Aim What is titration Write the completed neutralization







- Slides: 10

Aim: What is titration? Write the completed neutralization reaction for the following reactants. 1. Carbonic acid and potassium hydroxide 2. Phosphoric acid and sodium hydroxide 3. Nitric acid and calcium hydroxide

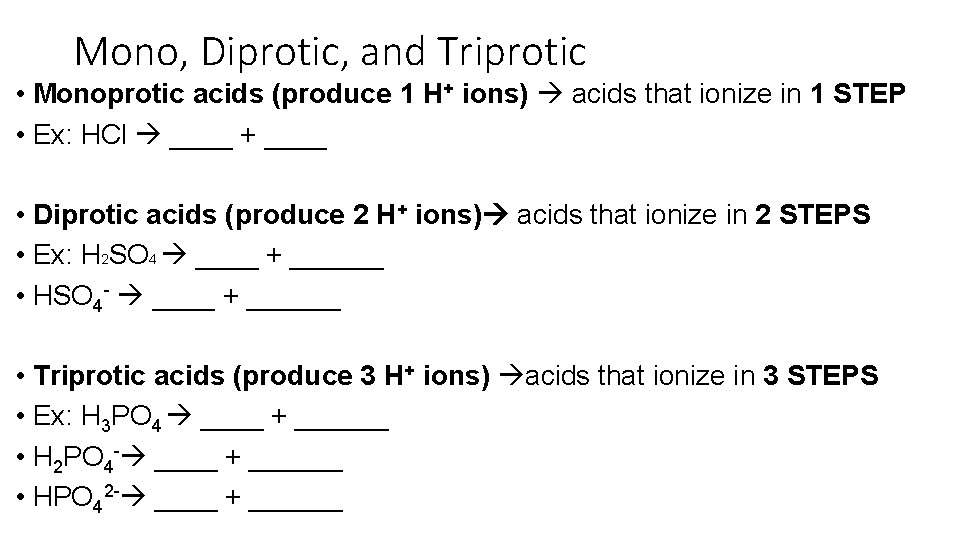
Mono, Diprotic, and Triprotic • Monoprotic acids (produce 1 H+ ions) acids that ionize in 1 STEP • Ex: HCl ____ + ____ • Diprotic acids (produce 2 H+ ions) acids that ionize in 2 STEPS • Ex: H 2 SO 4 ____ + ______ • HSO 4 - ____ + ______ • Triprotic acids (produce 3 H+ ions) acids that ionize in 3 STEPS • Ex: H 3 PO 4 ____ + ______ • H 2 PO 4 - ____ + ______ • HPO 42 - ____ + ______

• 1 M of HCl gives off 1 mole H+/liter • 2 M of HCl gives off ____ mole H+/liter • 1 M H 2 SO 4 gives off 2 mole H+/liter • 2 M H 2 SO 4 gives off ____ mole H+/liter • 1 M Na. OH gives off 1 mole OH-/liter • 2 M Na. OH gives off _____ mole OH-/liter • 1 M Ca(OH)2 gives off 2 mole OH-/liter • 2 M Ca(OH)2 gives off ____ mole OH-/liter

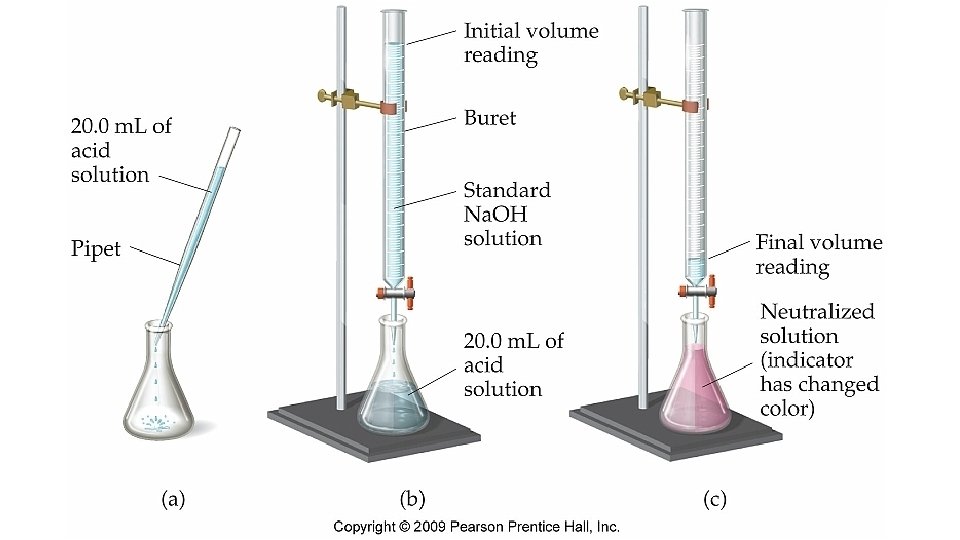
Titration: • Is used to find the molarity of an acid or a base • Is adding measured volumes of an acid or base of known molarity to a base or acid of unknown molarity until neutralization occurs (when # of H+ = # of OH-) and p. H of the solution is 7 (called equivalence point)

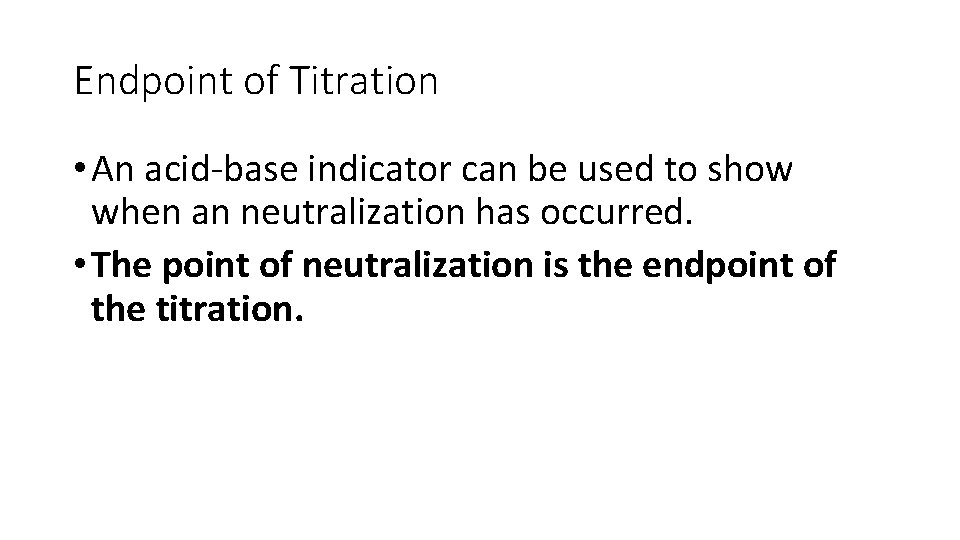
Endpoint of Titration • An acid-base indicator can be used to show when an neutralization has occurred. • The point of neutralization is the endpoint of the titration.

Titration • http: //www. mhhe. com/physsci/chemistry/animation s/chang_7 e_esp/crm 3 s 5_5. swf


Titration formula • Titration formula (Table T): MA V A = MB V B • Where: MA = molarity of acid (H+) • VA = volume of acid • MB = molarity of base (OH-) • VB = volume of base

Sample Problem 1: What is the concentration of a solution of HI if 0. 3 L is neutralized by 0. 6 L of 0. 2 M solution of KOH? Sample Problem 2: What is the concentration of a hydrochloric acid solution if 50. 0 m. L of a 0. 250 M KOH solution are needed to neutralize 20. 0 m. L of the HCl solution of unknown concentration?

Sample Problem 3: A particular acid has an H+ concentration of 0. 1 M and a volume of 100 m. L. What volume of a base with a 0. 5 M [OH-] will be required to neutralize the reaction? **Sample Problem 4: You have 50 m. L of 1. 0 M H 2 SO 4(aq). What volume of 0. 5 M Na. OH would be required to neutralize the acid? Remember Diprotic Acids yield 2 H+ ions in solution!