Chapter 15 Redox Titrations 15 1 The Shape

![§ Titration Curve has Three Regions: - Before the Equivalence Point: from [Fe 2+], § Titration Curve has Three Regions: - Before the Equivalence Point: from [Fe 2+],](https://slidetodoc.com/presentation_image_h/212d55d1d313dcae3f1a0e9ba88c2dfe/image-3.jpg)

- Slides: 19

Chapter 15 Redox Titrations

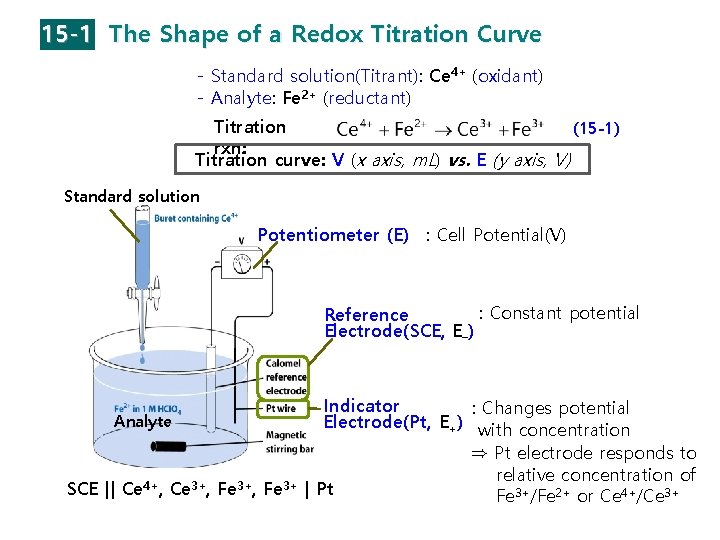
15 -1 The Shape of a Redox Titration Curve - Standard solution(Titrant): Ce 4+ (oxidant) - Analyte: Fe 2+ (reductant) Titration (15 -1) rxn: Titration curve: V (x axis, m. L) vs. E (y axis, V) Standard solution Potentiometer (E) : Cell Potential(V) : Constant potential Reference Electrode(SCE, E–) Analyte SCE || Ce 4+, Ce 3+, Fe 3+ Indicator : Changes potential Electrode(Pt, E+) with concentration ⇒ Pt electrode responds to relative concentration of | Pt Fe 3+/Fe 2+ or Ce 4+/Ce 3+
![Titration Curve has Three Regions Before the Equivalence Point from Fe 2 § Titration Curve has Three Regions: - Before the Equivalence Point: from [Fe 2+],](https://slidetodoc.com/presentation_image_h/212d55d1d313dcae3f1a0e9ba88c2dfe/image-3.jpg)
§ Titration Curve has Three Regions: - Before the Equivalence Point: from [Fe 2+], [Fe 3+] → E - At the Equivalence Point: [Ce 3+]=[Fe 3+], [Ce 4+]=[Fe 2+] → 2 E+ → E - After the Equivalence Point: from [Ce 3+], [Ce 4+] → E (15 -1) (15 -2) (15 -3) - Half cell potential for indicator electrode (E+ , Pt wire) (15 -9) E+ (1510) ⇒ During titrations: - Half potential for reference electrode (E– , SCE) E– (=ESCE) = constant = 0. 241 V ⇒ Cell potential for redox titration (15 -4) (For SCE)

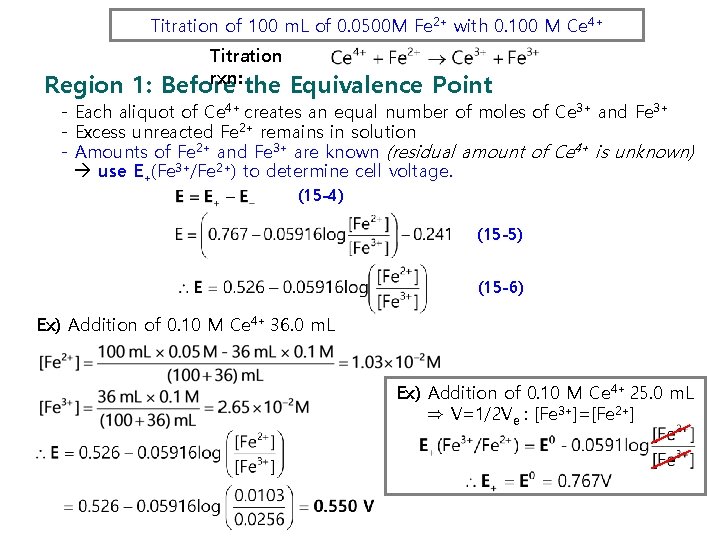
Titration of 100 m. L of 0. 0500 M Fe 2+ with 0. 100 M Ce 4+ Titration rxn: Region 1: Before the Equivalence Point - Each aliquot of Ce 4+ creates an equal number of moles of Ce 3+ and Fe 3+ - Excess unreacted Fe 2+ remains in solution - Amounts of Fe 2+ and Fe 3+ are known (residual amount of Ce 4+ is unknown) use E+(Fe 3+/Fe 2+) to determine cell voltage. (15 -4) (15 -5) (15 -6) Ex) Addition of 0. 10 M Ce 4+ 36. 0 m. L Ex) Addition of 0. 10 M Ce 4+ 25. 0 m. L ⇒ V=1/2 Ve : [Fe 3+]=[Fe 2+]

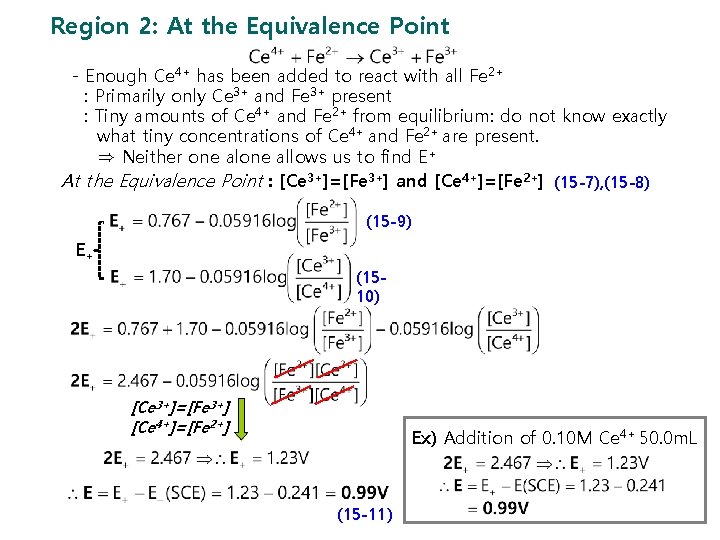
Region 2: At the Equivalence Point - Enough Ce 4+ has been added to react with all Fe 2+ : Primarily only Ce 3+ and Fe 3+ present : Tiny amounts of Ce 4+ and Fe 2+ from equilibrium: do not know exactly what tiny concentrations of Ce 4+ and Fe 2+ are present. ⇒ Neither one allows us to find E+ At the Equivalence Point : [Ce 3+]=[Fe 3+] and [Ce 4+]=[Fe 2+] (15 -7), (15 -8) (15 -9) E+ (1510) [Ce 3+]=[Fe 3+] [Ce 4+]=[Fe 2+] Ex) Addition of 0. 10 M Ce 4+ 50. 0 m. L (15 -11)

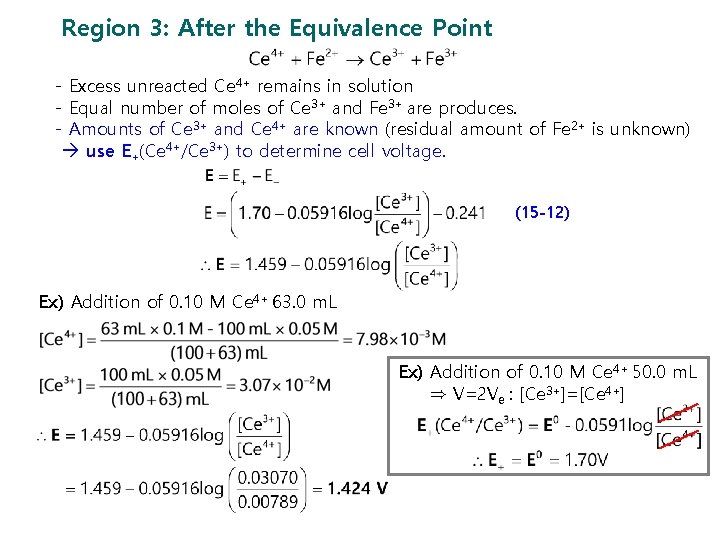
Region 3: After the Equivalence Point - Excess unreacted Ce 4+ remains in solution - Equal number of moles of Ce 3+ and Fe 3+ are produces. - Amounts of Ce 3+ and Ce 4+ are known (residual amount of Fe 2+ is unknown) use E+(Ce 4+/Ce 3+) to determine cell voltage. (15 -12) Ex) Addition of 0. 10 M Ce 4+ 63. 0 m. L Ex) Addition of 0. 10 M Ce 4+ 50. 0 m. L ⇒ V=2 Ve : [Ce 3+]=[Ce 4+]

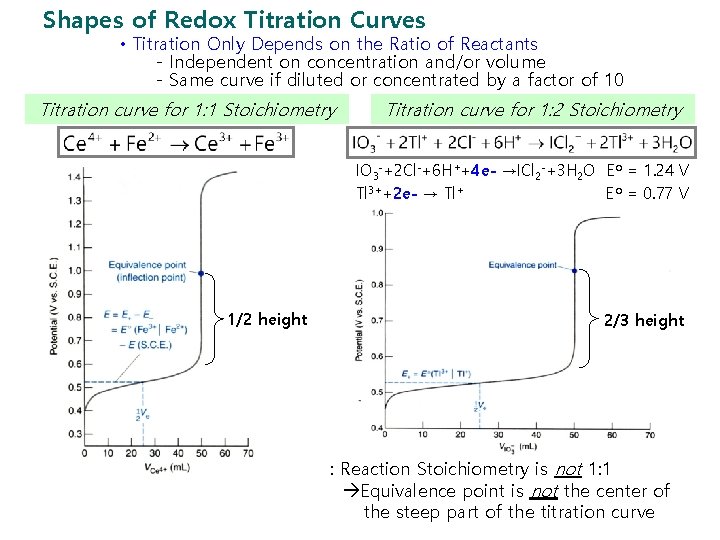
Shapes of Redox Titration Curves • Titration Only Depends on the Ratio of Reactants - Independent on concentration and/or volume - Same curve if diluted or concentrated by a factor of 10 Titration curve for 1: 1 Stoichiometry Titration curve for 1: 2 Stoichiometry IO 3 -+2 Cl-+6 H++4 e- →ICl 2 -+3 H 2 O Eo = 1. 24 V Tl 3++2 e- → Tl+ Eo = 0. 77 V 1/2 height 2/3 height : Reaction Stoichiometry is not 1: 1 Equivalence point is not the center of the steep part of the titration curve

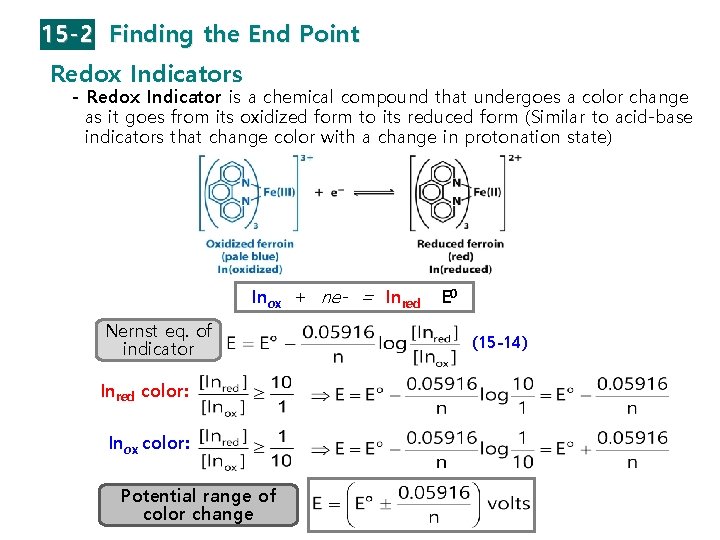
15 -2 Finding the End Point Redox Indicators - Redox Indicator is a chemical compound that undergoes a color change as it goes from its oxidized form to its reduced form (Similar to acid-base indicators that change color with a change in protonation state) Inox + ne- = Inred Nernst eq. of indicator Inred color: Inox color: Potential range of color change E 0 (15 -14)

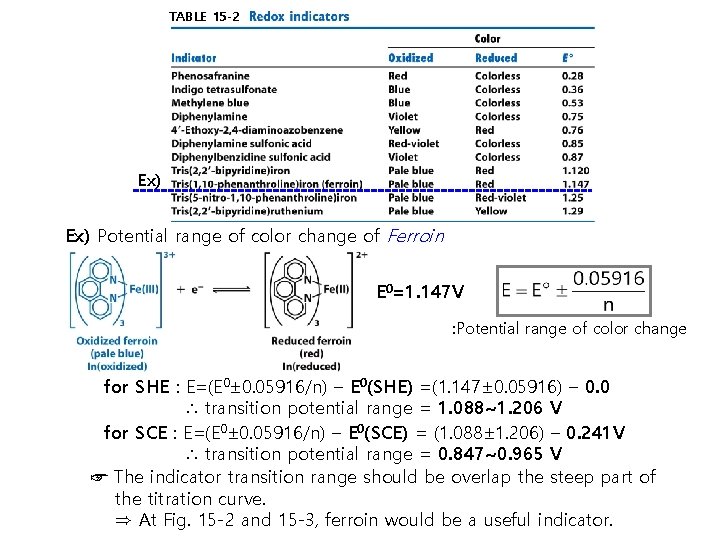
TABLE 15 -2 Ex) Potential range of color change of Ferroin E 0=1. 147 V : Potential range of color change for SHE : E=(E 0± 0. 05916/n) – E 0(SHE) =(1. 147± 0. 05916) – 0. 0 ∴ transition potential range = 1. 088~1. 206 V for SCE : E=(E 0± 0. 05916/n) – E 0(SCE) = (1. 088± 1. 206) – 0. 241 V ∴ transition potential range = 0. 847~0. 965 V ☞ The indicator transition range should be overlap the steep part of the titration curve. ⇒ At Fig. 15 -2 and 15 -3, ferroin would be a useful indicator.

The Starch-Iodine Complex - Commonly used as an indicator in redox titrations involving iodine - Reacts with iodine to form an intensely blue colored complex (starch-I 6) - Starch is not a redox indicator (Does not undergo a change in redox potential) - Starch is readily biodegraded, so it should be freshly dissolved or the solution be contain a preservative, such as Hg. I 2(~1 mg/100 m. L) or tymol. agent. - should A hydrolysis product of starch solution is glucose, which is a reducing Therefore, partially hydrolyzed starch solution ca be a source of error in a redox titration. Iodimetry – added iodine at the beginning of the titration Iodometry – added iodine near equivalence point

15 -3 Adjustment of Analyte Oxidation State • Before Redox Titrations, many compounds must be converted into a known oxidation state ⇒This step in the procedure is known as preoxidation or prereduction • Reagents for prereduction or preoxidation must: -Totally convert analyte into desired form - Be easy to remove from the reaction mixture - Avoid interfering in the titration Preoxidation ① Peroxydisulfate or persulfate (S 2 O 82 -) with Ag+ catalyst Powerful oxidants - Oxidizes Mn 2+, Ce 3+, Cr 3+, VO 2+ - excess S 2 O 82 - and Ag 2+ removed by boiling the solution ② Silver(II) oxide (Ag. O) in concentrated mineral acids - yields Ag 2+ - excess Ag 2+ removed by boiling ③ Hydrogen peroxide (H 2 O 2) is a good oxidant to use in basic solutions - Oxidizes Co 2+, Fe 2+, Mn 2+ - Reduces Cr 2 O 72 -, Mn. O 4 – in acidic solution - excess removed by boiling

Prereduction ① Stannous chloride (Sn. Cl 2) in hot HCl - Reduce Fe 3+ to Fe 2+ - excess removed by adding Hg. Cl 2 ② Chromous chloride(Cr. Cl 2) ③ Sulfur dioxide(SO 2), hydrogen sulfite(H 2 S) ④ Jones reductor (Zn + Zn amalgam) Amalgam: Zn powder + 2 wt% Hg. Cl 2(10 min) → washing - Fe 3+(in 1 M H 2 SO 4) → Fe 2+ ⑤ Walden reductor (Ag in 1 M HCl, Ag/Ag. Cl)

15 -4 Oxidation with Potassium Permangnate § Potassium Permanganate (KMn. O 4) - Common titrants for oxidation reactions - Strong oxidant - Own indicator (intense violet color) in acidic solution. - Should standardization with primary standard (Na 2 C 2 O 4, Fe) p. H ≤ 1 Violet p. H neutral or alkaline Violet Eo = 1. 692 V brown p. H strongly alkaline Violet colorless green Eo = 1. 507 V Eo = 0. 56 V

Preparation and Standardization Preparation of KMn. O 4 solution - Dissolve in distilled water→boil for an hour→filter with sintered-glass filter to remove solid Mn. O 2(Do not use filter paper) - Store in a dark glass bottle. - Aqueous is unstable by virtue of the reaction 4 Mn. O 4 - + 2 H 2 O → 4 Mn. O 2(s) + 3 O 2 + 4 OH– : slow in the absence of Mn. O 4–, Mn 2+, heat, light, acids, and bases Standardization of KMn. O 4 (Chapter 1) ① Na 2 C 2 O 4 (primary standard): dry at 105℃ for 2 h Standardization reaction( in 1 M H 2 SO 4): 5 H 2 C 2 O 4 + 2 Mn. O 4 - + 6 H+ → 10 CO 2 + 2 Mn+ + 8 H 2 O : add 90 -95% of the required KMn. O 4 solution at room temperature, then warm the solution to 55– 60℃ and complete the titration by slow addition of value KMn. O 4. : subtract a blank ② Pure Fe wire, Fe(NH 4)2(SO 4) 2∙ 6 H 2 O, Fe(H 3 NCH 2 NH 3)2(SO 4) 2∙ 2 H 2 O

15 -5 Oxidation with Ce 4+ - Commonly used in place of KMn. O 4 Works best in acidic solution Can be used in most applications in previous table Used to analyze some organic compounds Color change not distinct to be its own indicator use redox indicator Yellow colorless - Ce 4+ binds anions very strongly results in variation of formal potential 1. 70 V in 1 F HCl. O 4 (∵)Measure 1. 61 V in 1 F HNO 3 activity not Formal potential 1. 47 V in 1 F HCl concentration 1. 44 V in 1 F H 2 SO 4 Preparation and Standardization - Primary standard (NH 4)2 Ce(NO 3)6 can be dissolved in 1 M H 2 SO 4 and used directly. - If Primary standard is not available, standardization with primary standard (Na 2 C 2 O 4).

15 -6 Oxidation with Potassium Dichromate § Potassium Dichromate (K 2 Cr 2 O 7) - Powerful oxidant in strong acid Not as strong as KMn. O 4 or Ce 4+ Primarily used for the determination of Fe 2+ Color change not distinct to be its own indicator Potassium Dichromate (K 2 Cr 2 O 7) is primary standard orange green to violet 1. 00 V in 1 M HCl 1. 11 V in 2 M H 2 SO 4 - In basic solution, not an oxidant: Cr 2 O 72– is converted into Cr. O 42– Formal potential

15 -7 Methods Involving Iodine § Iodine (I 2) - Slightly soluble in water (solubility=1. 3× 10 -3 M at 20℃) ⇒ Soluble by complexation with iodide(I–) I 2(s) + I- = I 3– K=7× 102 Iodine Iodide triiodide : Triiodide(I 3–) is prepared by dissolving solid I 2 in excess KI. I 3– + 2 e- = 3 I– E 0 = 0. 535 V - Iodine act as reductant or oxidant Iodine as oxidant : I 3–(aq) + 2 e- = 3 IIodine as reductant : 3 I- = I 3–(aq) + 2 e- Use of Starch Indicator - Starch indicator for iodine : Starch-I 6 complex (Blue color) Iodimetry(titration with I 3 -): Iodine as oxidizing agent ⇒ starch added at the beginning of the titration : end point=Appearing blue color of starch-I 6 complex Iodometry(titration of I 3 -): Iodine as reducing agent ⇒ starch should not added until immediately before the end point : end point=Disappearing blue color of starch-I 6 complex to straw yellow

Preparation and Standardization of I 3 - Solutions ① Triiodide (I 3–) is prepared by dissolving solid I 2 in excess KI. Standardization with pure sample or standard Na 2 S 2 O 3. : Acidic solution of I 3– are unstable because I– is slowly oxidized by air : In natural solution, oxidation is insignificant : At p. H≥ 12, I 3– disproportionates to HOI, IO 3– , and I– ② Triiodide(I 3 -) is prepared by dissolving a weighed quantity of primary standard potassium iodate (KIO 3) to a small excess of KI. (15 -18) Use of Sodium Thiosulfate - Sodium thiosulfate(Na 2 S 2 O 3) is standard solution of iodometry (p. H<9) (15 -19) : Dissolving Na 2 S 2 O 3 in a boiled distilled water (∵remove CO 2) - Na 2 S 2 O 3 is standardized with primary standard KIO 3 + excess of KI (15 -18) (15 -19)

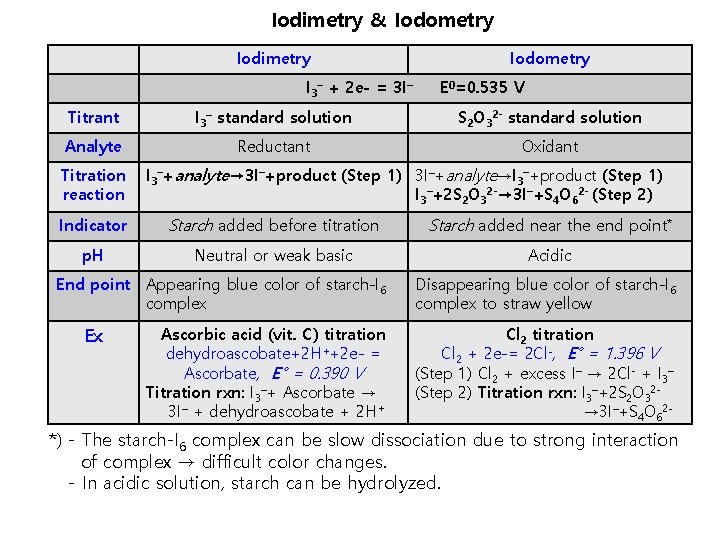
Iodimetry & Iodometry Iodimetry I 3– + 2 e- = 3 I– Iodometry E 0=0. 535 V Titrant I 3– standard solution S 2 O 32 - standard solution Analyte Reductant Oxidant Titration reaction I 3–+analyte→ 3 I–+product (Step 1) 3 I–+analyte→I 3–+product (Step 1) I 3–+2 S 2 O 32 -→ 3 I–+S 4 O 62 - (Step 2) Indicator Starch added before titration Starch added near the end point* p. H Neutral or weak basic Acidic End point Appearing blue color of starch-I 6 complex Ex Ascorbic acid (vit. C) titration dehydroascobate+2 H++2 e- = Ascorbate, E° = 0. 390 V Titration rxn: I 3–+ Ascorbate → 3 I– + dehydroascobate + 2 H+ Disappearing blue color of starch-I 6 complex to straw yellow Cl 2 titration Cl 2 + 2 e-= 2 Cl-, E° = 1. 396 V (Step 1) Cl 2 + excess I– → 2 Cl- + I 3– (Step 2) Titration rxn: I 3–+2 S 2 O 32→ 3 I–+S 4 O 62 - *) - The starch-I 6 complex can be slow dissociation due to strong interaction of complex → difficult color changes. - In acidic solution, starch can be hydrolyzed.