The Chemical Context of Life Chapter 2 Matter









- Slides: 19

The Chemical Context of Life Chapter 2

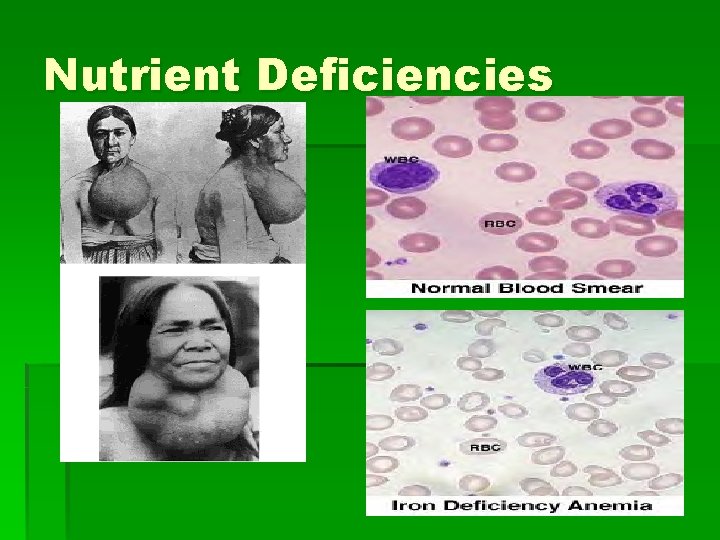
Matter § Matter consists of chemical elements in pure form and in combinations called compounds; living organisms are made of matter. § Matter -- Anything that takes up space and has mass. § Element -- A substance that cannot be broken down into other substances by chemical reactions; all matter made of elements. § Life requires about 25 chemical elements § 96% of living matter is composed of C, O, H, N. § Most of remaining 4% is P, S, Ca, K. § Trace element -- required by organisms in extremely small quantities: Cu, Fe, I, etc.

Matter cont. § Compound -- A pure substances made of two or more elements combined in a fixed ratio. § Have characterisitics different than the elements that make them up (emergent property). § Na and Cl have very different properties from Na. Cl. § Difference between mass and weight: § Mass -- measure of the amount of matter an object contains; constant. § Weight -- measure of how strongly an object is pulled by earth's gravity; varies.

Nutrient Deficiencies

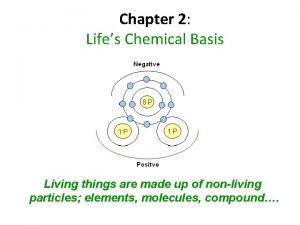
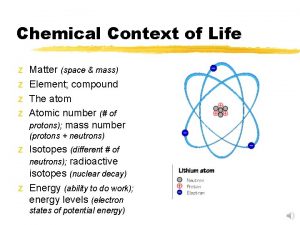
Atomic structure determines the behavior of an element § Atom -- Smallest possible unit of matter that retains the physical and chemical properties of its element. § Subatomic Particles § 1. Neutrons (no charge/neutral; found in nucleus; ~ 1 amu). § 2. Protons (+1 charge; found in nucleus; ~ 1 amu). § 3. Electrons (-1 charge; electron cloud; 1/2000 amu). § One amu approx equal to 1. 7 x 10 -24 g.

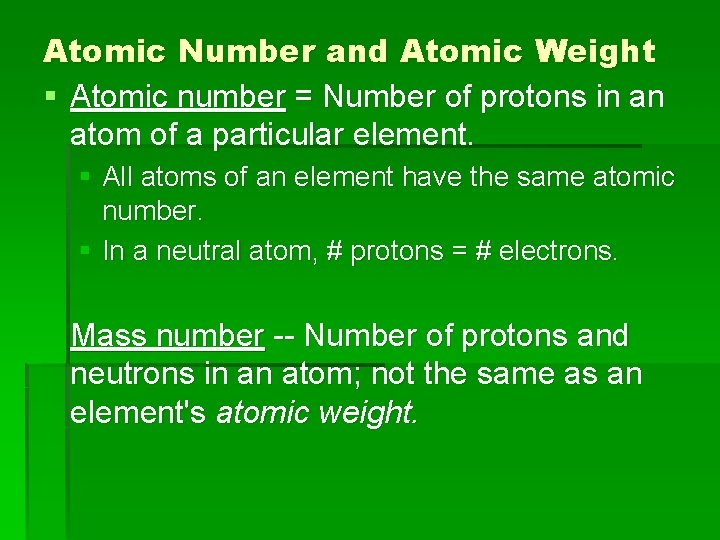
Atomic Number and Atomic Weight § Atomic number = Number of protons in an atom of a particular element. § All atoms of an element have the same atomic number. § In a neutral atom, # protons = # electrons. Mass number -- Number of protons and neutrons in an atom; not the same as an element's atomic weight.

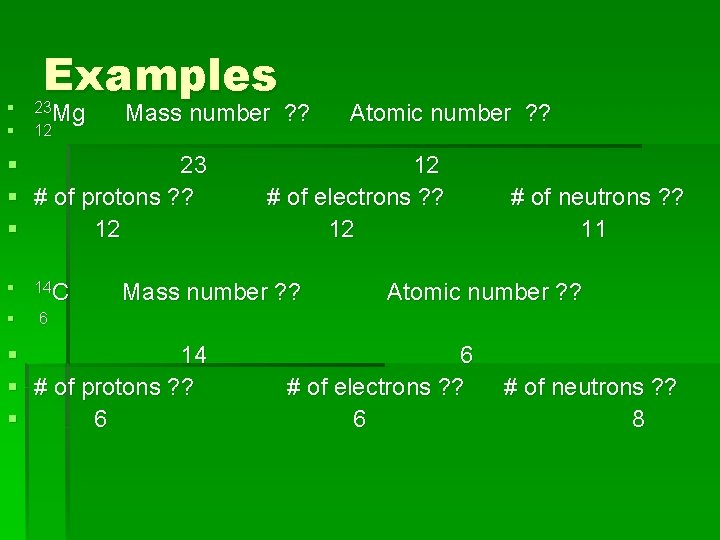
§ § Examples 23 Mg 12 Mass number ? ? § 23 § # of protons ? ? § 12 § 14 C § 6 12 # of electrons ? ? 12 Mass number ? ? § 14 § # of protons ? ? § 6 Atomic number ? ? # of neutrons ? ? 11 Atomic number ? ? 6 # of electrons ? ? 6 # of neutrons ? ? 8

Isotopes § Isotopes -- Atoms of an element that have the same atomic number but different mass number; different number of neutrons. § Half-life -- Time for 50% of radioactive atoms in a sample to decay. § Biological applications of radioactive isotopes include: § 1. Dating geological strata and fossils. § Radioactive decay is at a fixed rate; by comparing the ratio of radioactive and stable isotope, age can be estimated. in a fossil with the § Ratio of Carbon-14 to Carbon-12 is used to date fossils less than 50, 000 years old.

Isotopes cont. § 2. Radioactive tracers § Chemicals labelled with radioactive isotopes are used to trace the steps of a biochemical reaction or to determine the location of a particular substance within an organism. § Isotopes of P, N and H were used to determine DNA structure. § Used to diagnose disease. § 3. Treatment of cancer § Can be hazardous to cells.


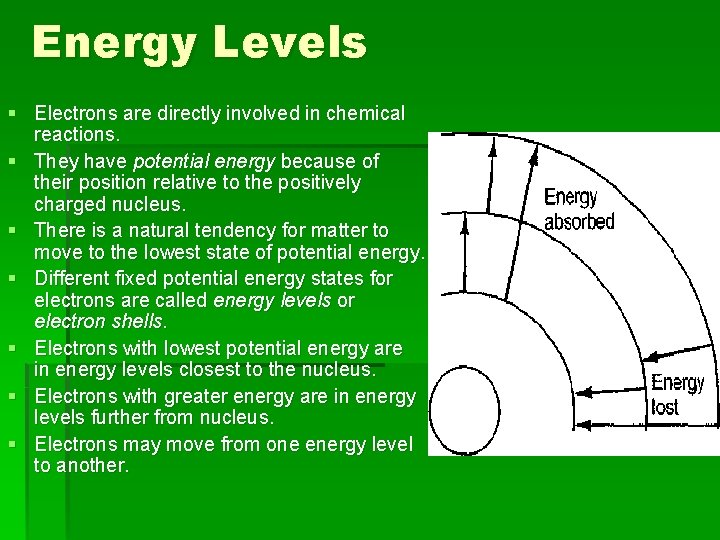
Energy Levels § Electrons are directly involved in chemical reactions. § They have potential energy because of their position relative to the positively charged nucleus. § There is a natural tendency for matter to move to the lowest state of potential energy. § Different fixed potential energy states for electrons are called energy levels or electron shells. § Electrons with lowest potential energy are in energy levels closest to the nucleus. § Electrons with greater energy are in energy levels further from nucleus. § Electrons may move from one energy level to another.

Electron Configuration and Chemical Properties § Electron configuration -- Distribution of electrons in an atom's electron shells; determines its chemical behavior. § Chemical properties of an atom depend upon the number of valence electrons (electrons in the outermost energy level. § Octet rule -- A valence shell is complete when it contains 8 electrons (except H and He). § An atom with an incomplete valence shell is chemically reactive (tends to form chemical bonds until it has 8 electrons to fill the valence shell). § Atoms with the same number of valence electrons show similar chemical behavior.

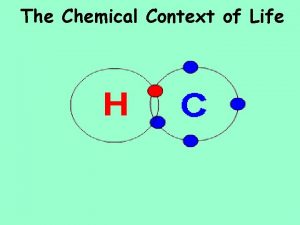
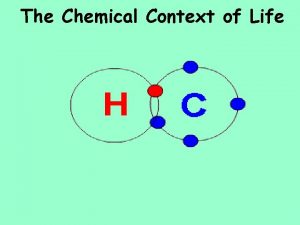
Bonding in Molecules § Chemical bonds -- Attractions that hold molecules together. § Molecules --Two or more atoms held together by chemical bonds. § Covalent bond -- formed between atoms by sharing a pair of valence electrons; common in organic compounds. § Single covalent bond -- Bond between atoms formed by sharing a single pair of valence electrons. § Double bond -- share two pairs of valence electrons. § Triple bond -- share three pairs of valence electrons. § Compound = A pure substance composed of two or more elements combined in a fixed ratio. § For example: water (H 2 O), methane (CH 4).


Nonpolar Covalent Bonds § Electronegativity -- Atom's ability to attract and hold electrons. § • The more electronegative an atom, the more strongly it attracts shared electrons. § • Scale determined by Linus Pauling: § O = 3. 5; N = 3. 0; S and C = 2. 5; P and H = 2. 1. § § Nonpolar bond -- Covalent bond formed by an equal sharing of electrons between atoms. § • Occurs when electronegativity of both atoms is about the same. § • Molecules made of one element usually have nonpolar covalent bonds (H 2 and O 2).

Polar Covalent Bonds § Polar bond -- Covalent bond formed by an unequal sharing of electrons between atoms. § • Occurs when the atoms involved have different electronegativities. § • In water, electrons spend more time around the oxygen than the hydrogens. This causes the oxygen atom to have a slight negative charge and the hydrogens to have a slight positive charge.


Ionic Bonds § Ion -- Charged atom or molecule. § Anion -- An atom that has gained one or more electrons from another atom; negatively charged. § Cation -- An atom that has lost one or more electrons; positively charged. § Ionic bond -- Bond formed by the electrostatic attraction after the complete transfer of an electron from a donor atom to an acceptor. § Strong bonds in crystals, but fragile bonds in water. § Ionic compounds are called salts (e. g. Na. Cl or table salt).

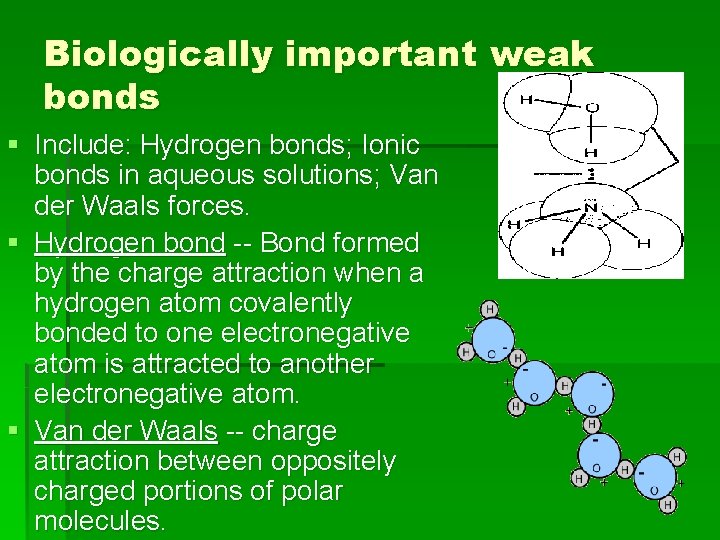
Biologically important weak bonds § Include: Hydrogen bonds; Ionic bonds in aqueous solutions; Van der Waals forces. § Hydrogen bond -- Bond formed by the charge attraction when a hydrogen atom covalently bonded to one electronegative atom is attracted to another electronegative atom. § Van der Waals -- charge attraction between oppositely charged portions of polar molecules.
 The chemical context of life chapter 2
The chemical context of life chapter 2 Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Chemical formulas and chemical compounds chapter 7
Chemical formulas and chemical compounds chapter 7 Are kc and kp equal
Are kc and kp equal Nonagist
Nonagist Communicating across generational differences
Communicating across generational differences Co text
Co text Verbal adalah
Verbal adalah Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter vs white matter
Gray matter vs white matter Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter What makes up the diencephalon
What makes up the diencephalon Section 1 composition of matter
Section 1 composition of matter Gray matter and white matter
Gray matter and white matter What is grey matter
What is grey matter Flow of energy vs flow of matter
Flow of energy vs flow of matter Chapter 2 life's chemical basis
Chapter 2 life's chemical basis Chapter 2 chemical basis of life
Chapter 2 chemical basis of life Physical property and chemical property
Physical property and chemical property