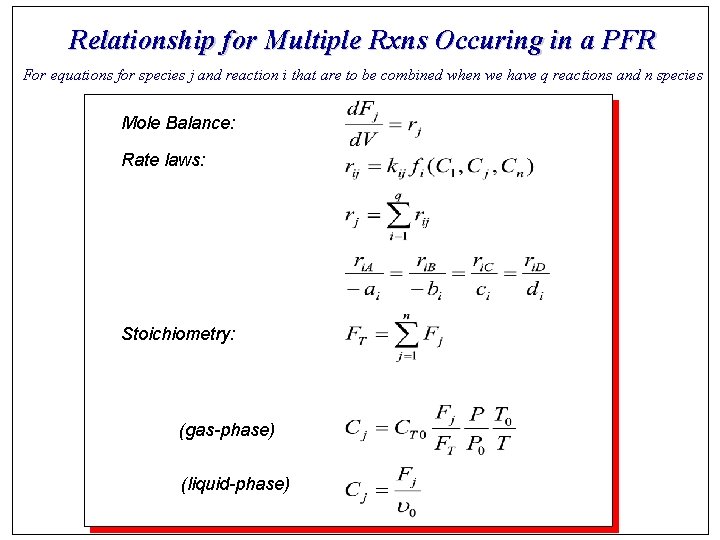
Relationship for Multiple Rxns Occuring in a PFR For equations for species j and reaction i that are to be combined when we have q reactions and n species Mole Balance: Rate laws: Stoichiometry: (gas-phase) (liquid-phase)

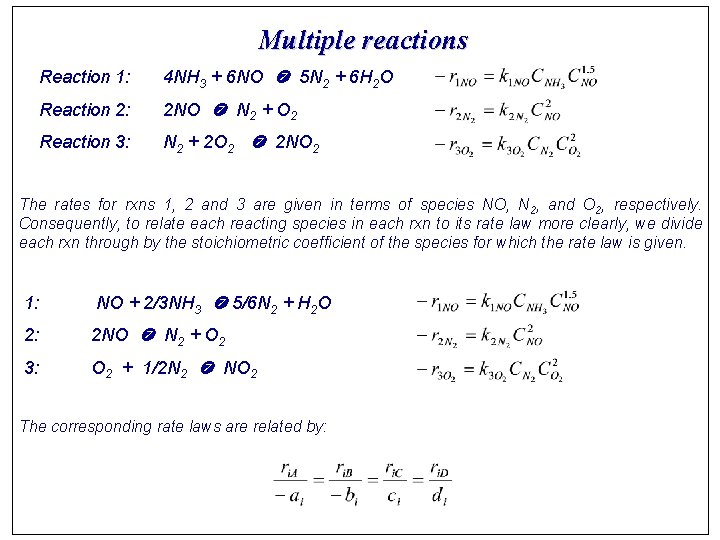
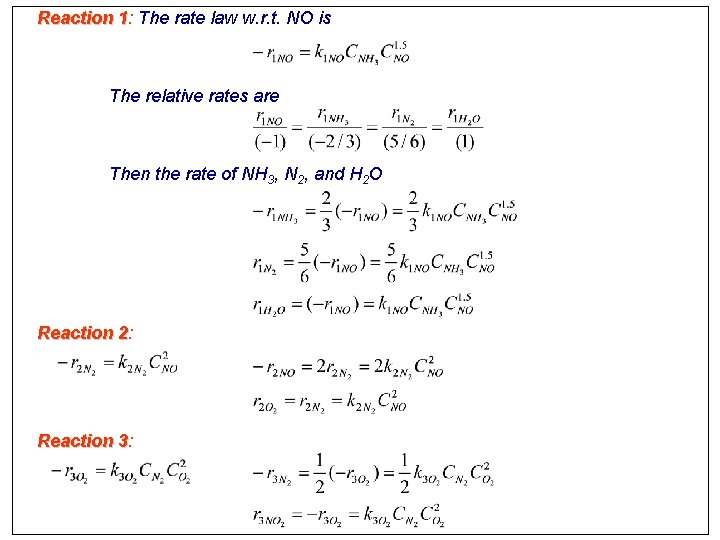
Multiple reactions Reaction 1: 4 NH 3 + 6 NO 5 N 2 + 6 H 2 O Reaction 2: 2 NO N 2 + O 2 Reaction 3: N 2 + 2 O 2 2 NO 2 The rates for rxns 1, 2 and 3 are given in terms of species NO, N 2, and O 2, respectively. Consequently, to relate each reacting species in each rxn to its rate law more clearly, we divide each rxn through by the stoichiometric coefficient of the species for which the rate law is given. 1: NO + 2/3 NH 3 5/6 N 2 + H 2 O 2: 2 NO N 2 + O 2 3: O 2 + 1/2 N 2 NO 2 The corresponding rate laws are related by:

Reaction 1: 1 The rate law w. r. t. NO is The relative rates are Then the rate of NH 3, N 2, and H 2 O Reaction 2: 2 Reaction 3: 3

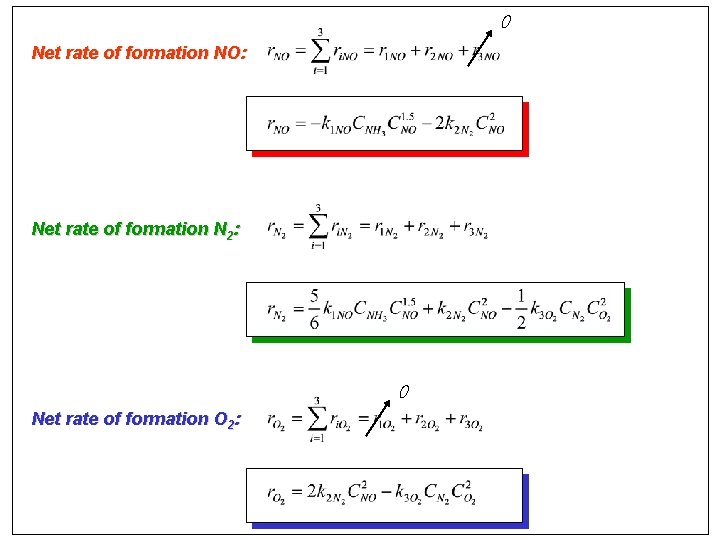
0 Net rate of formation NO: Net rate of formation N 2: 0 Net rate of formation O 2:

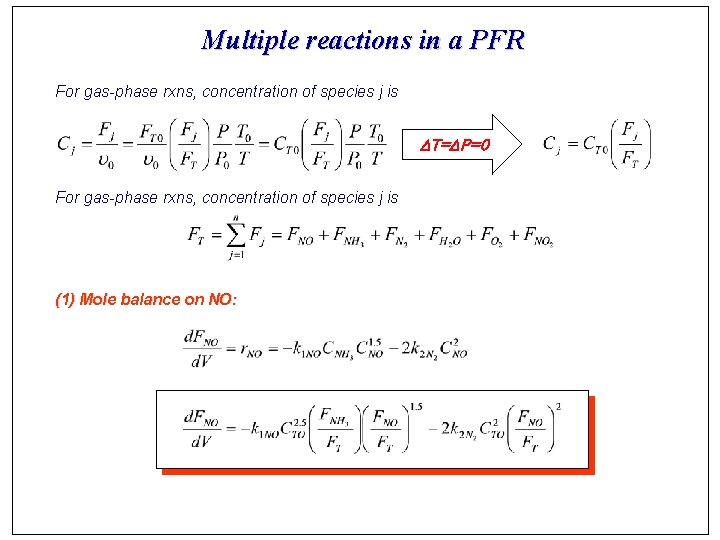
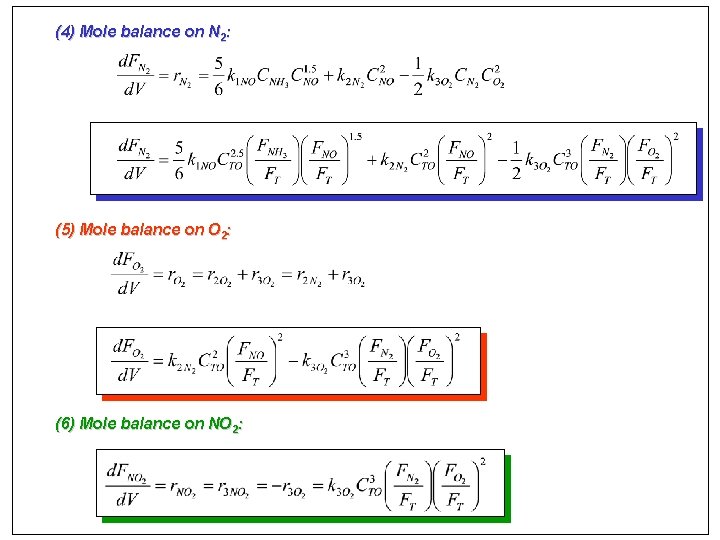
Multiple reactions in a PFR For gas-phase rxns, concentration of species j is DT=DP=0 For gas-phase rxns, concentration of species j is (1) Mole balance on NO:

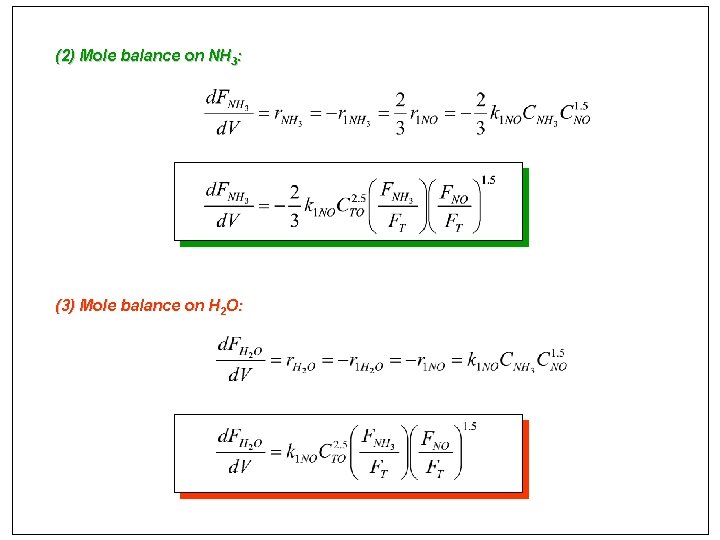
(2) Mole balance on NH 3: (3) Mole balance on H 2 O:

(4) Mole balance on N 2: (5) Mole balance on O 2: (6) Mole balance on NO 2:

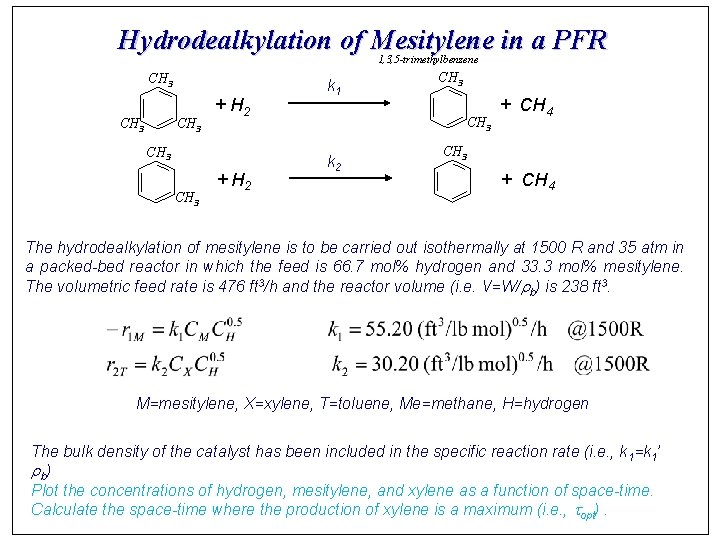
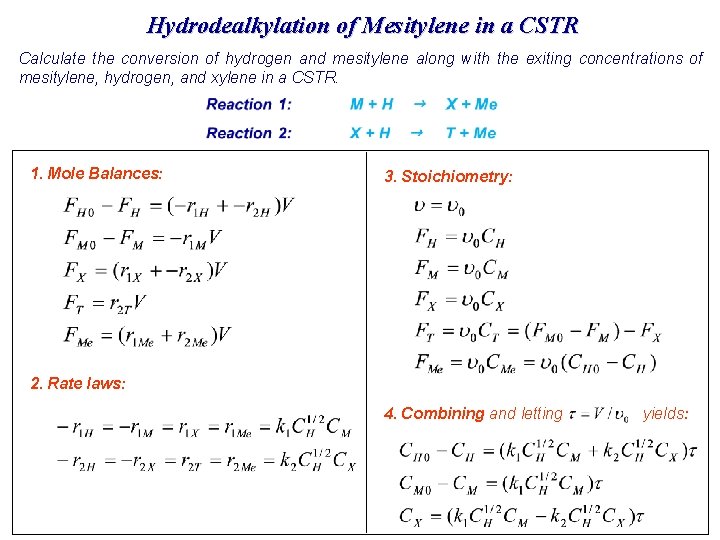
Hydrodealkylation of Mesitylene in a PFR 1, 3, 5 -trimethylbenzene CH 3 + H 2 CH 3 + H 2 k 1 CH 3 k 2 + CH 4 CH 3 + CH 4 The hydrodealkylation of mesitylene is to be carried out isothermally at 1500 R and 35 atm in a packed-bed reactor in which the feed is 66. 7 mol% hydrogen and 33. 3 mol% mesitylene. The volumetric feed rate is 476 ft 3/h and the reactor volume (i. e. V=W/rb) is 238 ft 3. M=mesitylene, X=xylene, T=toluene, Me=methane, H=hydrogen The bulk density of the catalyst has been included in the specific reaction rate (i. e. , k 1=k 1’ r b) Plot the concentrations of hydrogen, mesitylene, and xylene as a function of space-time. Calculate the space-time where the production of xylene is a maximum (i. e. , topt).

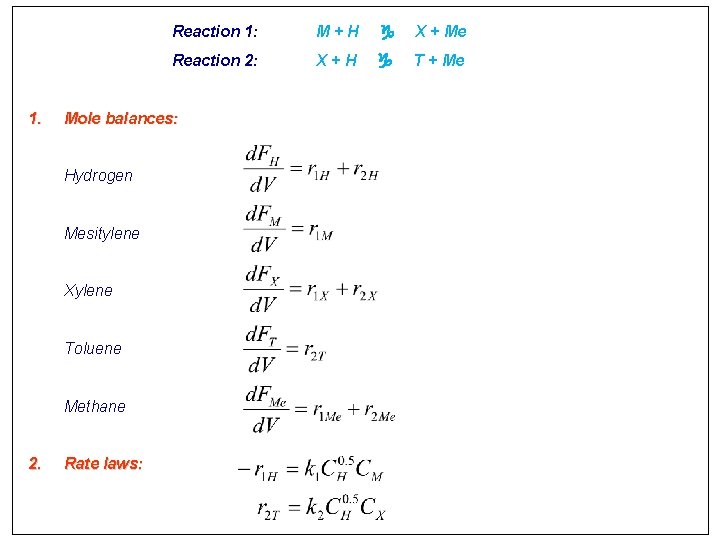
1. M+H X + Me Reaction 2: X+H T + Me Mole balances: Hydrogen Mesitylene Xylene Toluene Methane 2. Reaction 1: Rate laws:

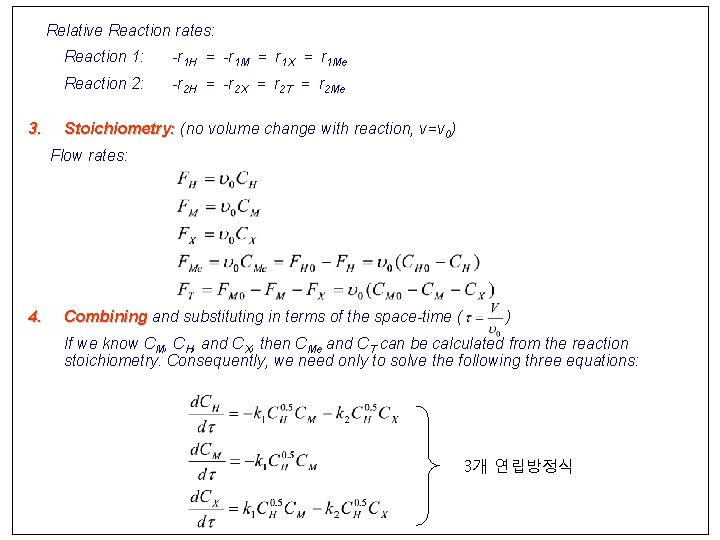
Relative Reaction rates: 3. Reaction 1: -r 1 H = -r 1 M = r 1 X = r 1 Me Reaction 2: -r 2 H = -r 2 X = r 2 T = r 2 Me Stoichiometry: (no volume change with reaction, v=v 0) Flow rates: 4. Combining and substituting in terms of the space-time ( ) If we know CM, CH, and CX, then CMe and CT can be calculated from the reaction stoichiometry. Consequently, we need only to solve the following three equations: 3개 연립방정식

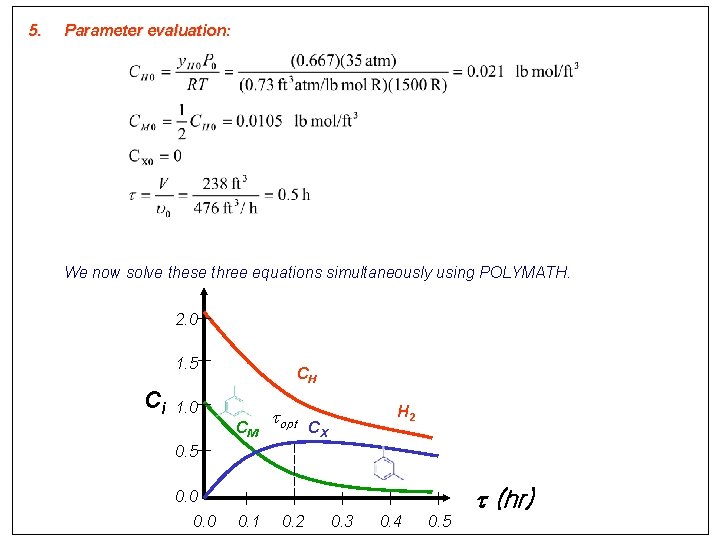
5. Parameter evaluation: We now solve these three equations simultaneously using POLYMATH. 2. 0 1. 5 Ci CH 1. 0 CM topt H 2 CX 0. 5 0. 0 0. 1 0. 2 0. 3 0. 4 0. 5 t (hr)

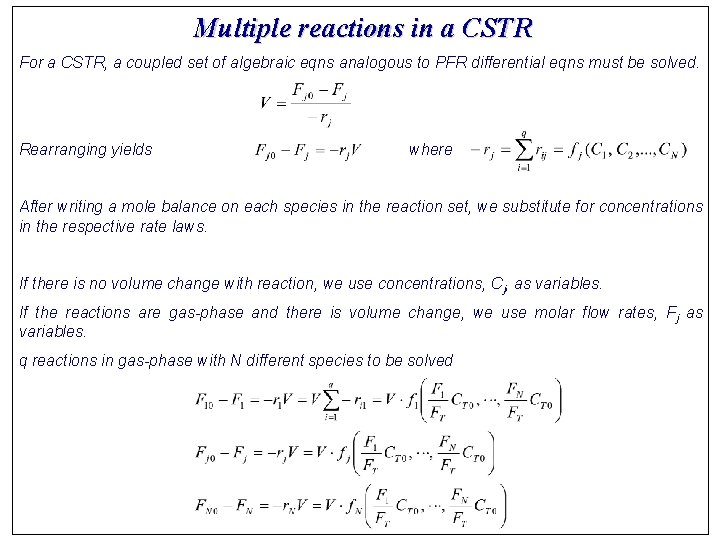
Multiple reactions in a CSTR For a CSTR, a coupled set of algebraic eqns analogous to PFR differential eqns must be solved. Rearranging yields where After writing a mole balance on each species in the reaction set, we substitute for concentrations in the respective rate laws. If there is no volume change with reaction, we use concentrations, Cj, as variables. If the reactions are gas-phase and there is volume change, we use molar flow rates, Fj as variables. q reactions in gas-phase with N different species to be solved

Hydrodealkylation of Mesitylene in a CSTR Calculate the conversion of hydrogen and mesitylene along with the exiting concentrations of mesitylene, hydrogen, and xylene in a CSTR. 1. Mole Balances: 3. Stoichiometry: 2. Rate laws: 4. Combining and letting yields:

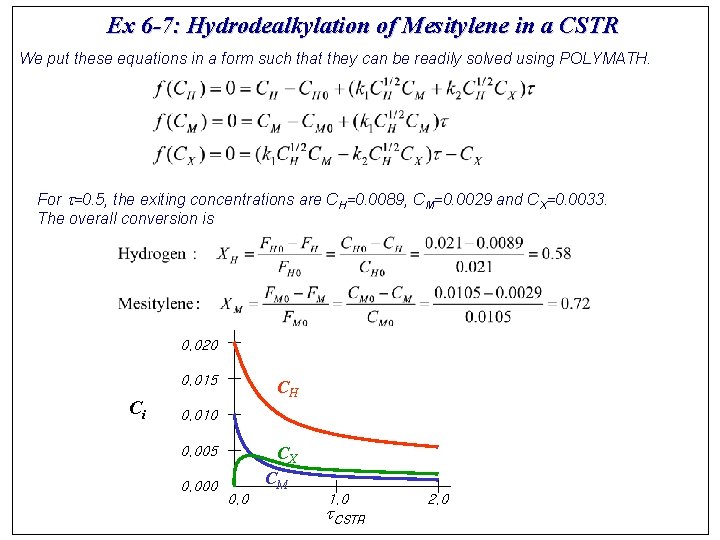
Ex 6 -7: Hydrodealkylation of Mesitylene in a CSTR We put these equations in a form such that they can be readily solved using POLYMATH. For t=0. 5, the exiting concentrations are CH=0. 0089, CM=0. 0029 and CX=0. 0033. The overall conversion is 0. 020 0. 015 Ci CH 0. 010 CX 0. 005 0. 000 CM 0. 0 1. 0 t. CSTR 2. 0

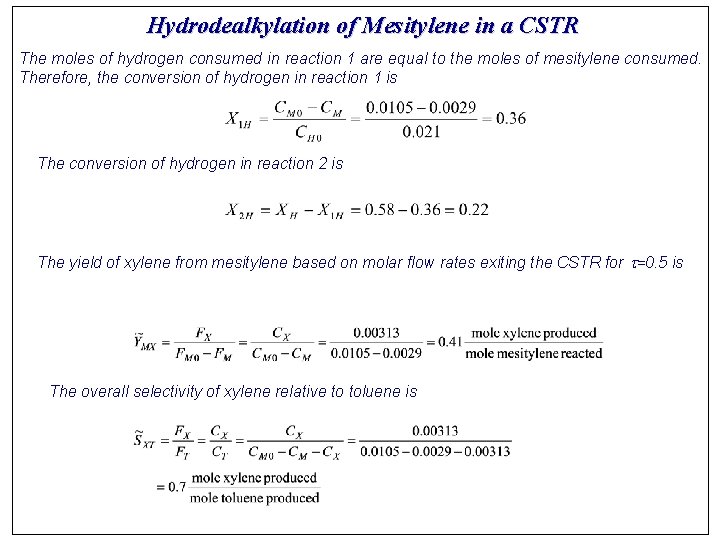
Hydrodealkylation of Mesitylene in a CSTR The moles of hydrogen consumed in reaction 1 are equal to the moles of mesitylene consumed. Therefore, the conversion of hydrogen in reaction 1 is The conversion of hydrogen in reaction 2 is The yield of xylene from mesitylene based on molar flow rates exiting the CSTR for t=0. 5 is The overall selectivity of xylene relative to toluene is

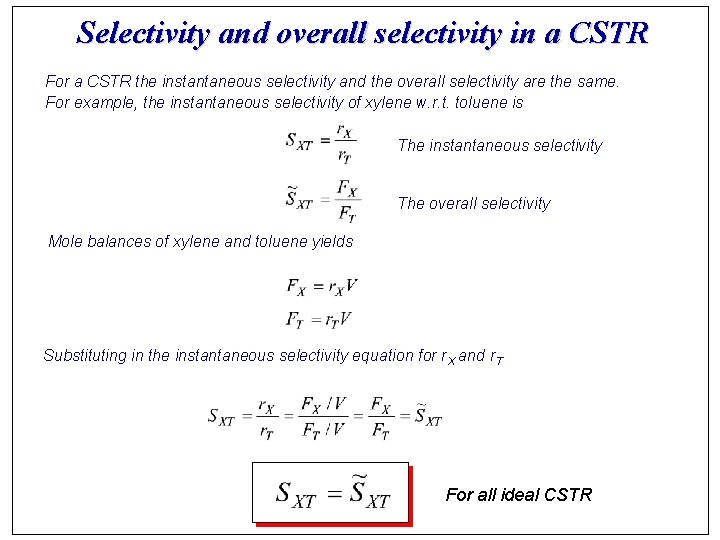
Selectivity and overall selectivity in a CSTR For a CSTR the instantaneous selectivity and the overall selectivity are the same. For example, the instantaneous selectivity of xylene w. r. t. toluene is The instantaneous selectivity The overall selectivity Mole balances of xylene and toluene yields Substituting in the instantaneous selectivity equation for r. X and r. T For all ideal CSTR

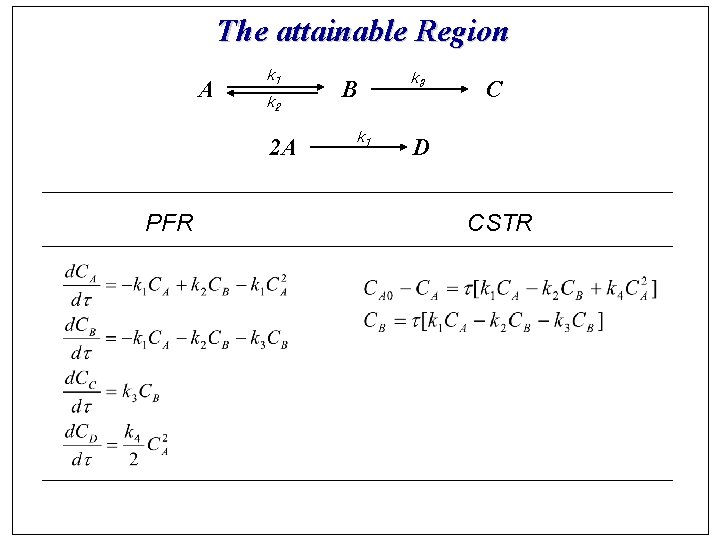
The attainable Region A k 1 k 2 2 A PFR B k 1 k 3 C D CSTR

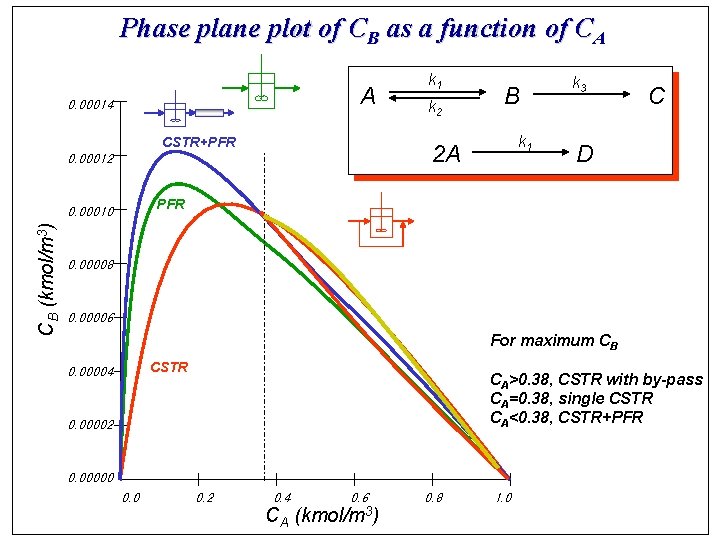
Phase plane plot of CB as a function of CA A 0. 00014 CSTR+PFR k 2 B k 1 2 A 0. 00012 k 3 C D PFR 0. 00010 CB (kmol/m 3) k 1 0. 00008 0. 00006 For maximum CB CSTR 0. 00004 CA>0. 38, CSTR with by-pass CA=0. 38, single CSTR CA<0. 38, CSTR+PFR 0. 00002 0. 00000 0. 2 0. 4 CA 0. 6 (kmol/m 3) 0. 8 1. 0

8. 6 Membrane Reactors to Improve Selectivity in Multiple Reactions In addition to using membrane reactors to remove a reaction product in order to shift the equilibrium toward completion, we can use membrane reactors to increase selectivity in multiple reactions. This increase can be achieved by injecting one of the reactants along the length of the reactor. It is particularly effective in partial oxidation of hydrocarbons, chlorination, ethoxylation, hydrogenation, nitration, and sulfunation reactions to name a few.

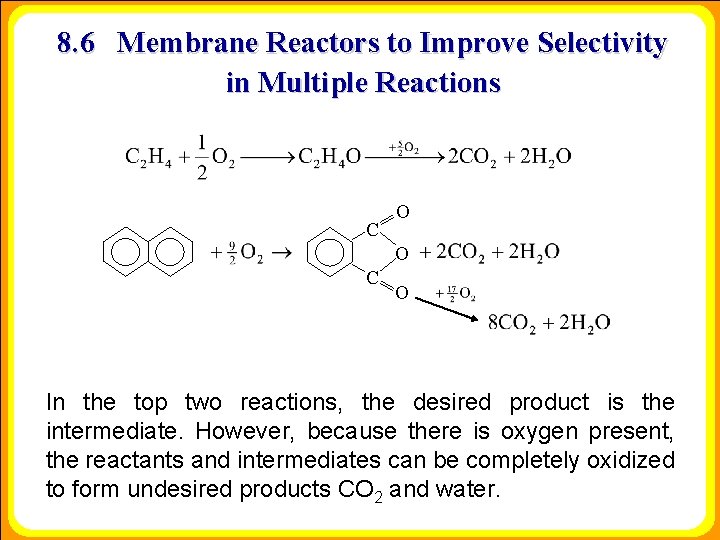
8. 6 Membrane Reactors to Improve Selectivity in Multiple Reactions C O O C O In the top two reactions, the desired product is the intermediate. However, because there is oxygen present, the reactants and intermediates can be completely oxidized to form undesired products CO 2 and water.

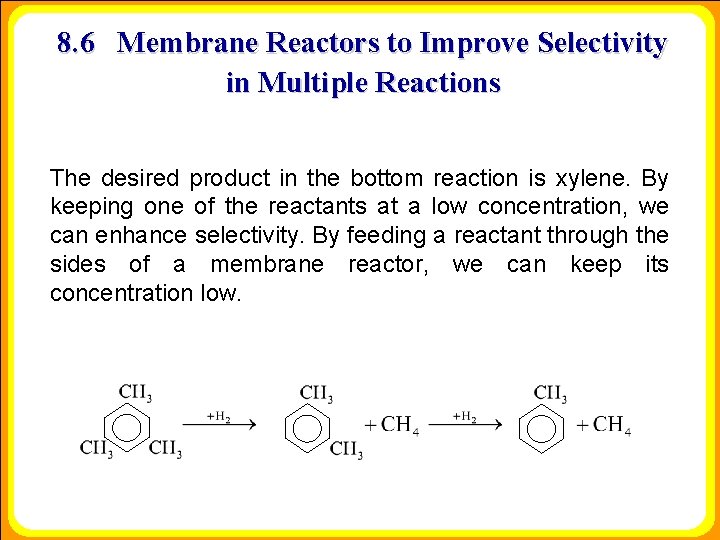
8. 6 Membrane Reactors to Improve Selectivity in Multiple Reactions The desired product in the bottom reaction is xylene. By keeping one of the reactants at a low concentration, we can enhance selectivity. By feeding a reactant through the sides of a membrane reactor, we can keep its concentration low.

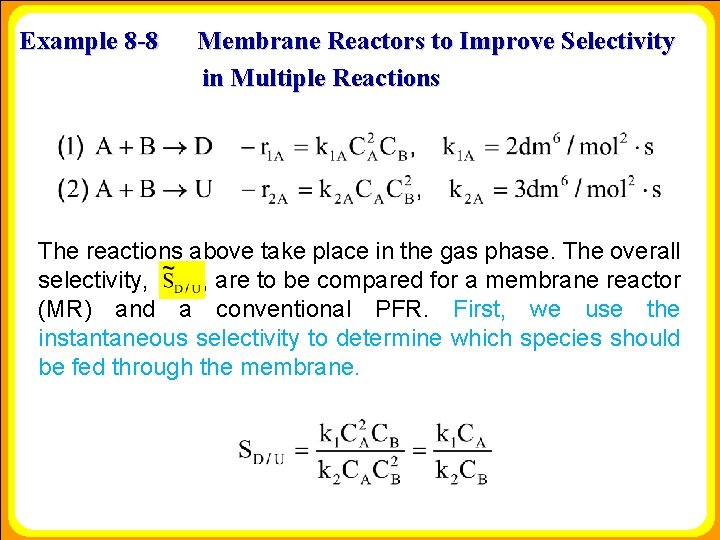
Example 8 -8 Membrane Reactors to Improve Selectivity in Multiple Reactions The reactions above take place in the gas phase. The overall selectivity, , are to be compared for a membrane reactor (MR) and a conventional PFR. First, we use the instantaneous selectivity to determine which species should be fed through the membrane.

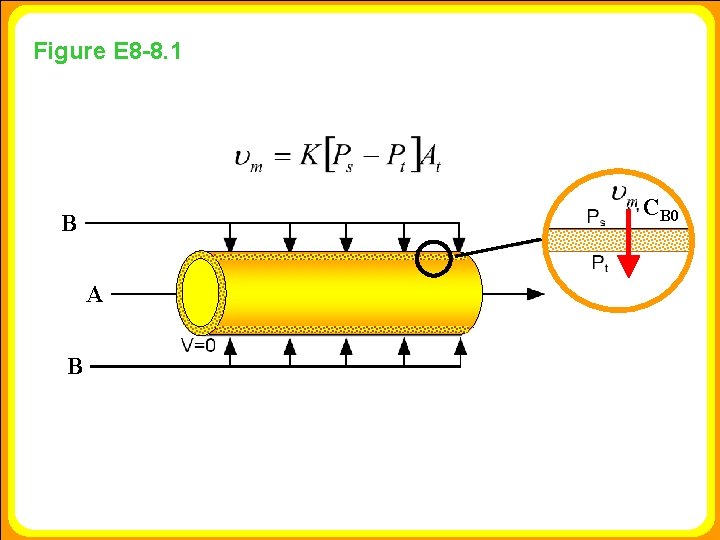
Example 8 -8 Membrane Reactors to Improve Selectivity in Multiple Reactions We see that to maximize SD/U we need to keep the concentration of A high and the concentration of B low; therefore, we feed B through the membrane. The molar flow rate of A entering the reactor is 4 mol/s and that of B entering through the membrane is 4 mol/s as shown in Figure E 8. 8. 1. For the PFR, B enters along with A. The reactor volume is 50 dm 3 and the entering total concentration is 0. 8 mol/dm 3. Plot the molar flow rates and overall selectivity, function of reactor volume for both the MR and PFR. , as a

Figure E 8 -8. 1 CB 0 B A B

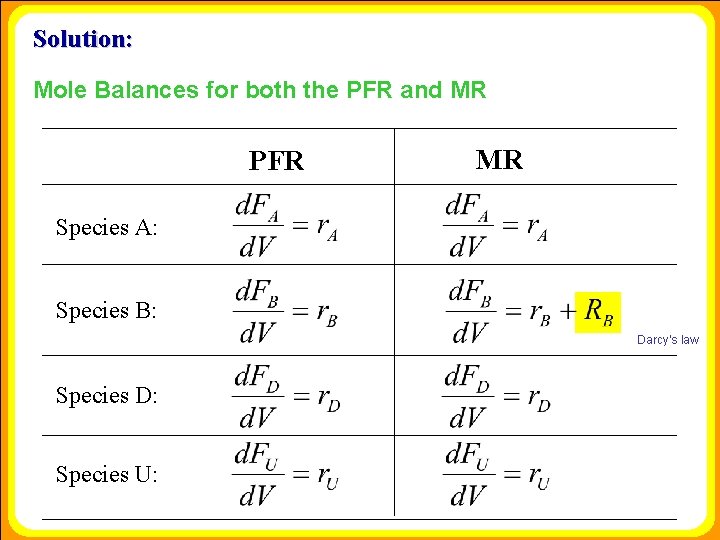
Solution: Mole Balances for both the PFR and MR PFR MR Species A: Species B: Darcy’s law Species D: Species U:

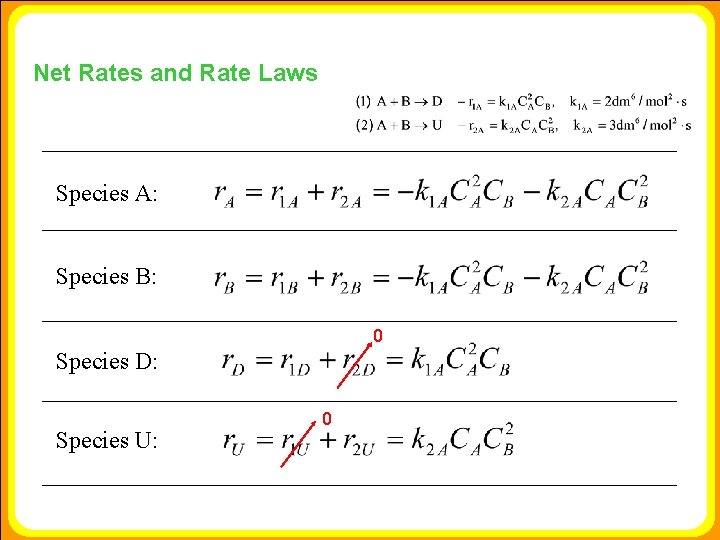
Net Rates and Rate Laws Species A: Species B: 0 Species D: Species U: 0

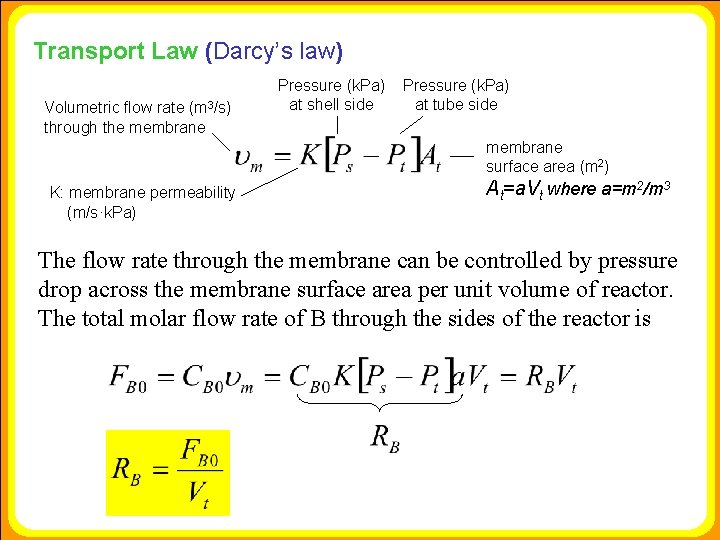
Transport Law (Darcy’s law) Volumetric flow rate (m 3/s) through the membrane Pressure (k. Pa) at shell side Pressure (k. Pa) at tube side membrane surface area (m 2) K: membrane permeability (m/s·k. Pa) At=a. Vt where a=m 2/m 3 The flow rate through the membrane can be controlled by pressure drop across the membrane surface area per unit volume of reactor. The total molar flow rate of B through the sides of the reactor is

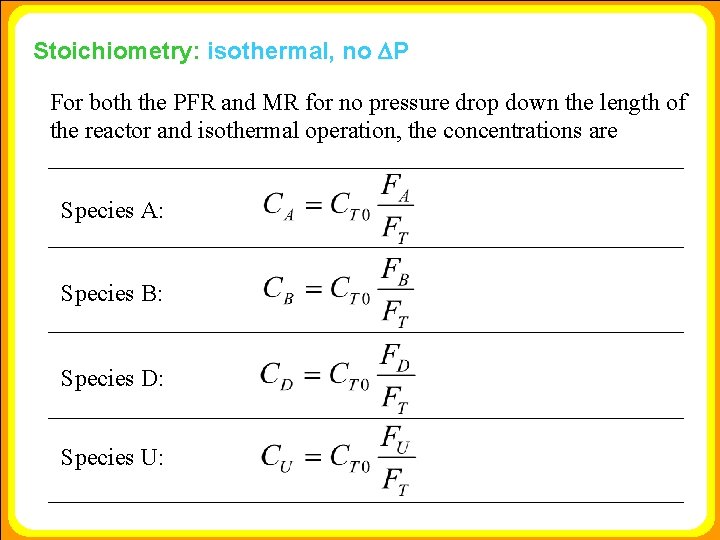
Stoichiometry: isothermal, no DP For both the PFR and MR for no pressure drop down the length of the reactor and isothermal operation, the concentrations are Species A: Species B: Species D: Species U:

Combine The Polymath Program will combine the mole balance, net rates, and stoichiometric equations to solve for the molar flow rate and selectivity profiles for both the conventional PFR and the MR and also the selectivity profile. A note of caution on calculating the overall selectivity. We have to fool Polymath because at the entrance of the reactor FU=0. Polymath will look at and will not run because it will say you are dividing by zero. Therefore, we need to add a very small number to the denominator, say 0. 0001; that is

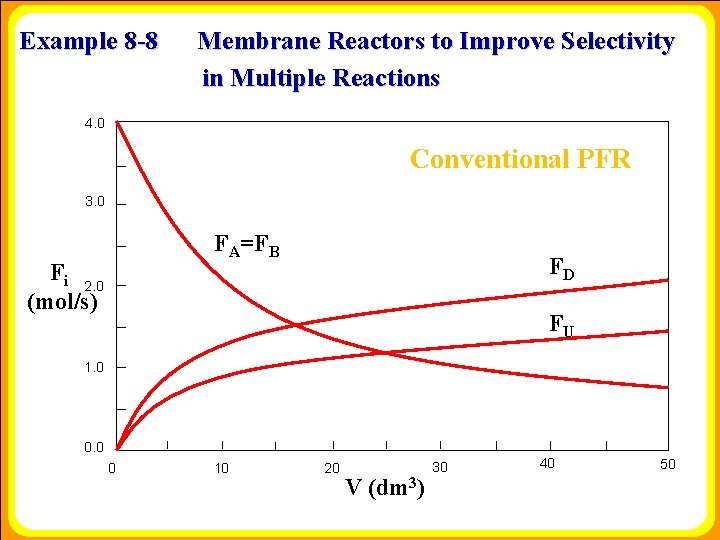
Example 8 -8 Membrane Reactors to Improve Selectivity in Multiple Reactions 4. 0 Conventional PFR 3. 0 FA=FB Fi 2. 0 (mol/s) FD FU 1. 0 0 10 20 V (dm 3) 30 40 50

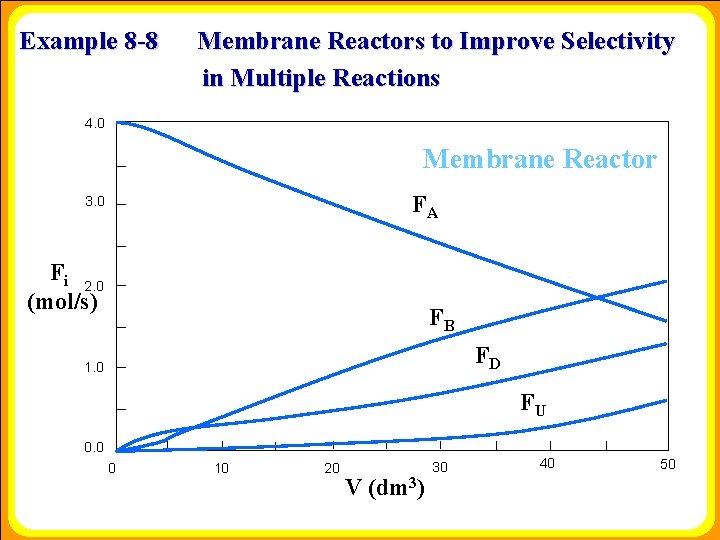
Example 8 -8 Membrane Reactors to Improve Selectivity in Multiple Reactions 4. 0 Membrane Reactor FA 3. 0 Fi 2. 0 (mol/s) FB FD 1. 0 FU 0. 0 0 10 20 V (dm 3) 30 40 50

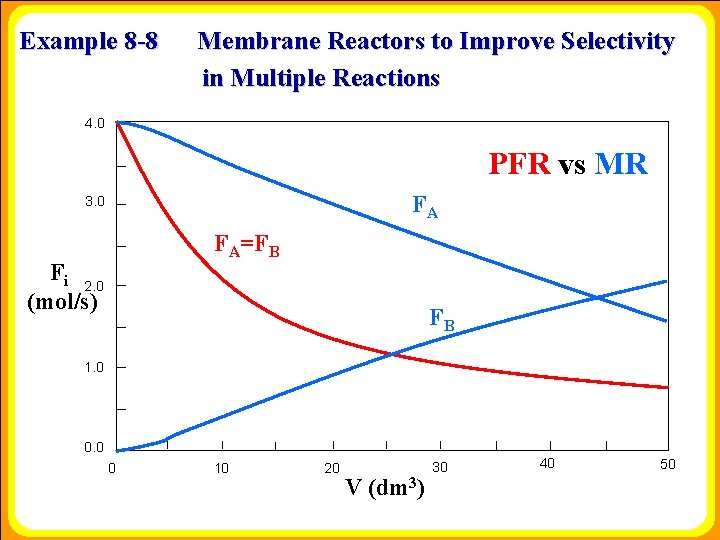
Example 8 -8 Membrane Reactors to Improve Selectivity in Multiple Reactions 4. 0 PFR vs MR FA 3. 0 FA=FB Fi 2. 0 (mol/s) FB 1. 0 0 10 20 V (dm 3) 30 40 50

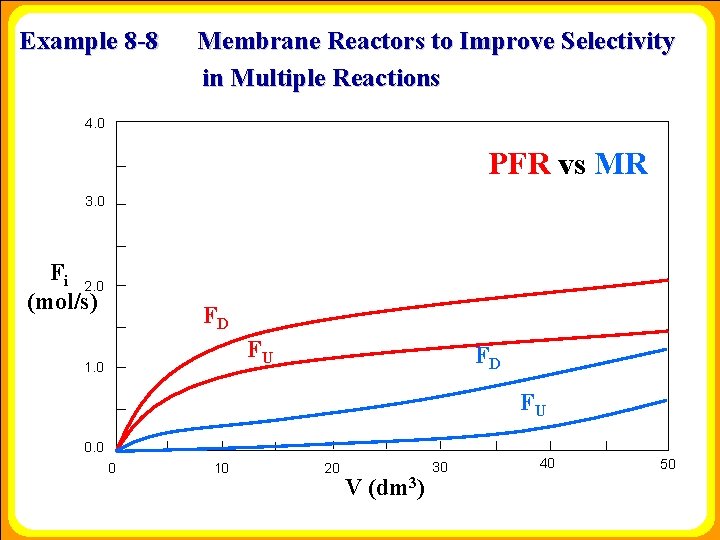
Example 8 -8 Membrane Reactors to Improve Selectivity in Multiple Reactions 4. 0 PFR vs MR 3. 0 Fi 2. 0 (mol/s) FD FU 1. 0 FD FU 0. 0 0 10 20 V (dm 3) 30 40 50

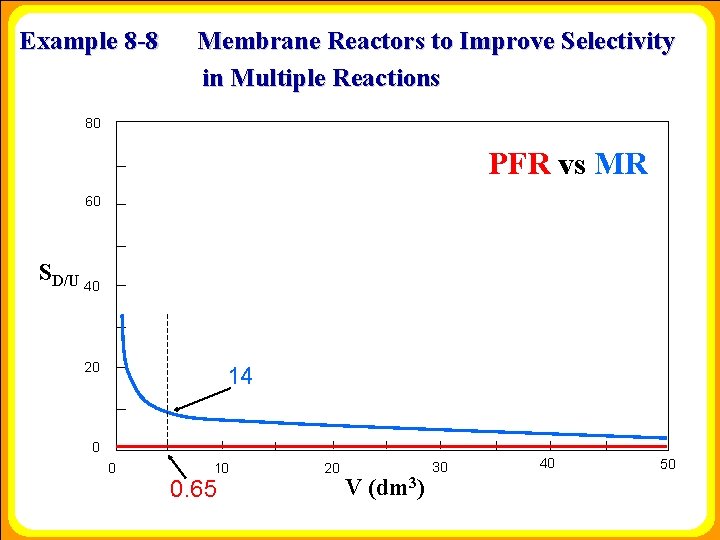
Example 8 -8 Membrane Reactors to Improve Selectivity in Multiple Reactions 80 PFR vs MR 60 SD/U 40 20 14 0 0 10 0. 65 20 V (dm 3) 30 40 50
 Reactor design pattern
Reactor design pattern Rbmk reactor design flaws
Rbmk reactor design flaws Non isothermal reactor design problems
Non isothermal reactor design problems Reactor space time
Reactor space time Reactor design project
Reactor design project Isothermal
Isothermal Reactor design pattern
Reactor design pattern L
L Reactor sizing
Reactor sizing Safurex chemical composition
Safurex chemical composition Reactor pattern java
Reactor pattern java Event
Event Fission reactor nuclearcraft
Fission reactor nuclearcraft Artur korneyev
Artur korneyev Magical reactor 3
Magical reactor 3 Reactor working principle
Reactor working principle Boiling water reactor
Boiling water reactor Nuclear reactor anatomy
Nuclear reactor anatomy Reactor pattern
Reactor pattern Tunem
Tunem Como calcular la inductancia
Como calcular la inductancia Boiling water reactor
Boiling water reactor Psychoda alternata
Psychoda alternata Sequencing batch reactor
Sequencing batch reactor Plug flow reactor animation
Plug flow reactor animation Phomenon
Phomenon Mit reactor tour
Mit reactor tour Ipwr reactor
Ipwr reactor Language reactor
Language reactor D.t.r
D.t.r Candu reactor construction
Candu reactor construction Language reactor
Language reactor Reactor heat exchanger
Reactor heat exchanger Language reactor
Language reactor Gapped core shunt reactor
Gapped core shunt reactor Tritium breeding blanket
Tritium breeding blanket Shunt compensation
Shunt compensation Tmsr
Tmsr Shunt reactors for power factor correction
Shunt reactors for power factor correction Pressurized water reactor
Pressurized water reactor Pressurized water reactor
Pressurized water reactor Nucular reactor
Nucular reactor Osirak nuclear reactor
Osirak nuclear reactor Multi tubular reactor
Multi tubular reactor Phwr reactor in india
Phwr reactor in india Reactor for eps material
Reactor for eps material High temperature gas cooled reactor
High temperature gas cooled reactor Molar balance
Molar balance Reactor de columna de burbujeo
Reactor de columna de burbujeo Flow rate concentration equation
Flow rate concentration equation Reactor sinks
Reactor sinks Hazop analysis for separator
Hazop analysis for separator Two film theory
Two film theory What is a nuclear reactor
What is a nuclear reactor Sequencing batch reactor advantages and disadvantages
Sequencing batch reactor advantages and disadvantages Inin reactor nuclear
Inin reactor nuclear Neutralization reactor
Neutralization reactor West lee middle school
West lee middle school Lee shau kee primary school
Lee shau kee primary school Lbms attendance line
Lbms attendance line Jaggers nutrition menu
Jaggers nutrition menu Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Formula of love
Formula of love Are kc and kp equal
Are kc and kp equal School architecture change chemical
School architecture change chemical Hitman lee pokemon
Hitman lee pokemon Mary lee timeline
Mary lee timeline Merdeka talks
Merdeka talks Lee vor luv beispiele
Lee vor luv beispiele Onel de guzman
Onel de guzman Roy kenshin lee
Roy kenshin lee Summary to kill a mockingbird
Summary to kill a mockingbird Harper lee timeline
Harper lee timeline What is the genre of the novel to kill a mockingbird
What is the genre of the novel to kill a mockingbird Harper lee biography to kill a mockingbird
Harper lee biography to kill a mockingbird What is symbolic about the streetlamp that starks bought
What is symbolic about the streetlamp that starks bought Lee diamond sherwin williams
Lee diamond sherwin williams Lsvt loud video
Lsvt loud video Kendall lee notation
Kendall lee notation Lee su ann
Lee su ann Assertive discipline theory
Assertive discipline theory Silvia lee a su abuelo
Silvia lee a su abuelo Kevin lee yates
Kevin lee yates Quality lee
Quality lee Ace lip puff that hung
Ace lip puff that hung Algal classification by lee
Algal classification by lee Branly
Branly Relative lee
Relative lee Yoonsang lee
Yoonsang lee Lee wee sun
Lee wee sun Jeremy lee australia
Jeremy lee australia 2020 y tres enteros con cuatro décimos
2020 y tres enteros con cuatro décimos Tai sing lee
Tai sing lee