CHAPTER 2 CHEMICAL CONTEXT OF LIFE 1 Matter











- Slides: 32

CHAPTER 2 – CHEMICAL CONTEXT OF LIFE 1

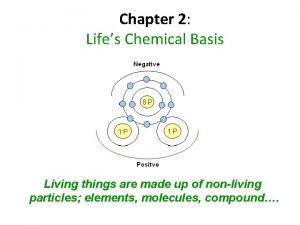
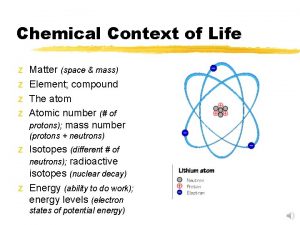
Matter/ Elements/ Compounds Matter anything that takes up space and has mass Element a substance that cannot be broken down (periodic table) Compound 2 or more different elements (Ex. H 20, Na. Cl); O 2 is NOT a compound 4 elements that make up 96% of living things: C, H, N, O 2

Trace vs. Essential Elements � � Essential Elements necessary for living things (C, H, N, O); make up 96% of all living things Trace Elements required by an organism but only in minute quantities 3

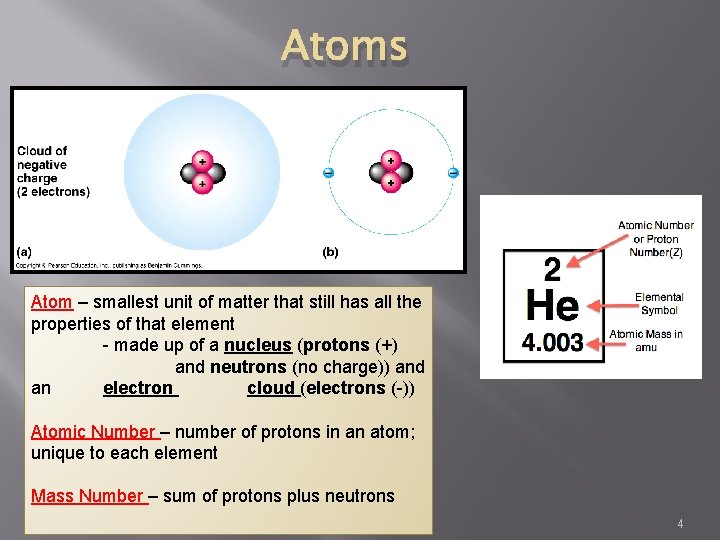
Atoms Atom – smallest unit of matter that still has all the properties of that element - made up of a nucleus (protons (+) and neutrons (no charge)) and an electron cloud (electrons (-)) Atomic Number – number of protons in an atom; unique to each element Mass Number – sum of protons plus neutrons 4

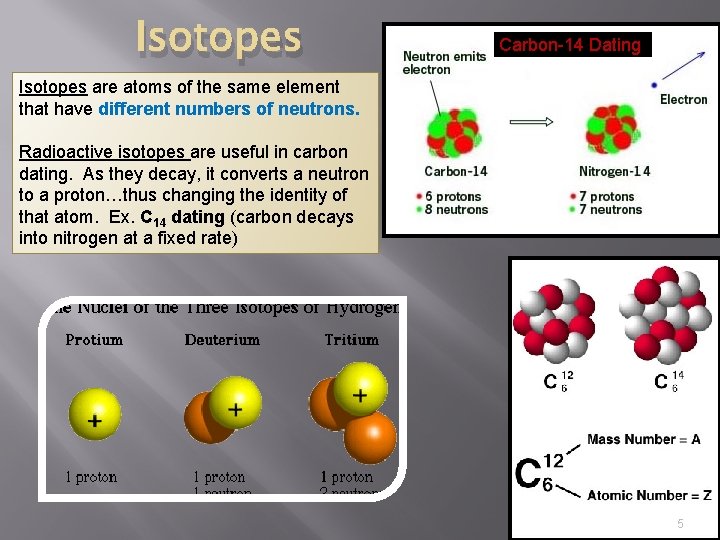
Isotopes Carbon-14 Dating Isotopes are atoms of the same element that have different numbers of neutrons. Radioactive isotopes are useful in carbon dating. As they decay, it converts a neutron to a proton…thus changing the identity of that atom. Ex. C 14 dating (carbon decays into nitrogen at a fixed rate) 5

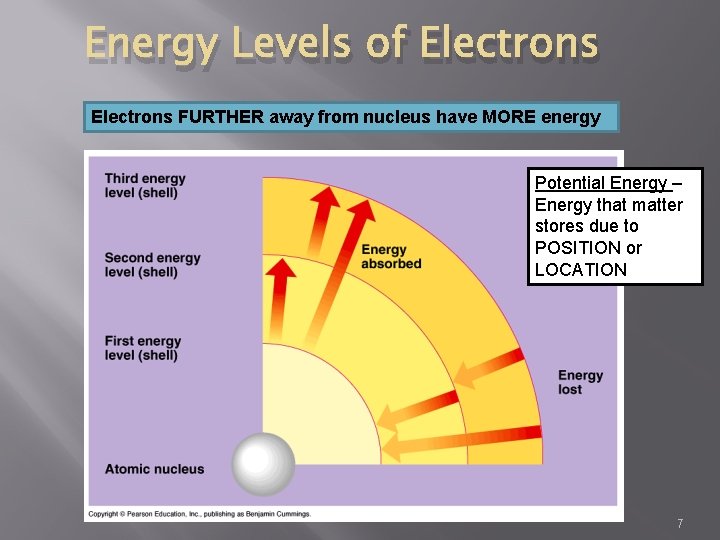
Energy � � � Energy ability to cause change by doing work Potential Energy energy that matter stores due to structure or location (stored in BONDS!) Kinetic Energy energy of motion 6

Energy Levels of Electrons FURTHER away from nucleus have MORE energy Potential Energy – Energy that matter stores due to POSITION or LOCATION 7

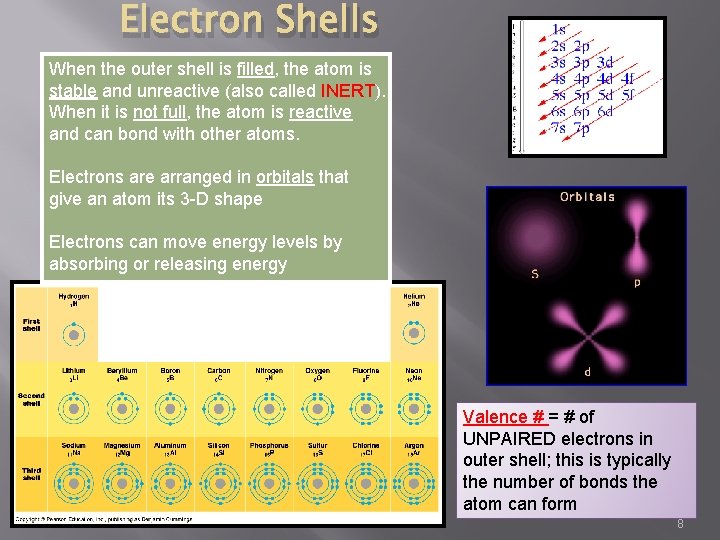
Electron Shells When the outer shell is filled, the atom is stable and unreactive (also called INERT). When it is not full, the atom is reactive and can bond with other atoms. Electrons are arranged in orbitals that give an atom its 3 -D shape Electrons can move energy levels by absorbing or releasing energy Valence # = # of UNPAIRED electrons in outer shell; this is typically the number of bonds the atom can form 8

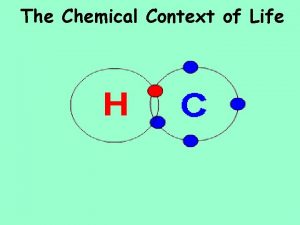
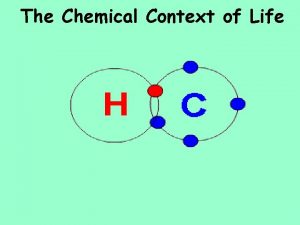
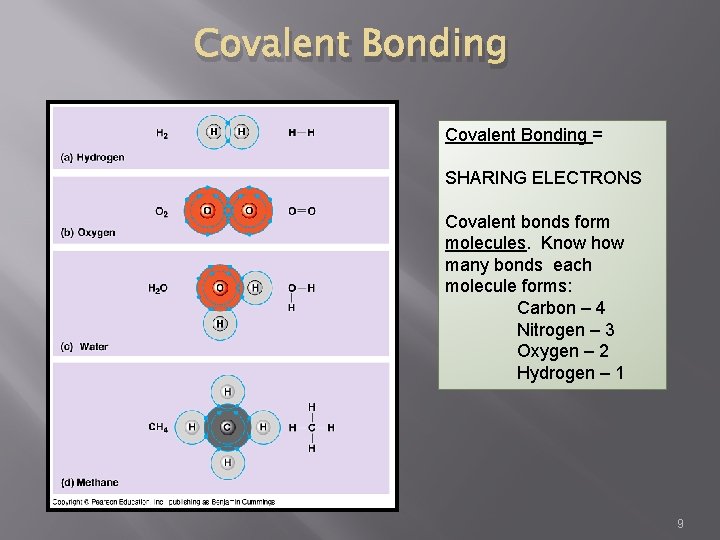
Covalent Bonding = SHARING ELECTRONS Covalent bonds form molecules. Know how many bonds each molecule forms: Carbon – 4 Nitrogen – 3 Oxygen – 2 Hydrogen – 1 9

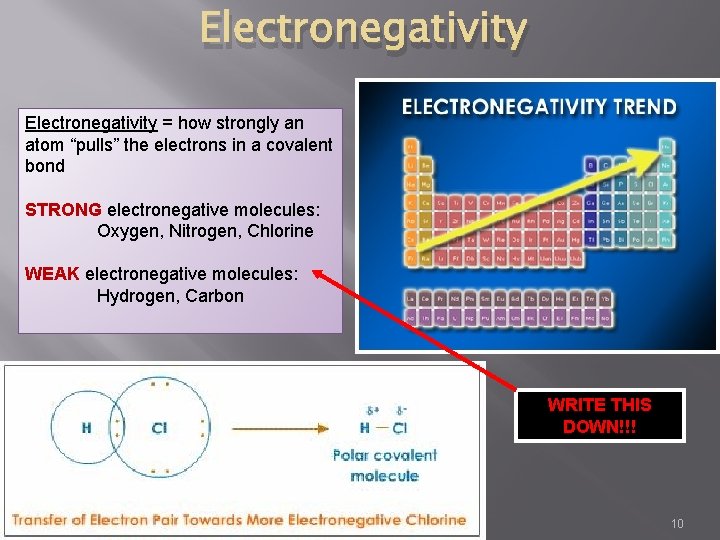
Electronegativity = how strongly an atom “pulls” the electrons in a covalent bond STRONG electronegative molecules: Oxygen, Nitrogen, Chlorine WEAK electronegative molecules: Hydrogen, Carbon WRITE THIS DOWN!!! 10

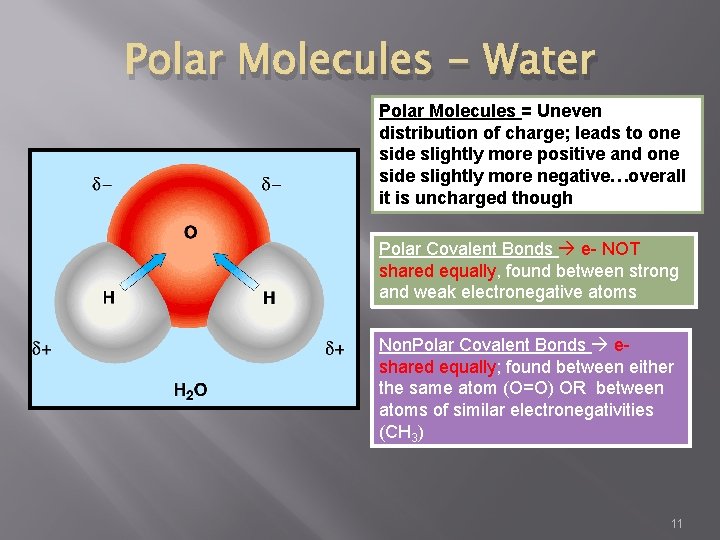
Polar Molecules - Water Polar Molecules = Uneven distribution of charge; leads to one side slightly more positive and one side slightly more negative…overall it is uncharged though Polar Covalent Bonds e- NOT shared equally, found between strong and weak electronegative atoms Non. Polar Covalent Bonds eshared equally; found between either the same atom (O=O) OR between atoms of similar electronegativities (CH 3) 11

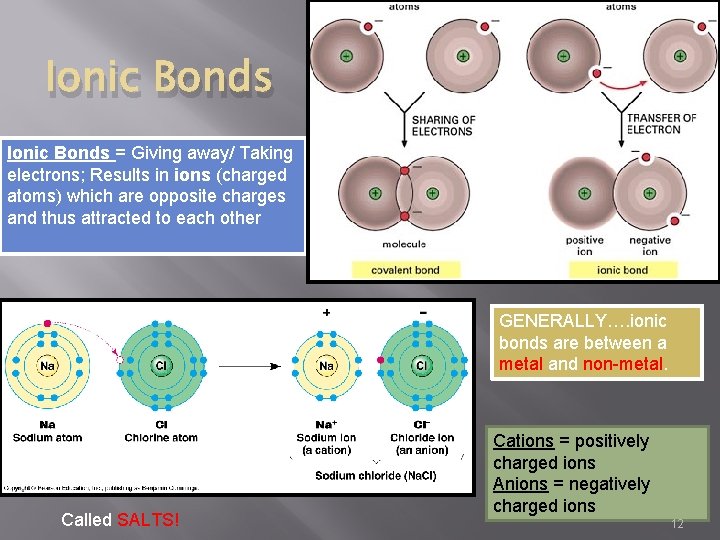
Ionic Bonds = Giving away/ Taking electrons; Results in ions (charged atoms) which are opposite charges and thus attracted to each other GENERALLY…. ionic bonds are between a metal and non-metal. Called SALTS! Cations = positively charged ions Anions = negatively charged ions 12

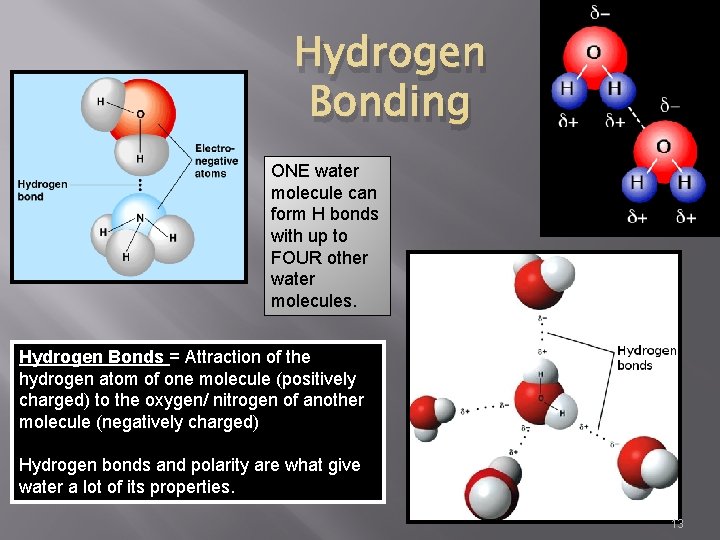
Hydrogen Bonding ONE water molecule can form H bonds with up to FOUR other water molecules. Hydrogen Bonds = Attraction of the hydrogen atom of one molecule (positively charged) to the oxygen/ nitrogen of another molecule (negatively charged) Hydrogen bonds and polarity are what give water a lot of its properties. 13

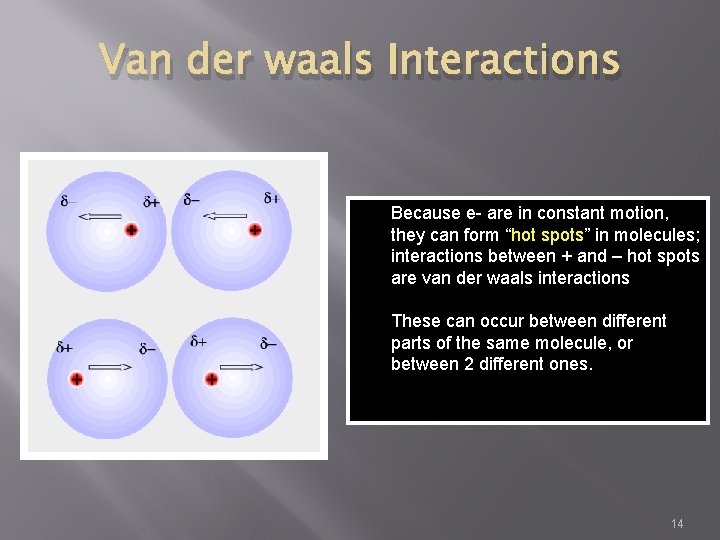
Van der waals Interactions Because e- are in constant motion, they can form “hot spots” in molecules; interactions between + and – hot spots are van der waals interactions These can occur between different parts of the same molecule, or between 2 different ones. 14

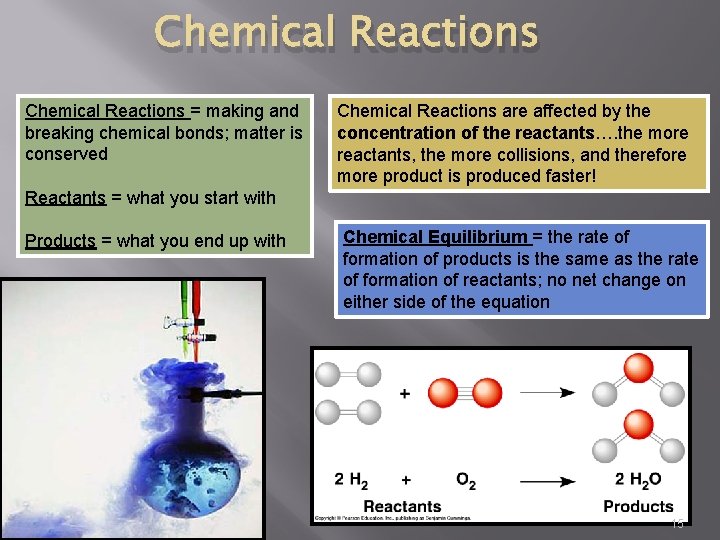
Chemical Reactions = making and breaking chemical bonds; matter is conserved Chemical Reactions are affected by the concentration of the reactants…. the more reactants, the more collisions, and therefore more product is produced faster! Reactants = what you start with Products = what you end up with Chemical Equilibrium = the rate of formation of products is the same as the rate of formation of reactants; no net change on either side of the equation 15

CHAPTER 3 - WATER AND THE FITNESS OF THE ENVIRONMENT 16

Water – UNIQUE!! � � � ¾ of the Earth’s surface is covered in water Cells are 70 -95% water Water is unusual because it exists in 3 states of matter: solid (ice), liquid, gas (water vapor) Water is a reactant in many chemical reactions Water is the reason we have life on Earth! 17

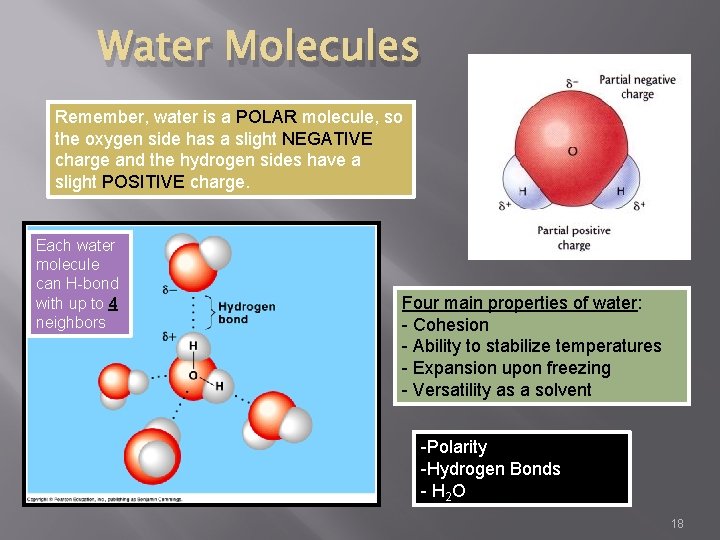
Water Molecules Remember, water is a POLAR molecule, so the oxygen side has a slight NEGATIVE charge and the hydrogen sides have a slight POSITIVE charge. Each water molecule can H-bond with up to 4 neighbors Four main properties of water: - Cohesion - Ability to stabilize temperatures - Expansion upon freezing - Versatility as a solvent -Polarity -Hydrogen Bonds - H 2 O 18

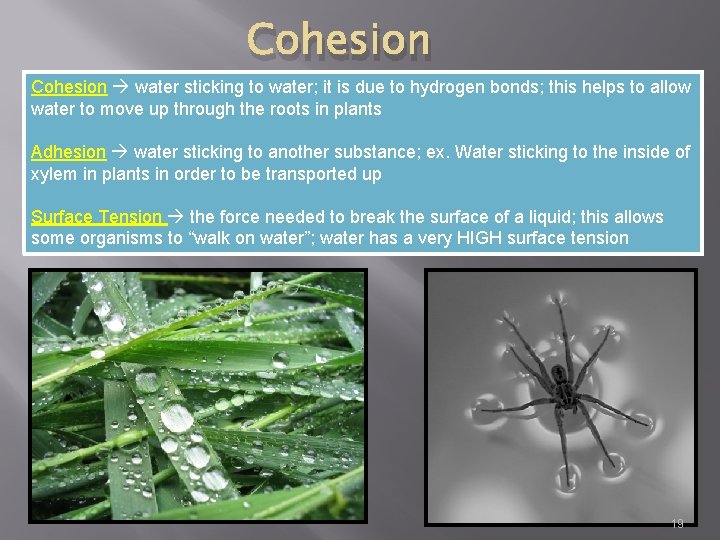
Cohesion water sticking to water; it is due to hydrogen bonds; this helps to allow water to move up through the roots in plants Adhesion water sticking to another substance; ex. Water sticking to the inside of xylem in plants in order to be transported up Surface Tension the force needed to break the surface of a liquid; this allows some organisms to “walk on water”; water has a very HIGH surface tension 19

Ability to Stabilize Temperature Kinetic Energy = Energy of motion (all molecules are in constant motion, so they have kinetic energy) Heat = TOTAL quantity of kinetic energy of a body of matter Temperature = AVERAGE kinetic energy in a body of matter Specific Heat = Amount of heat needed to change 1 gram of a substance by 1 ºC Measuring Temperature Calorie = amount of heat it takes to raise 1 g of water 1º C Kilocalorie (kcal) = amount of heat it takes to raise 1000 g (1 kg) of water 1º C Joule = 1 J = 0. 239 cal 20

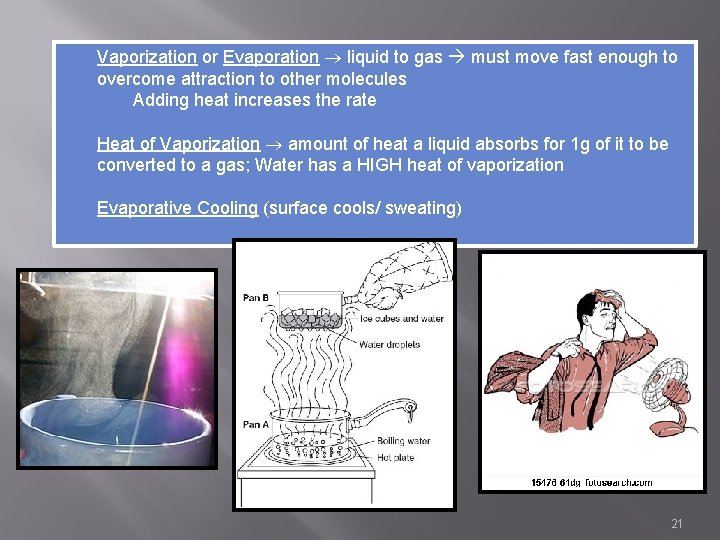
Vaporization or Evaporation liquid to gas must move fast enough to overcome attraction to other molecules Adding heat increases the rate Heat of Vaporization amount of heat a liquid absorbs for 1 g of it to be converted to a gas; Water has a HIGH heat of vaporization Evaporative Cooling (surface cools/ sweating) 21

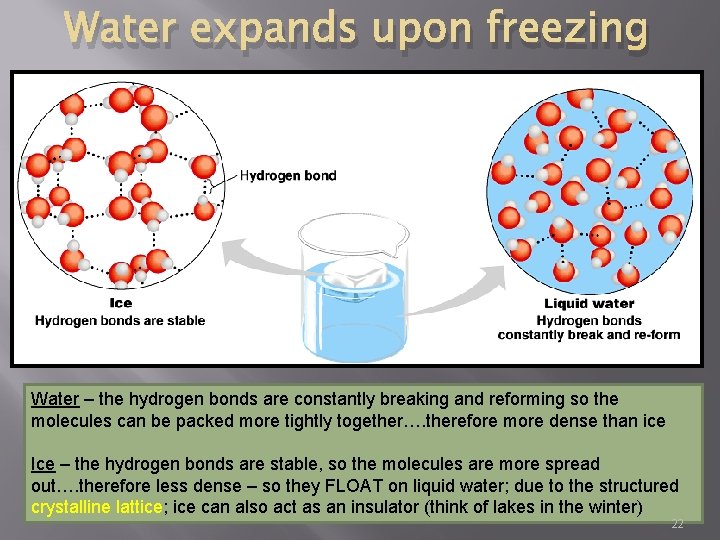
Water expands upon freezing Water – the hydrogen bonds are constantly breaking and reforming so the molecules can be packed more tightly together…. therefore more dense than ice Ice – the hydrogen bonds are stable, so the molecules are more spread out…. therefore less dense – so they FLOAT on liquid water; due to the structured crystalline lattice; ice can also act as an insulator (think of lakes in the winter) 22

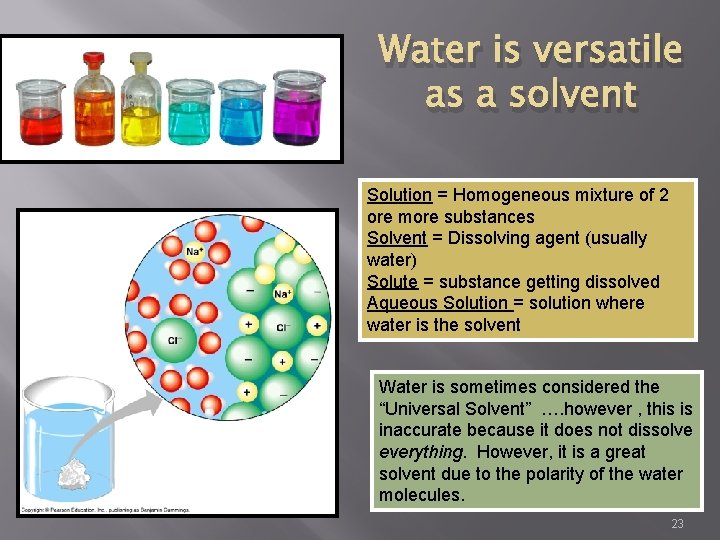
Water is versatile as a solvent Solution = Homogeneous mixture of 2 ore more substances Solvent = Dissolving agent (usually water) Solute = substance getting dissolved Aqueous Solution = solution where water is the solvent Water is sometimes considered the “Universal Solvent” …. however , this is inaccurate because it does not dissolve everything. However, it is a great solvent due to the polarity of the water molecules. 23

Hydrophobic vs. Hydrophilic = water-loving; ionic and polar bonds are hydrophilic; will dissolve in water Hydrophobic = water-fearing; non-ionic and non-polar substances; does not mix with water (ex. oil) Waxy cuticle of a plant is hydrophobic 24

Moles Mole (mol) = grams of a substance equal to the molecular weight Molecular weight = sum of all the weights of all the atoms in a molecule The advantage of measuring in moles is that one mole of any substance has exactly the same number of molecules as 1 mole of any other substance - The # of molecules in a mole = Avogadro’s Number = 6. 02 x 1023 SO…. one mole of any substance will have the same # of molecules, but will weigh different amounts! 25

Example from Notes How to make a 1 M solution of Sucrose - First find the molecular weight of the molecule - Then you would dissolve 342 g of sucrose in water until the whole solution reached 1 liter. That would give you a 1 M solution. - IF you wanted to make a 2 M solution, you would multiply the molecular weight by 2 (684 g), dissolve it in water, and bring the solution to 1 liter. - IF you wanted to make a. 5 M solution, you would multiply the molecular weight by. 5 and then add that much solvent (it would be 171 g), etc 26

PRACTICE! � Make a 1 M solution of C 6 H 12 O 6 �C = 12 daltons x 6 = � H = 1 dalton x 12 = 12 � O = 16 daltons x 6 = 96 72 180 g = molecular weight So, dissolve 180 g of C 6 H 12 O 6 in water and fill it up to 1 liter. 27

PRACTICE! � Make a. 5 M solution of C 12 H 22 O 11 C = 12 daltons x 12 = 144 H = 1 dalton x 22 = 22 O = 16 daltons x 11 = 176 342 g = molecular weight So, multiply 342 g by. 5 (171 g) and then dissolve 171 g of C 12 H 22 O 11 in water and fill it up to 1 liter. 28

PRACTICE! � Make a 1. 5 M solution of C 4 H 6 NO 2 �C = 12 daltons x 4 = � H = 1 dalton x 6 = 6 � N = 14 daltons x 1 = � O = 16 daltons x 2 = 32 48 14 100 g = molecular weight So, multiply 100 g by 1. 5 (150 g) and then dissolve 150 g of C 4 H 6 NO 2 in water and fill it up to 1 liter. 29

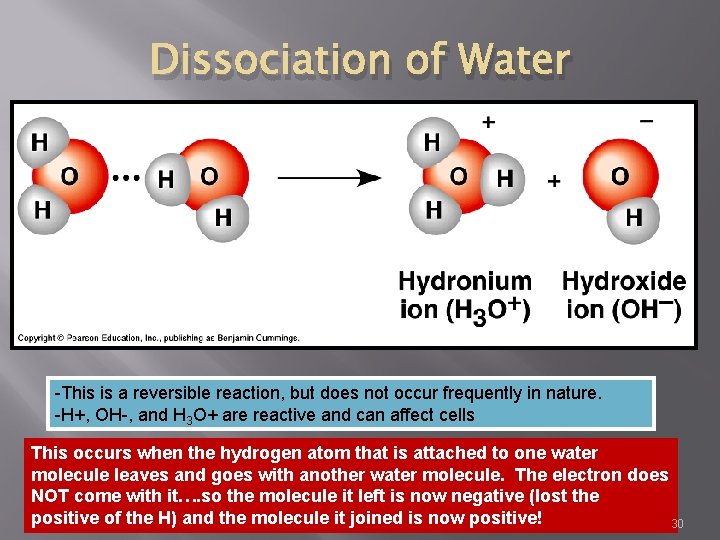
Dissociation of Water -This is a reversible reaction, but does not occur frequently in nature. -H+, OH-, and H 3 O+ are reactive and can affect cells This occurs when the hydrogen atom that is attached to one water molecule leaves and goes with another water molecule. The electron does NOT come with it…. so the molecule it left is now negative (lost the positive of the H) and the molecule it joined is now positive! 30

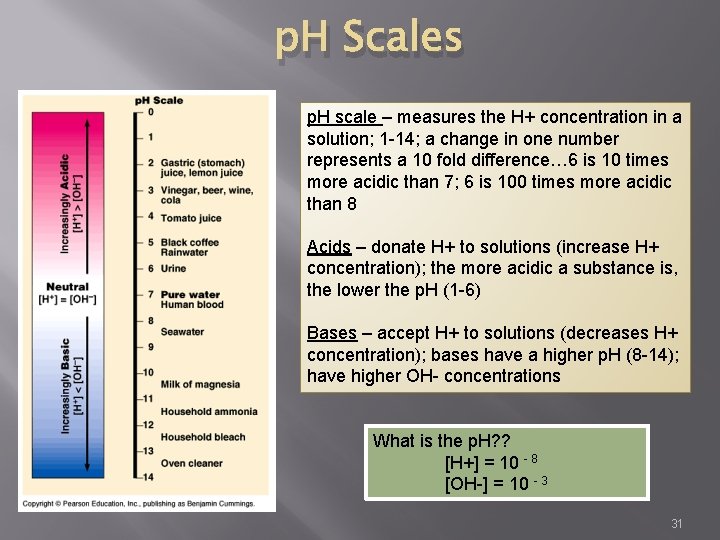
p. H Scales p. H scale – measures the H+ concentration in a solution; 1 -14; a change in one number represents a 10 fold difference… 6 is 10 times more acidic than 7; 6 is 100 times more acidic than 8 Acids – donate H+ to solutions (increase H+ concentration); the more acidic a substance is, the lower the p. H (1 -6) Bases – accept H+ to solutions (decreases H+ concentration); bases have a higher p. H (8 -14); have higher OH- concentrations What is the p. H? ? [H+] = 10 - 8 [OH-] = 10 - 3 31

Buffers are solutions that can act as acids or bases (donate or accept H+) to minimize changes in p. H. They are very important in living systems. One common buffer in human blood is carbonic acid. It acts as an acid and base pair either accepting OR donating H+ depending on the need of the blood. Can act as an acid and DONATE H+ Can act as a base and ACCEPT H+ Response to a RISE in p. H → H 2 CO 3 ↔ HCO 3 - + H+ ←Response to a DROP in p. H H+ donor (acid) Carbonic Acid H+ acceptor (base) Hydrogen ion Bicarbonate ion 32
 Chapter 2 the chemical context of life
Chapter 2 the chemical context of life Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Love formula in chemistry
Love formula in chemistry Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Nonagist
Nonagist High context vs low context culture ppt
High context vs low context culture ppt Physical context and linguistic context
Physical context and linguistic context Soal essay komunikasi verbal
Soal essay komunikasi verbal Section 1 composition of matter
Section 1 composition of matter Grey matter
Grey matter Section 1 composition of matter
Section 1 composition of matter Brain falx
Brain falx Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter Frontal and parietal lobes
Frontal and parietal lobes Flow energy review
Flow energy review Chemical properties of atoms
Chemical properties of atoms Chapter 2 chemical basis of life
Chapter 2 chemical basis of life Chemical and physical properties
Chemical and physical properties Measurable properties examples
Measurable properties examples What are chemical properties of matter
What are chemical properties of matter Eating food physical or chemical change
Eating food physical or chemical change Chemical equation with states of matter
Chemical equation with states of matter Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Chemical names and formulas chapter 9
Chemical names and formulas chapter 9 Matter life
Matter life All life is composed of matter
All life is composed of matter Chemical reactions in everyday life
Chemical reactions in everyday life