Chemical Reactions Mr Kinton Honors Chemistry Enloe High




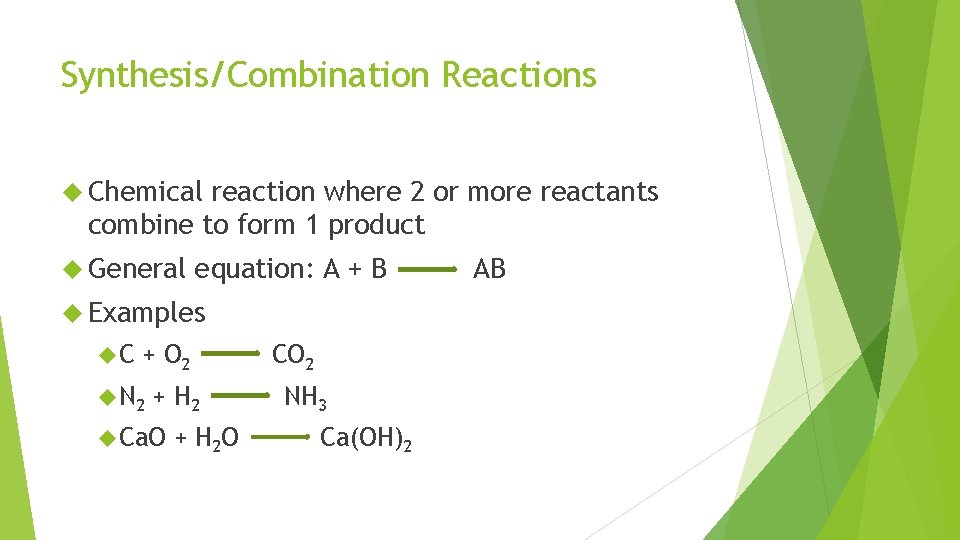




- Slides: 33

Chemical Reactions Mr. Kinton Honors Chemistry Enloe High School

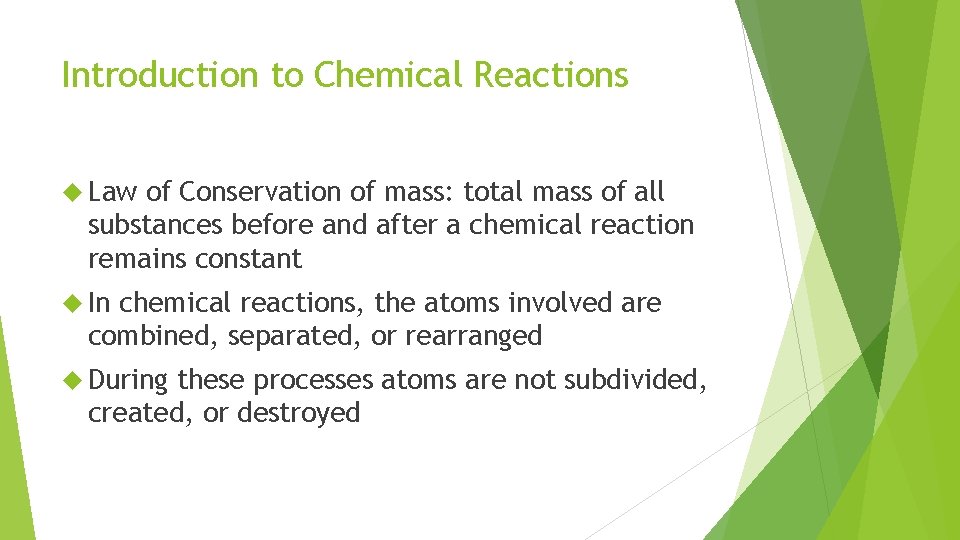
Introduction to Chemical Reactions Law of Conservation of mass: total mass of all substances before and after a chemical reaction remains constant In chemical reactions, the atoms involved are combined, separated, or rearranged During these processes atoms are not subdivided, created, or destroyed

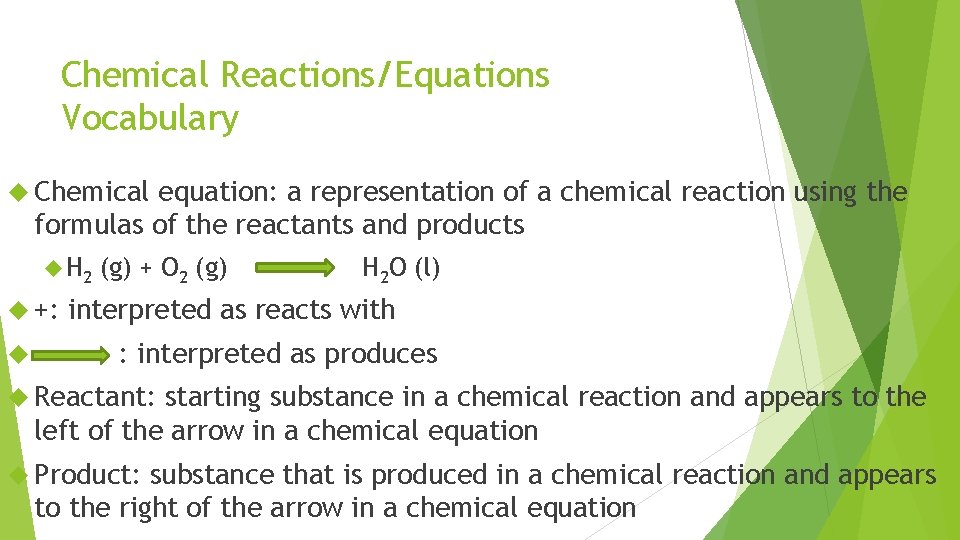
Chemical Reactions/Equations Vocabulary Chemical equation: a representation of a chemical reaction using the formulas of the reactants and products H 2 +: (g) + O 2 (g) H 2 O (l) interpreted as reacts with : interpreted as produces Reactant: starting substance in a chemical reaction and appears to the left of the arrow in a chemical equation Product: substance that is produced in a chemical reaction and appears to the right of the arrow in a chemical equation

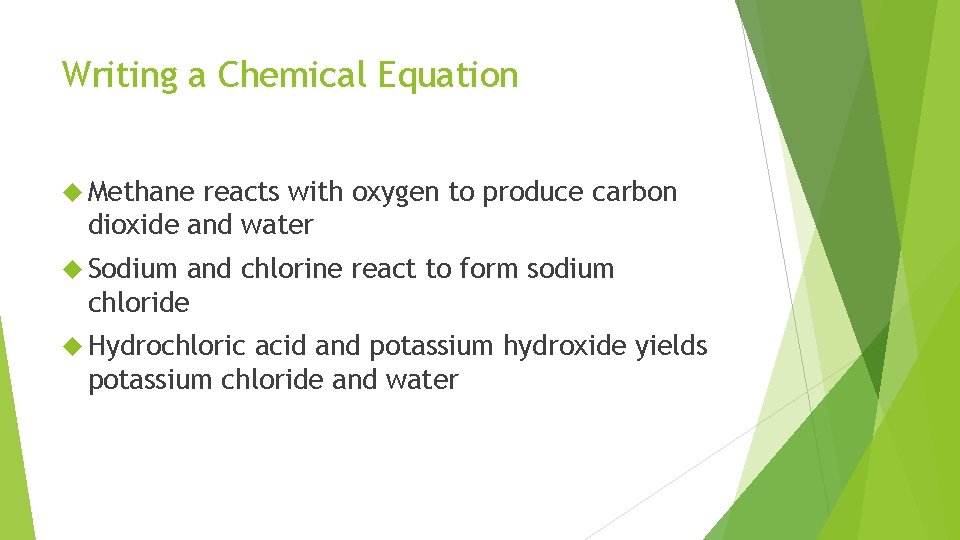
Writing a Chemical Equation Methane reacts with oxygen to produce carbon dioxide and water Sodium and chlorine react to form sodium chloride Hydrochloric acid and potassium hydroxide yields potassium chloride and water

Chemical Reactions/Equations Vocabulary Balanced: used to describe an equation when the total number of atoms on each side are equal Coefficients: numbers that are placed in front chemical formulas in order to balance an equation Skeleton equation: another term used to describe an unbalanced chemical equation

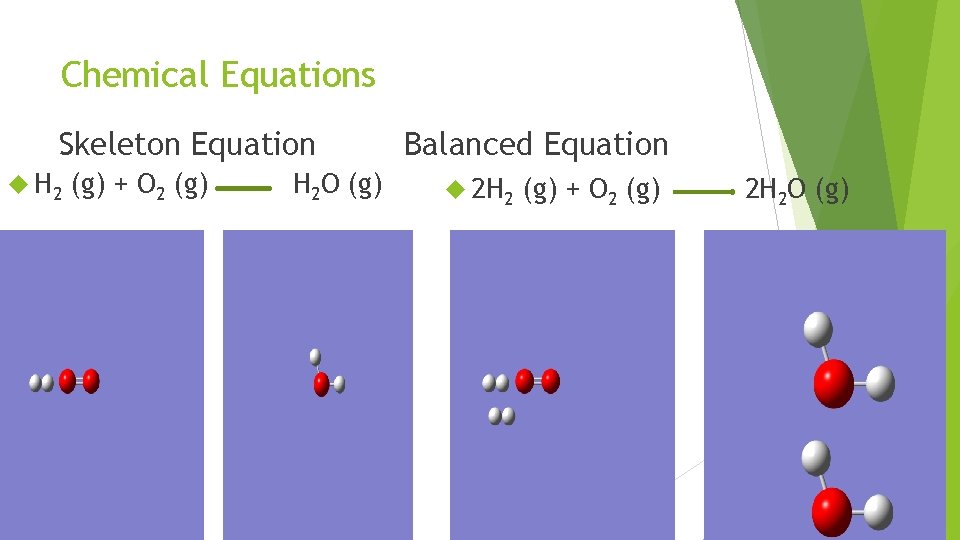
Chemical Equations Skeleton Equation H 2 (g) + O 2 (g) H 2 O (g) Balanced Equation 2 H 2 (g) + O 2 (g) 2 H 2 O (g)

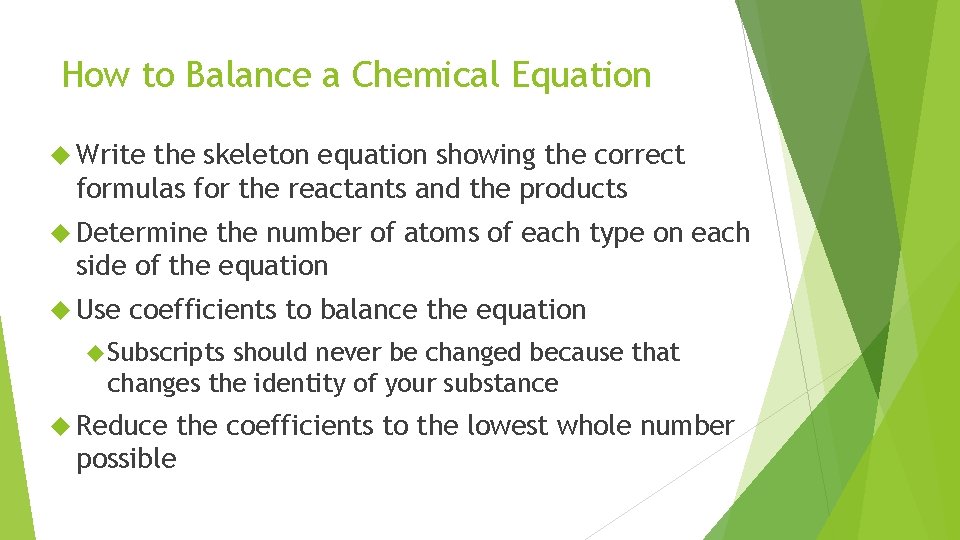
How to Balance a Chemical Equation Write the skeleton equation showing the correct formulas for the reactants and the products Determine the number of atoms of each type on each side of the equation Use coefficients to balance the equation Subscripts should never be changed because that changes the identity of your substance Reduce the coefficients to the lowest whole number possible

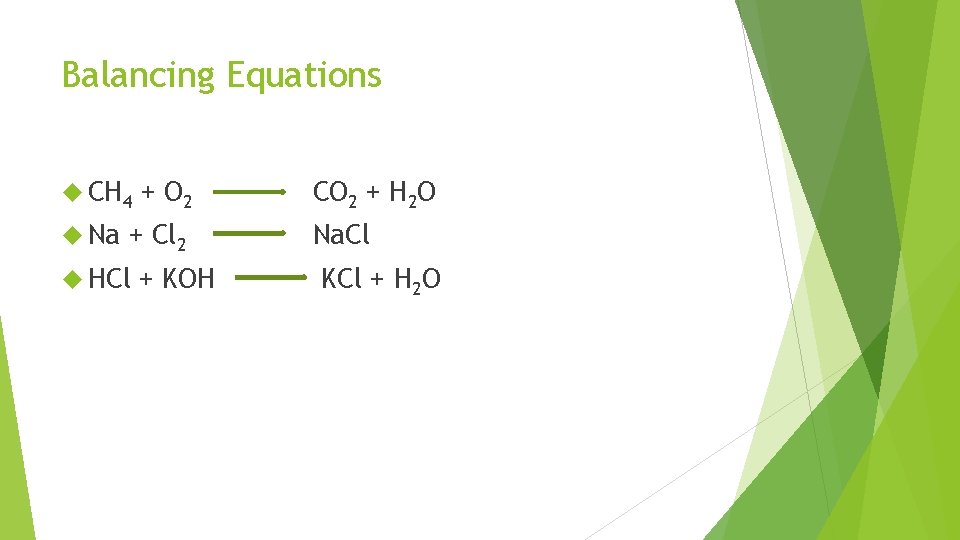
Balancing Equations CH 4 Na + O 2 + Cl 2 HCl + KOH CO 2 + H 2 O Na. Cl KCl + H 2 O

Physical States and Chemical Reactions We can also include the physical state of our element or compound in our equation Liquids (l): only 2 elements are liquids at room temperature; Hg and Br Gases (g): H, N, O, F, Cl, He, Ne, Ar, Kr, Xe, and Rn are the only elements that exist in the gas phase at room temperature Solids (s): Every other element is a solid at room temperature

Physical States and Chemical Reactions Ionic Compounds exist as solids at room temperature Covalent compounds with a molecular weight less than 100 amu will be gases Aqueous (aq): substance that has been dissolved in water (ionic compounds, acids, bases) As we encounter other compounds of interest we will discuss their physical states

Other Information Given in Chemical Equations Additionally, reactions that require energy, light, or electricity can also be indicated in a chemical equation Heat: Δ Electricity: Light: These hν things can be found above the arrow

Indicators of a Chemical Reaction During a chemical reactions a chemical change is taking place That means that the process is not easily reversible When a chemical reaction occurs one or more of the following could be observed Formation of a gas Formation of a precipitate Liberation of heat or light Color change

How to Identify a Gas In the chemical reactions we will examine the most common gases produced are H 2, O 2, and CO 2 The following tests can be used to identify the following gases: H 2 - take a burning splint and listen for a “pop” O 2 - take a glowing splint and observe it get brighter CO 2 - take a burning splint and observe the flame be extinguished

Types of Chemical Reactions For our class all chemical reactions will fall into one of 5 categories: Synthesis or Combination Decomposition Combustion Single-replacement/displacement Double-replacement/displacement Acid-base neutralization
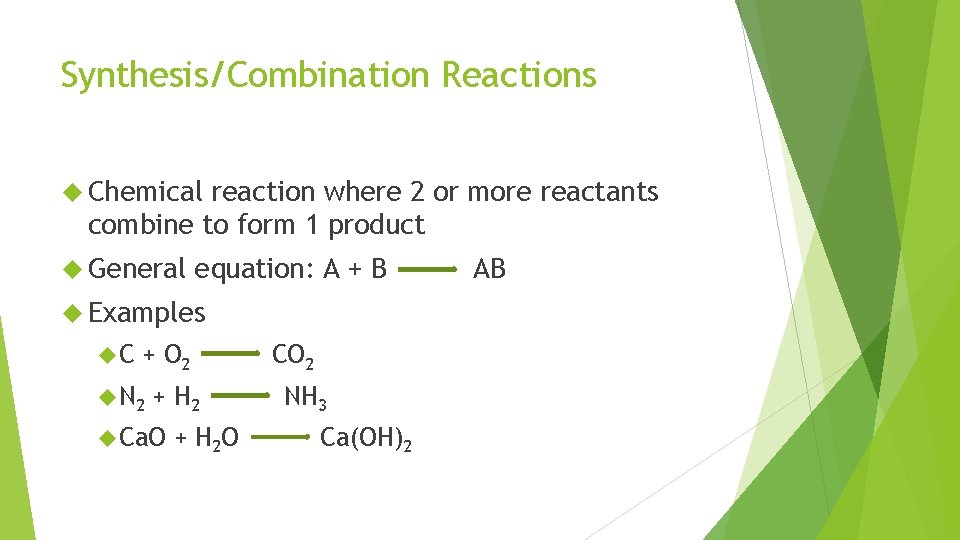
Synthesis/Combination Reactions Chemical reaction where 2 or more reactants combine to form 1 product General equation: A + B Examples C + O 2 N 2 + H 2 Ca. O + H 2 O CO 2 NH 3 Ca(OH)2 AB

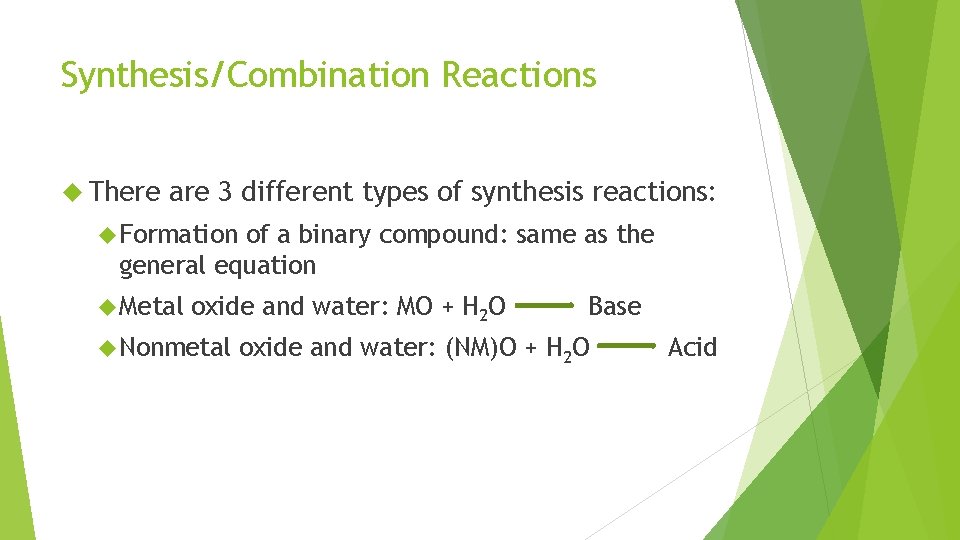
Synthesis/Combination Reactions There are 3 different types of synthesis reactions: Formation of a binary compound: same as the general equation Metal oxide and water: MO + H 2 O Nonmetal Base oxide and water: (NM)O + H 2 O Acid

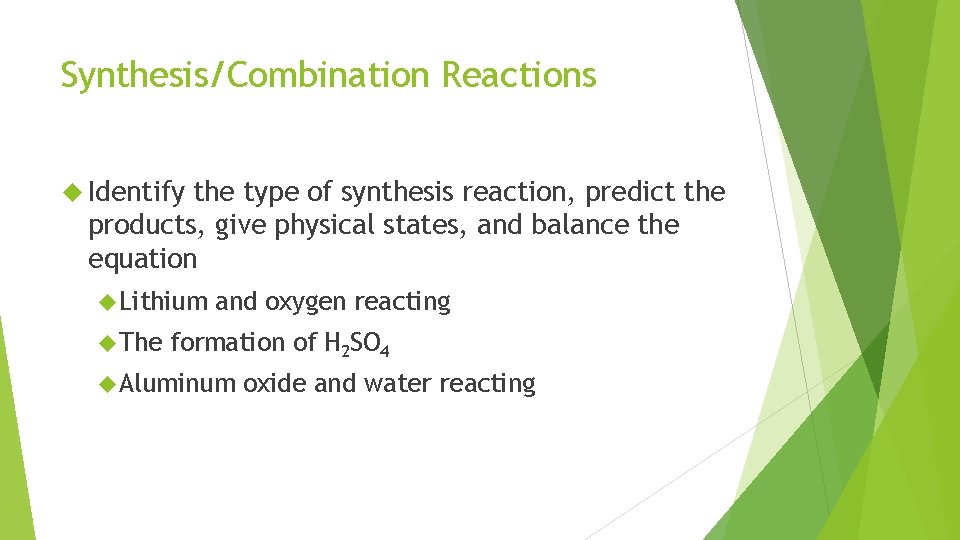
Synthesis/Combination Reactions Identify the type of synthesis reaction, predict the products, give physical states, and balance the equation Lithium The and oxygen reacting formation of H 2 SO 4 Aluminum oxide and water reacting

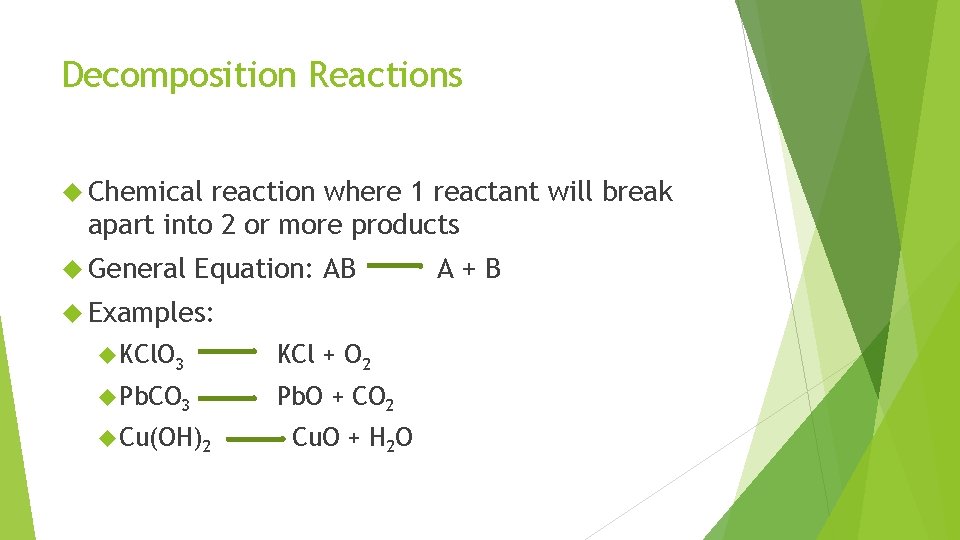
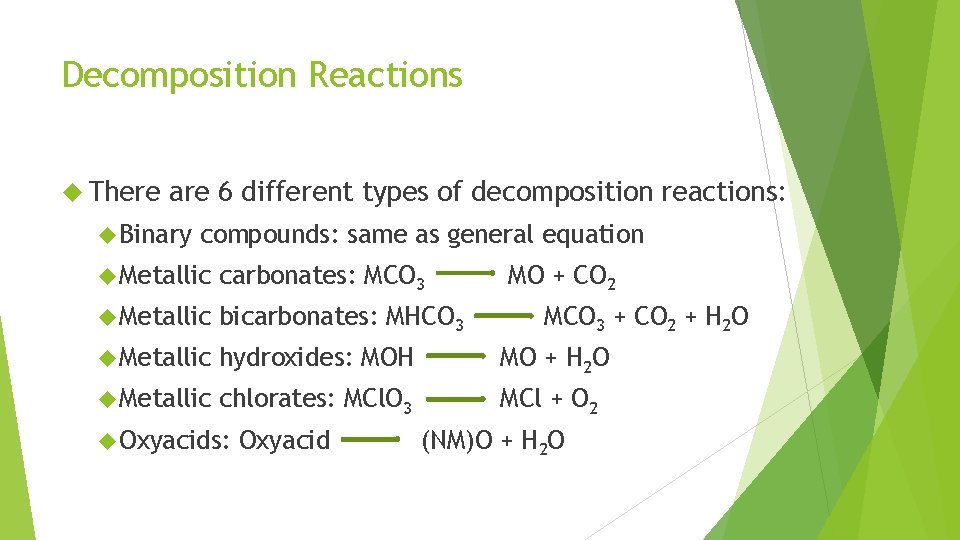
Decomposition Reactions Chemical reaction where 1 reactant will break apart into 2 or more products General Equation: AB Examples: KCl. O 3 KCl + O 2 Pb. CO 3 Pb. O + CO 2 Cu(OH)2 Cu. O + H 2 O A+B

Decomposition Reactions There are 6 different types of decomposition reactions: Binary compounds: same as general equation Metallic carbonates: MCO 3 Metallic bicarbonates: MHCO 3 Metallic hydroxides: MOH MO + H 2 O Metallic chlorates: MCl. O 3 MCl + O 2 Oxyacids: Oxyacid MO + CO 2 MCO 3 + CO 2 + H 2 O (NM)O + H 2 O

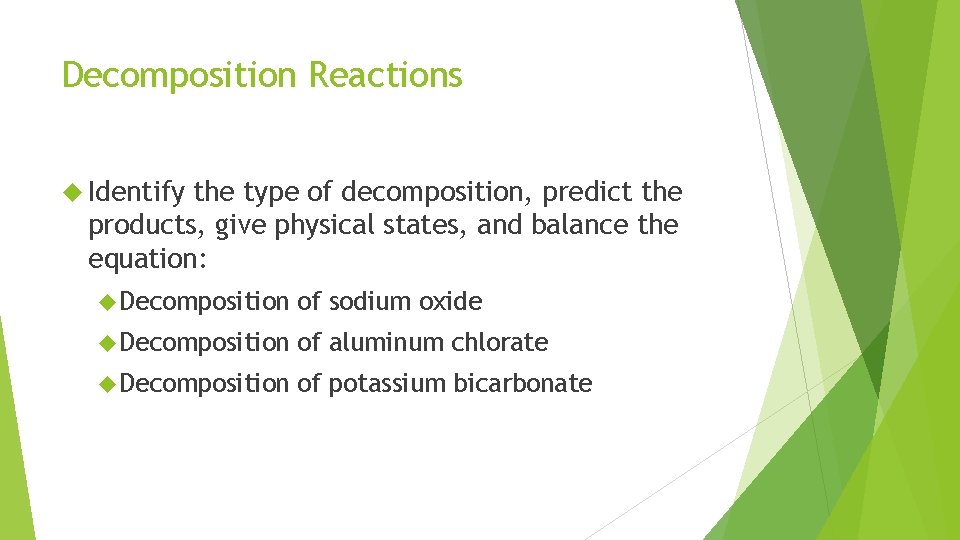
Decomposition Reactions Identify the type of decomposition, predict the products, give physical states, and balance the equation: Decomposition of sodium oxide Decomposition of aluminum chlorate Decomposition of potassium bicarbonate

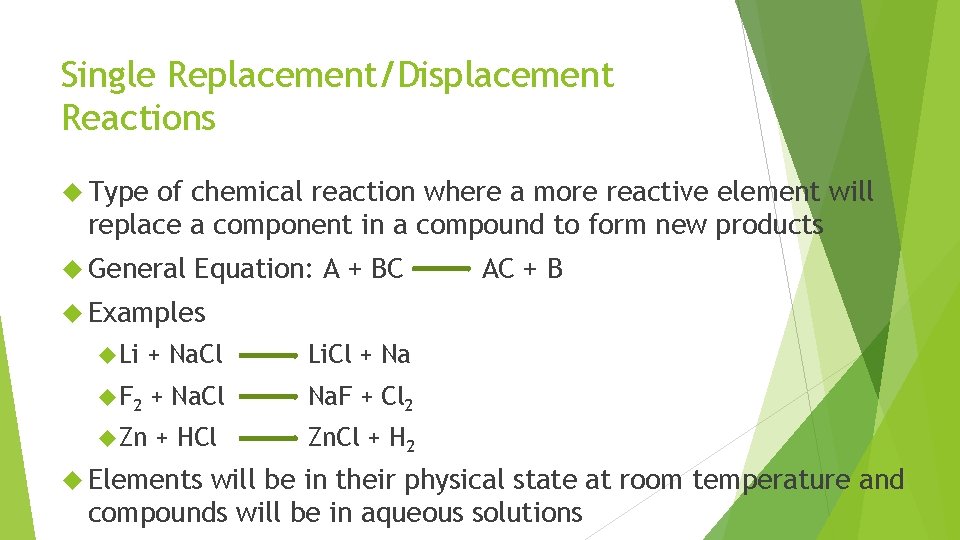
Single Replacement/Displacement Reactions Type of chemical reaction where a more reactive element will replace a component in a compound to form new products General Equation: A + BC AC + B Examples Li + Na. Cl Li. Cl + Na F 2 + Na. Cl Na. F + Cl 2 Zn + HCl Zn. Cl + H 2 Elements will be in their physical state at room temperature and compounds will be in aqueous solutions

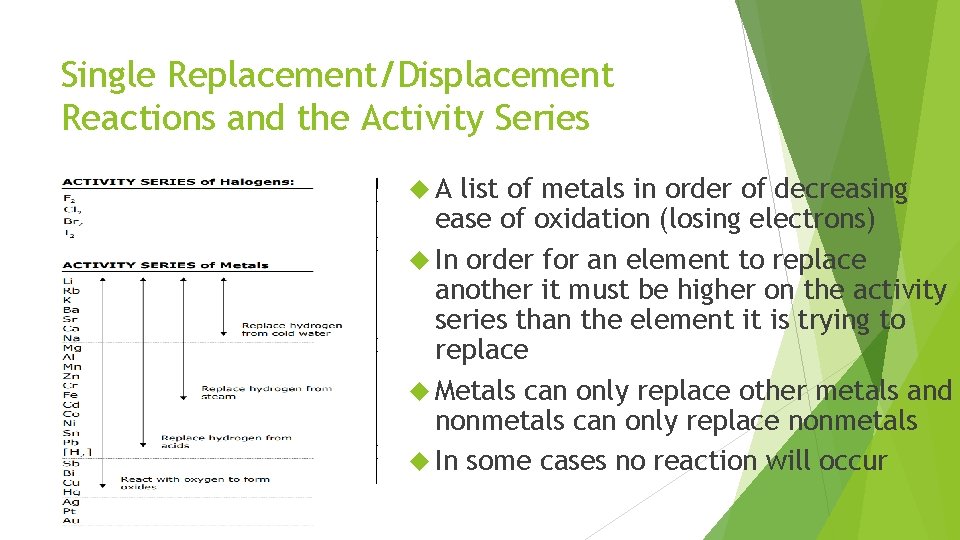
Single Replacement/Displacement Reactions and the Activity Series A list of metals in order of decreasing ease of oxidation (losing electrons) In order for an element to replace another it must be higher on the activity series than the element it is trying to replace Metals can only replace other metals and nonmetals can only replace nonmetals In some cases no reaction will occur

Activity Series Determine whether or not the following element can replace the other: K replacing H Mn replacing Ca Cu replacing Au I replacing Cl

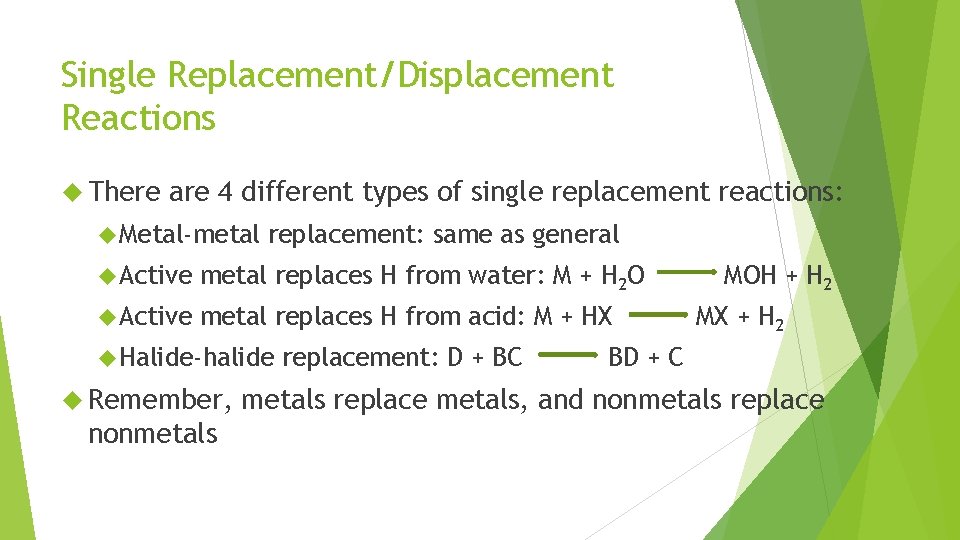
Single Replacement/Displacement Reactions There are 4 different types of single replacement reactions: Metal-metal replacement: same as general Active metal replaces H from water: M + H 2 O Active metal replaces H from acid: M + HX Halide-halide Remember, nonmetals replacement: D + BC MOH + H 2 MX + H 2 BD + C metals replace metals, and nonmetals replace

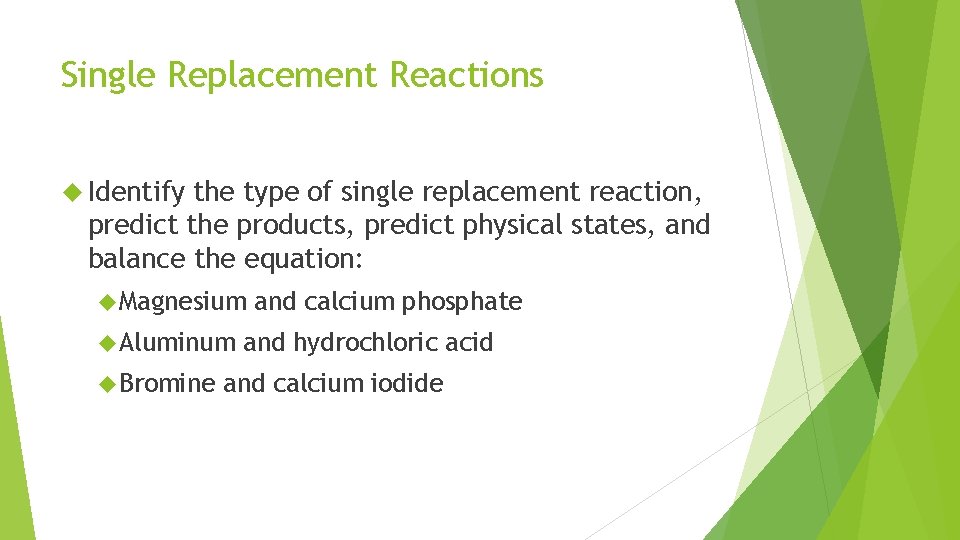
Single Replacement Reactions Identify the type of single replacement reaction, predict the products, predict physical states, and balance the equation: Magnesium Aluminum Bromine and calcium phosphate and hydrochloric acid and calcium iodide

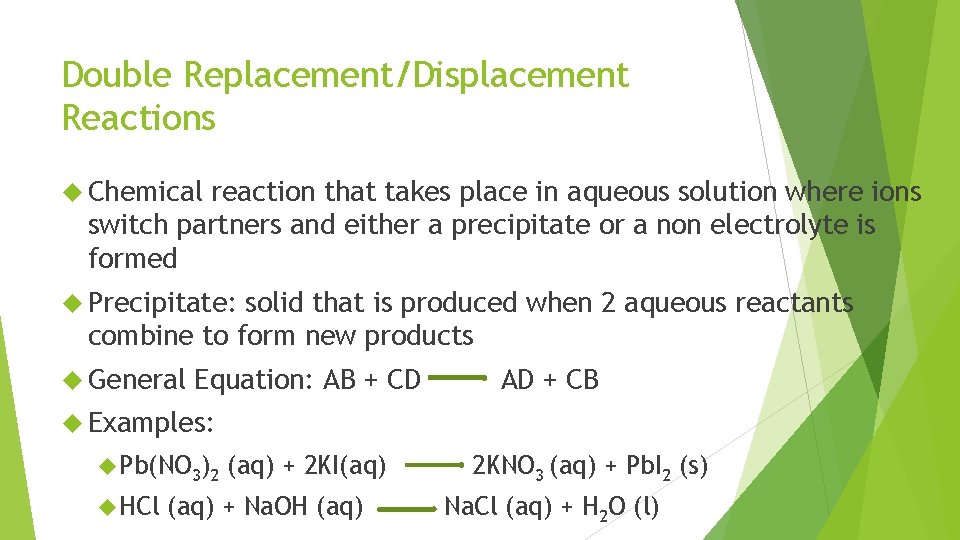
Double Replacement/Displacement Reactions Chemical reaction that takes place in aqueous solution where ions switch partners and either a precipitate or a non electrolyte is formed Precipitate: solid that is produced when 2 aqueous reactants combine to form new products General Equation: AB + CD AD + CB Examples: Pb(NO 3)2 HCl (aq) + 2 KI(aq) + Na. OH (aq) 2 KNO 3 (aq) + Pb. I 2 (s) Na. Cl (aq) + H 2 O (l)

Double Replacement/Displacement Reactions and Solubility Rules Not all substances are soluble in water When all of your reactants and products are aqueous, no reaction takes place Use these rules to help determine the physical states of double replacement reactions only

Solubility Determine whether the following compounds would be soluble in water: NH 4 NO 3 Ca. F 2 Li 2 SO 4 Ca. CO 3 Sr(OH)2

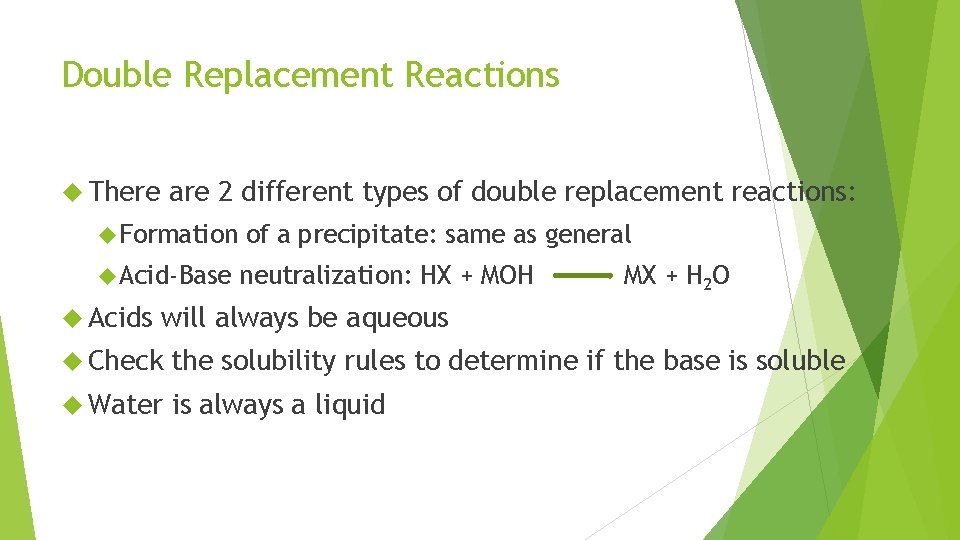
Double Replacement Reactions There are 2 different types of double replacement reactions: Formation of a precipitate: same as general Acid-Base neutralization: HX + MOH Acids MX + H 2 O will always be aqueous Check the solubility rules to determine if the base is soluble Water is always a liquid

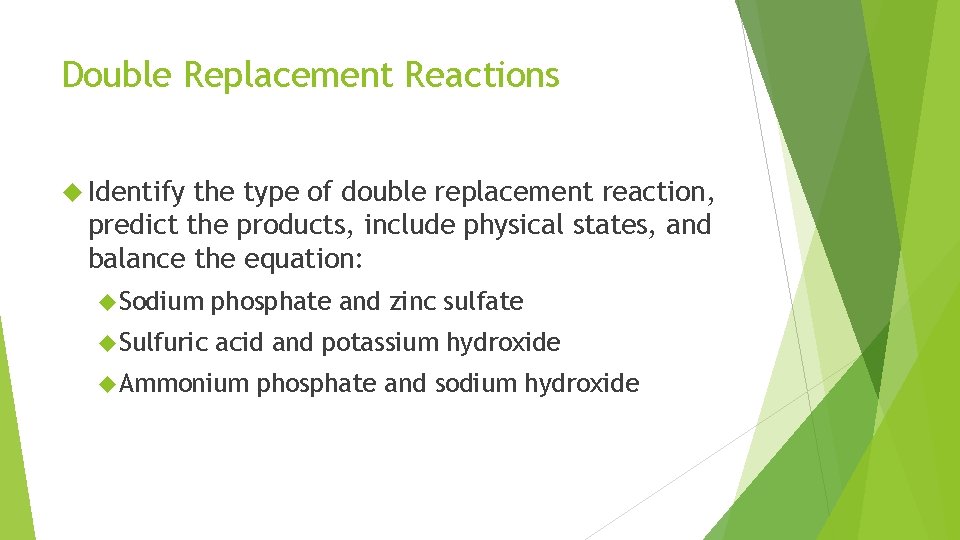
Double Replacement Reactions Identify the type of double replacement reaction, predict the products, include physical states, and balance the equation: Sodium phosphate and zinc sulfate Sulfuric acid and potassium hydroxide Ammonium phosphate and sodium hydroxide

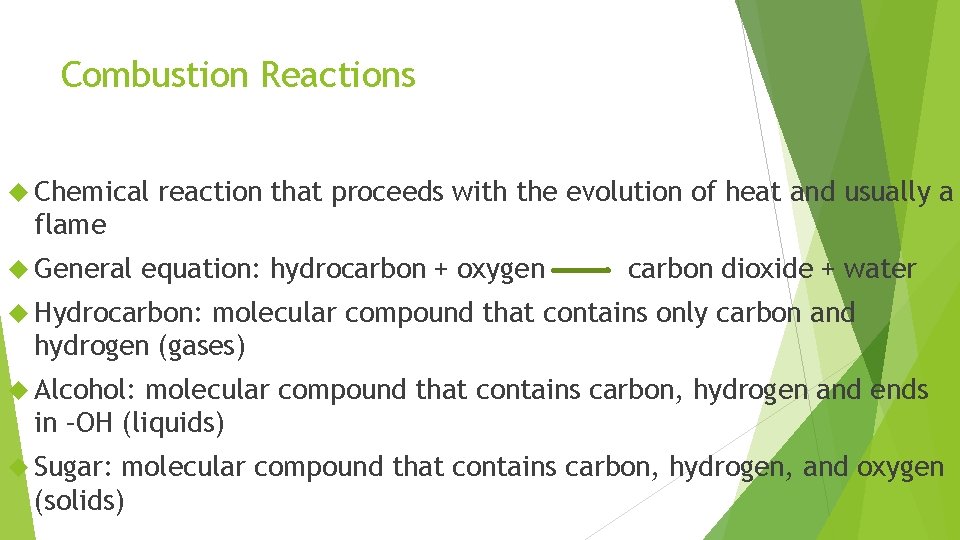
Combustion Reactions Chemical reaction that proceeds with the evolution of heat and usually a flame General equation: hydrocarbon + oxygen carbon dioxide + water Hydrocarbon: molecular compound that contains only carbon and hydrogen (gases) Alcohol: molecular compound that contains carbon, hydrogen and ends in –OH (liquids) Sugar: molecular compound that contains carbon, hydrogen, and oxygen (solids)

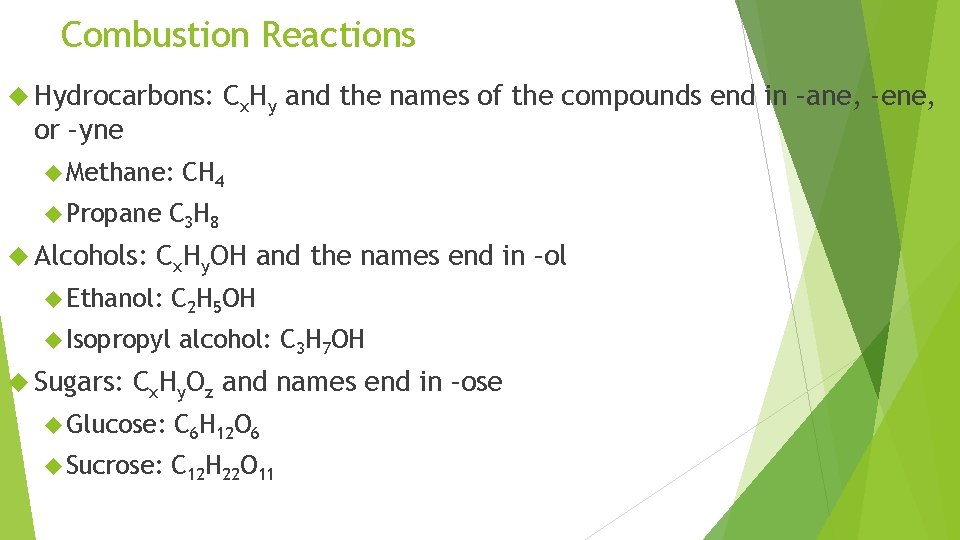
Combustion Reactions Hydrocarbons: or –yne Methane: Propane Alcohols: CH 4 C 3 H 8 Cx. Hy. OH and the names end in –ol Ethanol: Isopropyl Sugars: Cx. Hy and the names of the compounds end in –ane, -ene, C 2 H 5 OH alcohol: C 3 H 7 OH Cx. Hy. Oz and names end in –ose Glucose: C 6 H 12 O 6 Sucrose: C 12 H 22 O 11

Combustion Reactions Identify the type of hydrocarbon present, predict the products, include physical states, and balance the equation: C 2 H 6 C 6 H 5 OH C 6 H 12 O 6
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Enloe flight school
Enloe flight school Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Section 1 atoms elements and compounds
Section 1 atoms elements and compounds Chapter 6 section 1 atoms elements and compounds
Chapter 6 section 1 atoms elements and compounds Section 1 chemical changes
Section 1 chemical changes Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Kuei honors chemistry
Kuei honors chemistry Chemistry unit 4 review answers
Chemistry unit 4 review answers Honors chemistry summer assignment
Honors chemistry summer assignment Honors chemistry
Honors chemistry Deped order no. 36, s. 2016
Deped order no. 36, s. 2016 Enloe dam
Enloe dam Dr enloe arc
Dr enloe arc Enloe cardiology
Enloe cardiology How to write half reactions
How to write half reactions Types of reactions chemistry
Types of reactions chemistry 5 types of reactions in chemistry
5 types of reactions in chemistry Type of reactions chemistry
Type of reactions chemistry Reaction type synthesis
Reaction type synthesis Slidetodoc.com
Slidetodoc.com Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Balancing chemical equations definition
Balancing chemical equations definition Types of chemical reactions redox
Types of chemical reactions redox Types of reactions
Types of reactions Types of reactions
Types of reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions