Do Now We have learned the concept of




















- Slides: 30

Do Now We have learned the concept of acid and base. Discuss following questions with your partner. 1. How to identify acid and base? 2. Identify (list) following as acids and bases. H 2 SO 4, CH 3 COOH, NH 4 OH, H 2 S, H 3 PO 4, Na. OH, HCl. 3. Write an equation for the ionization of an acid and for the dissociation of a base in water.

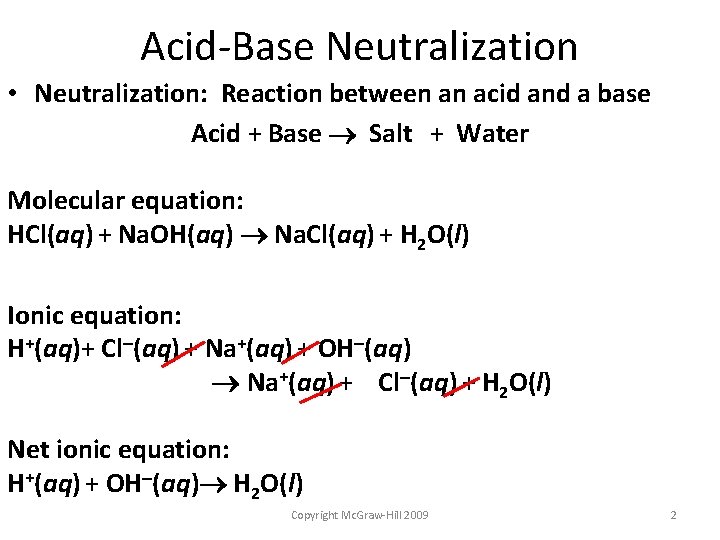
Acid-Base Neutralization • Neutralization: Reaction between an acid and a base Acid + Base Salt + Water Molecular equation: HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) Ionic equation: H+(aq)+ Cl (aq) + Na+(aq) + OH (aq) Na+(aq) + Cl (aq) + H 2 O(l) Net ionic equation: H+(aq) + OH (aq) H 2 O(l) Copyright Mc. Graw-Hill 2009 2

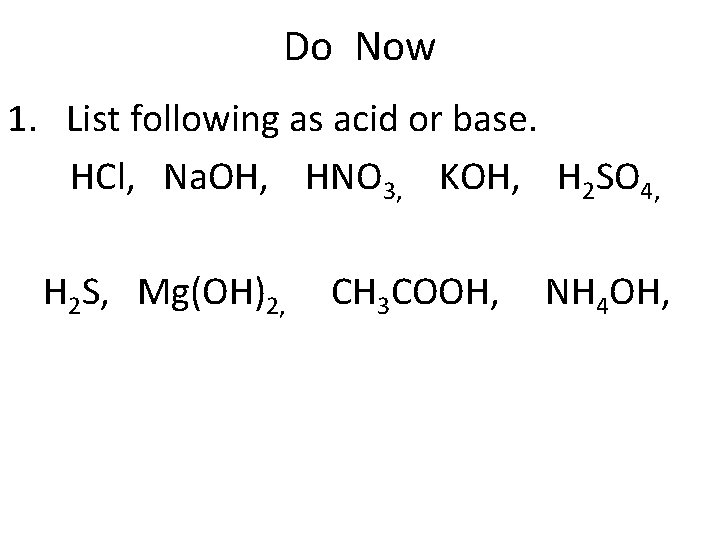
Do Now 1. List following as acid or base. HCl, Na. OH, HNO 3, KOH, H 2 SO 4, H 2 S, Mg(OH)2, CH 3 COOH, NH 4 OH,

Activity 1. Students sit in pre-determined group. 2. Each student writes one paragraph that compare and contrast the acid properties with base properties. 3. The teacher will demonstrate the properties of acid and base while students read their writing to the class.

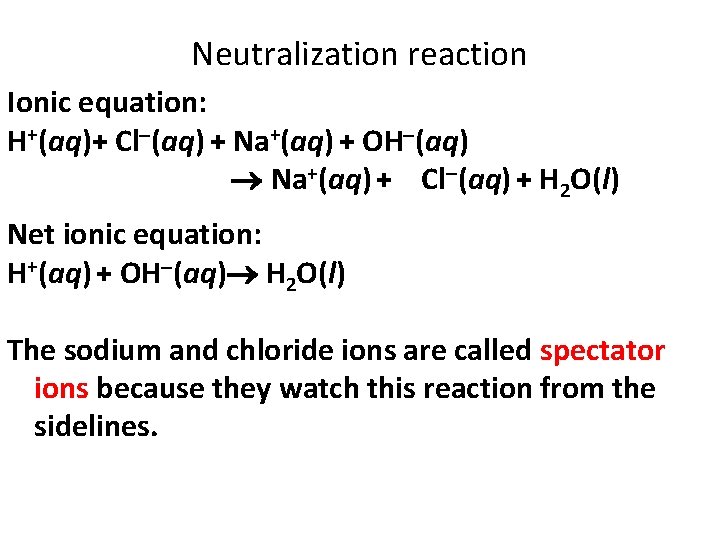
Neutralization reaction Ionic equation: H+(aq)+ Cl (aq) + Na+(aq) + OH (aq) Na+(aq) + Cl (aq) + H 2 O(l) Net ionic equation: H+(aq) + OH (aq) H 2 O(l) The sodium and chloride ions are called spectator ions because they watch this reaction from the sidelines.

Writing neutralization equations When acids and bases are mixed, a salt forms Na. OH + HCl H 2 O + Na. Cl base + acid water + salt Ca(OH)2 + H 2 SO 4 2 H 2 O + Ca. SO 4

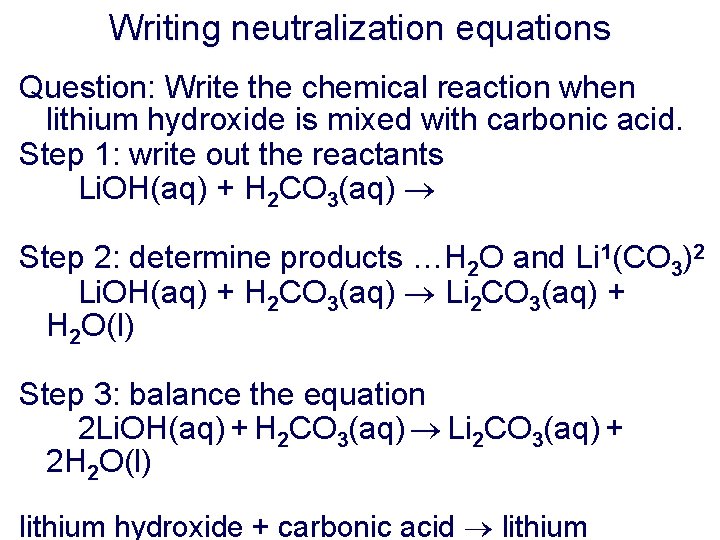
Writing neutralization equations Question: Write the chemical reaction when lithium hydroxide is mixed with carbonic acid. Step 1: write out the reactants Li. OH(aq) + H 2 CO 3(aq) Step 2: determine products …H 2 O and Li 1(CO 3)2 Li. OH(aq) + H 2 CO 3(aq) Li 2 CO 3(aq) + H 2 O(l) Step 3: balance the equation 2 Li. OH(aq) + H 2 CO 3(aq) Li 2 CO 3(aq) + 2 H 2 O(l) lithium hydroxide + carbonic acid lithium

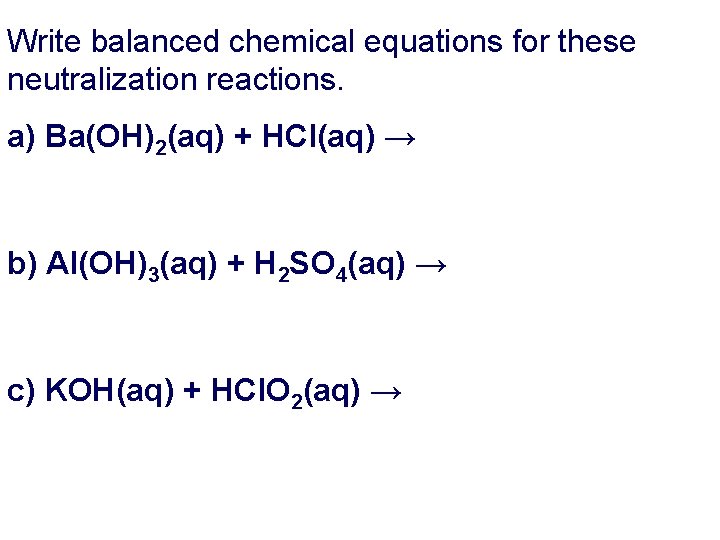
Write balanced chemical equations for these neutralization reactions. a) Ba(OH)2(aq) + HCl(aq) → b) Al(OH)3(aq) + H 2 SO 4(aq) → c) KOH(aq) + HCl. O 2(aq) →

Write balanced chemical equations for these neutralization reactions. d) iron(II) hydroxide + phosphoric acid e) calcium hydroxide + nitric acid f) ammonium hydroxide + hydrosulfuric acid

a) 3 Fe(OH)2(aq) + 2 H 3 PO 4(aq) Fe 3(PO 4)2(aq) + 6 H 2 O(l) iron(II) hydroxide + phosphoric acid iron (II) phosphate b) Ba(OH)2(aq) + 2 HCl(aq) Ba. Cl 2 (aq) + 2 H 2 O(l) barium hydroxide + hydrochloric acid barium chloride c) Ca(OH)2(aq) + 2 HNO 3(aq) Ca(NO 3)2(aq) + 2 H 2 O(l) calcium hydroxide + nitric acid calcium nitrate d) 2 Al(OH)3(aq) + 3 H 2 SO 4(aq) Al 2(SO 4)3(aq) + 6 H 2 O(l) aluminum hydroxide + sulfuric acid aluminum sulfate


Do Now Pure water is actually a mixture of water molecules, hydronium ions, and hydroxide ions. 2 H 2 O → H 3 O+ + OHHow does the concentration of hydroxide ions compare to the concentration of hydronium ions in pure water?

Neutralization Formula H+(aq) + OH (aq) H 2 O(l) the number of H+ = the number of OH 6. 02 x 1023 H+ = 6. 02 x 1023 OHthe mole of H+ = the mole of OHRecall the Molarity (M) = mole/volume, So mole = M x V Ma Va =Mb Vb

Methods of Solving Neutralizaion Problems: using the formula a. Ma. Va=b. Mb. Vb. #H+ x MA x VA = #OH- x MB x VB Sample problem 1: Determine the concentration of H 3 PO 4 if a 90 m. L sample is neutralized by 30 m. L of 0. 9 M Ca(OH)2.

Sample Problem 2: How much 3. 0 M H 2 SO 4 is needed to neutralize 50 m. L of 1. 2 M Al(OH)3?

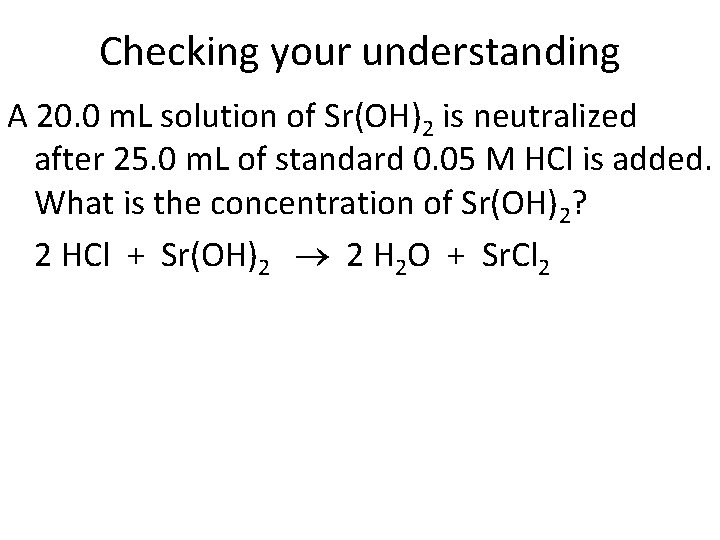
Checking your understanding A 20. 0 m. L solution of Sr(OH)2 is neutralized after 25. 0 m. L of standard 0. 05 M HCl is added. What is the concentration of Sr(OH)2? 2 HCl + Sr(OH)2 2 H 2 O + Sr. Cl 2

Check your understanding 1. What is the concentration of HCl if 30. 0 m. L of 0. 10 M Na. OH neutralizes 50. 0 m. L HCl? Na. OH + HCl H 2 O + Na. Cl 2. How many moles of HCl were used? Hint: #moles= Ma. Va , but convert the volume to L( 50 m. L=0. 05 L).


Titration: • A laboratory method for determining the concentration of an unknown acid or base using a neutralization reaction. • A standard solution, (a solution of known concentration), is used.

Buret Valve

Titration Acid with Phenolpthalein End-Point

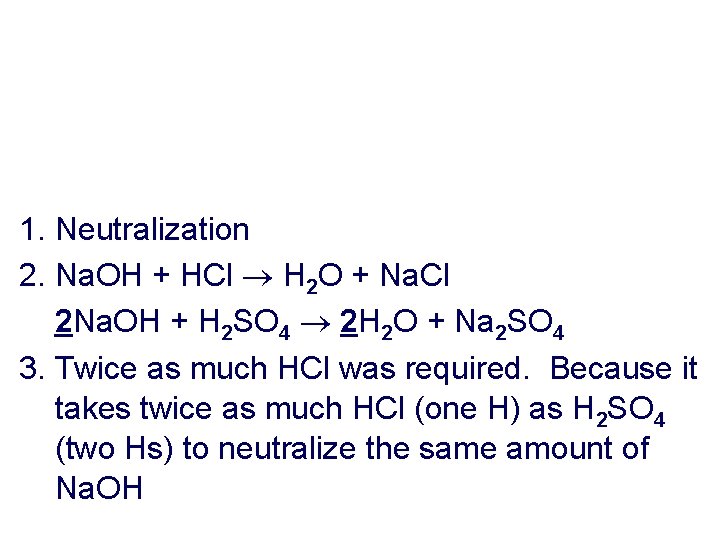
1. Neutralization 2. Na. OH + HCl H 2 O + Na. Cl 2 Na. OH + H 2 SO 4 2 H 2 O + Na 2 SO 4 3. Twice as much HCl was required. Because it takes twice as much HCl (one H) as H 2 SO 4 (two Hs) to neutralize the same amount of Na. OH

Equivalence Point • The point at which there are stoichiometrically equivalent amounts of acid and base. • [H+] = [OH-]

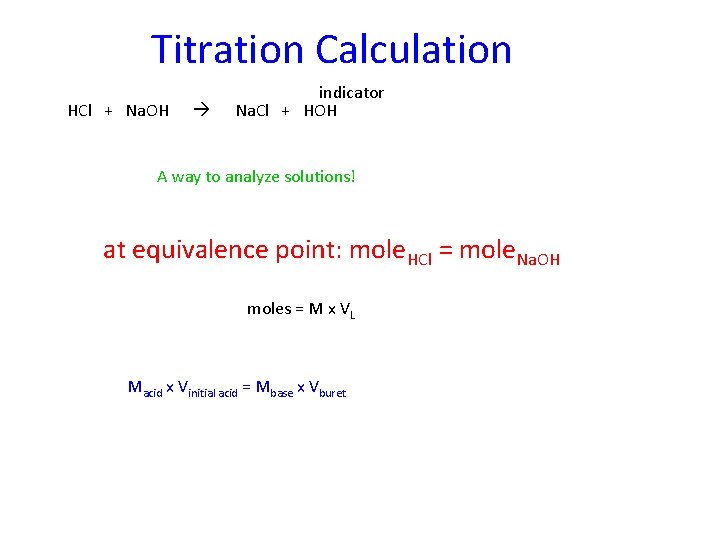
Titration Calculation HCl + Na. OH indicator Na. Cl + HOH A way to analyze solutions! at equivalence point: mole. HCl = mole. Na. OH moles = M x VL Macid x Vinitial acid = Mbase x Vburet


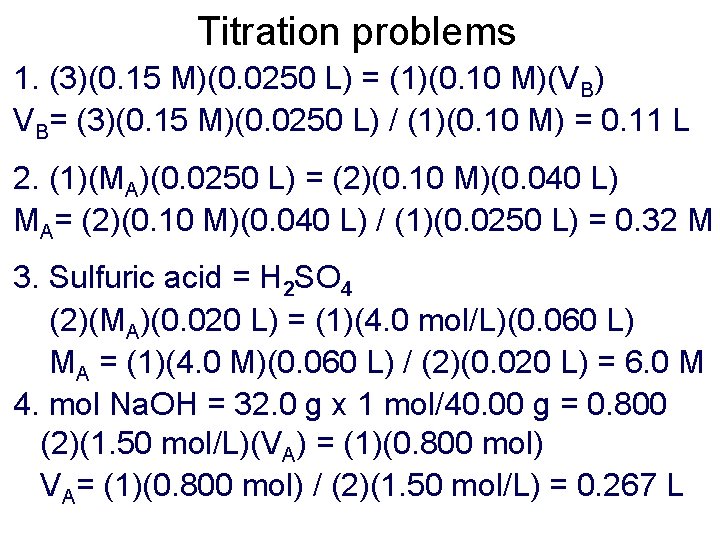
Titration problems 1. What volume of 0. 10 mol/L Na. OH is needed to neutralize 25. 0 m. L of 0. 15 mol/L H 3 PO 4? 2. 25. 0 m. L of HCl(aq) was neutralized by 40. 0 m. L of 0. 10 mol/L Ca(OH)2 solution. What was the concentration of HCl?

3. A truck carrying sulfuric acid is in an accident. A laboratory analyzes a sample of the spilled acid and finds that 20 m. L of acid is neutral-ized by 60 m. L of 4. 0 mol/L Na. OH solution. What is the concentration of the acid? 4. What volume of 1. 50 mol/L H 2 S will neutralize a solution containing 32. 0 g Na. OH?

Titration problems 1. (3)(0. 15 M)(0. 0250 L) = (1)(0. 10 M)(VB) VB= (3)(0. 15 M)(0. 0250 L) / (1)(0. 10 M) = 0. 11 L 2. (1)(MA)(0. 0250 L) = (2)(0. 10 M)(0. 040 L) MA= (2)(0. 10 M)(0. 040 L) / (1)(0. 0250 L) = 0. 32 M 3. Sulfuric acid = H 2 SO 4 (2)(MA)(0. 020 L) = (1)(4. 0 mol/L)(0. 060 L) MA = (1)(4. 0 M)(0. 060 L) / (2)(0. 020 L) = 6. 0 M 4. mol Na. OH = 32. 0 g x 1 mol/40. 00 g = 0. 800 (2)(1. 50 mol/L)(VA) = (1)(0. 800 mol) VA= (1)(0. 800 mol) / (2)(1. 50 mol/L) = 0. 267 L

Ex. 5 • How many m. L of 0. 20 M H 3 PO 4 are needed to neutralize 55. 0 m. L of a 0. 10 M solution of Na. OH?

Ex. 6 • What volume of 0. 20 M Ca(OH)2 will neutralize 45. 0 m. L of a 1 M solution of HCl. O 3?