Ch 6 Good Titrations a A t T







- Slides: 27

Ch 6: Good Titrations

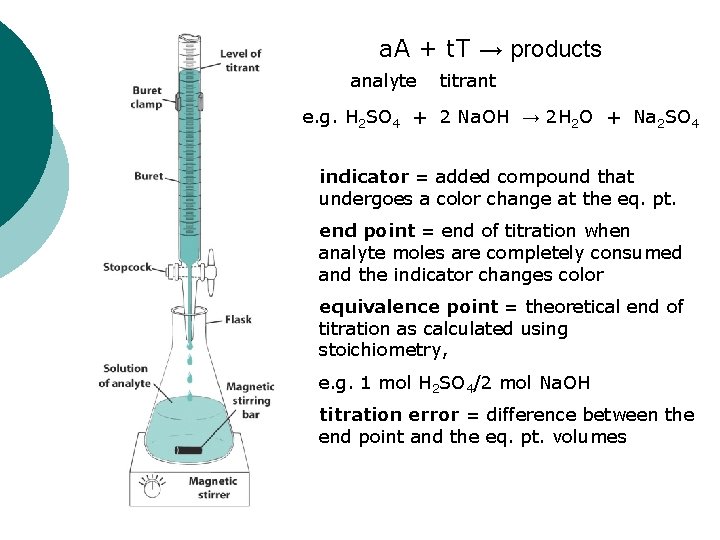
a. A + t. T → products analyte titrant e. g. H 2 SO 4 + 2 Na. OH → 2 H 2 O + Na 2 SO 4 indicator = added compound that undergoes a color change at the eq. pt. end point = end of titration when analyte moles are completely consumed and the indicator changes color equivalence point = theoretical end of titration as calculated using stoichiometry, e. g. 1 mol H 2 SO 4/2 mol Na. OH titration error = difference between the end point and the eq. pt. volumes

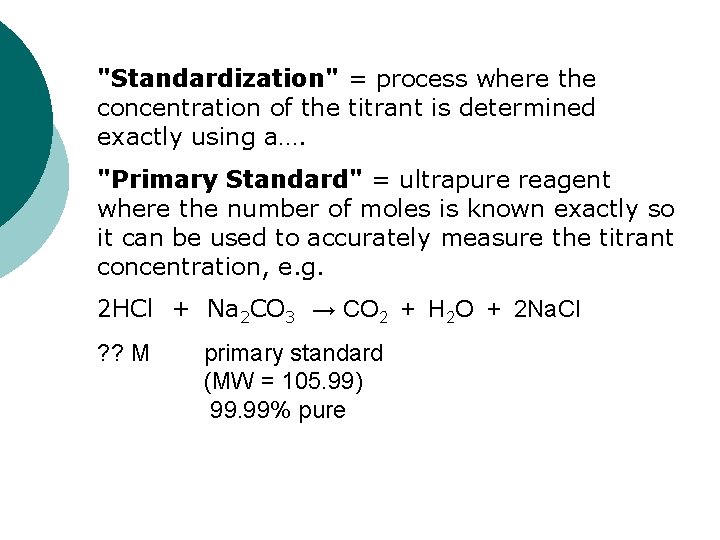
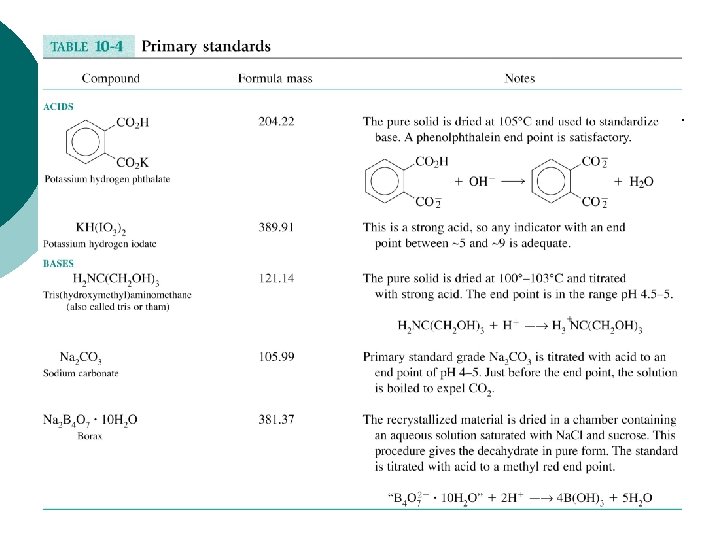
"Standardization" = process where the concentration of the titrant is determined exactly using a…. "Primary Standard" = ultrapure reagent where the number of moles is known exactly so it can be used to accurately measure the titrant concentration, e. g. 2 HCl + Na 2 CO 3 → CO 2 + H 2 O + 2 Na. Cl ? ? M primary standard (MW = 105. 99) 99. 99% pure


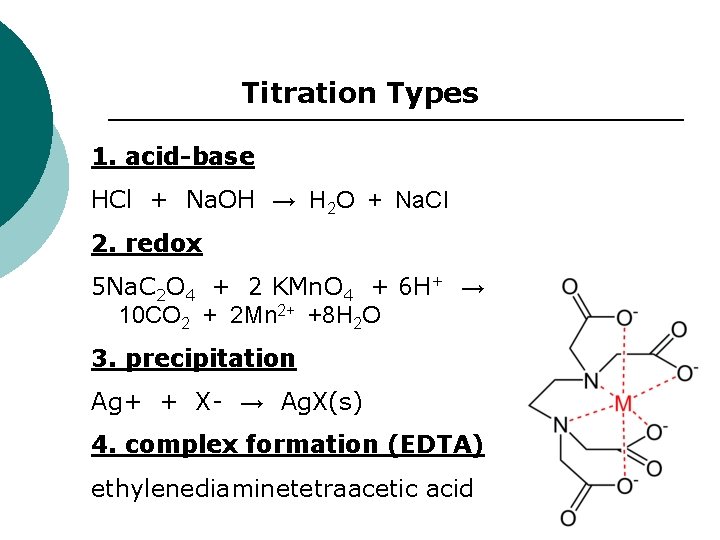
Titration Types 1. acid-base HCl + Na. OH → H 2 O + Na. Cl 2. redox 5 Na. C 2 O 4 + 2 KMn. O 4 + 6 H+ → 10 CO 2 + 2 Mn 2+ +8 H 2 O 3. precipitation Ag+ + X- → Ag. X(s) 4. complex formation (EDTA) ethylenediaminetetraacetic acid

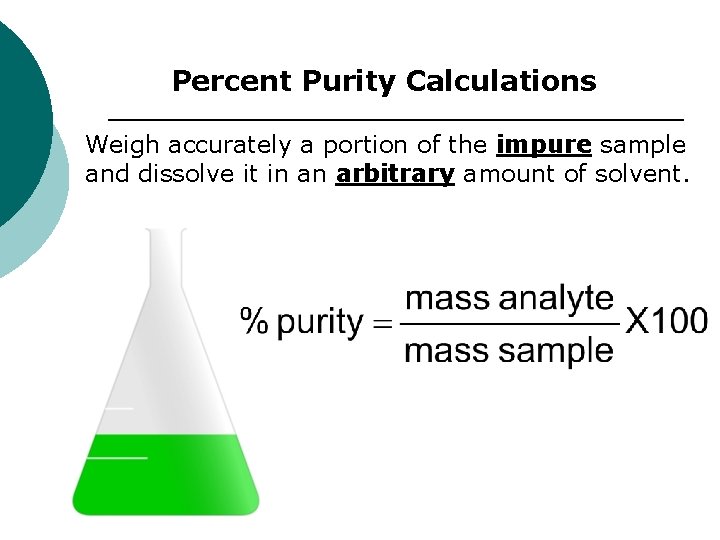
Percent Purity Calculations Weigh accurately a portion of the impure sample and dissolve it in an arbitrary amount of solvent.

Lab #2 %Purity Calculation A sample of impure KHP weighing 2. 1283 g required 42. 58 m. L of a 0. 1084 M Na. OH solution for titration to the phenolphthalein end point. Calculate the %purity of the sample.

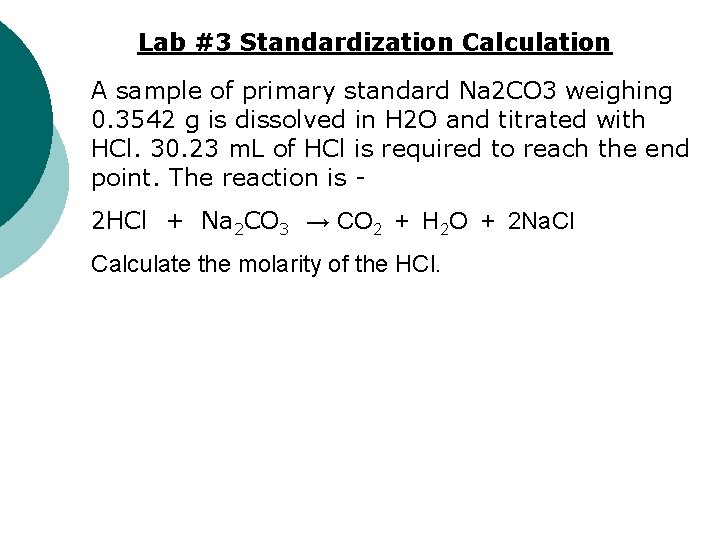
Lab #3 Standardization Calculation A sample of primary standard Na 2 CO 3 weighing 0. 3542 g is dissolved in H 2 O and titrated with HCl. 30. 23 m. L of HCl is required to reach the end point. The reaction is 2 HCl + Na 2 CO 3 → CO 2 + H 2 O + 2 Na. Cl Calculate the molarity of the HCl.

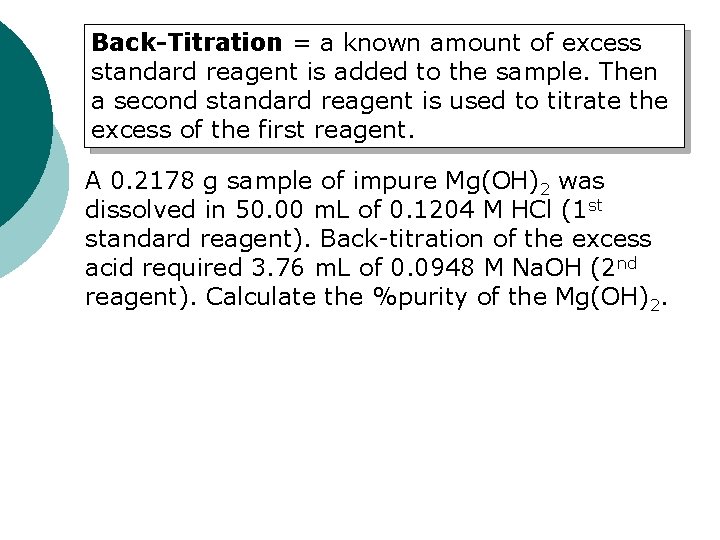
Back-Titration = a known amount of excess standard reagent is added to the sample. Then a second standard reagent is used to titrate the excess of the first reagent. A 0. 2178 g sample of impure Mg(OH)2 was dissolved in 50. 00 m. L of 0. 1204 M HCl (1 st standard reagent). Back-titration of the excess acid required 3. 76 m. L of 0. 0948 M Na. OH (2 nd reagent). Calculate the %purity of the Mg(OH)2.

Ch 7: Gravimetric and Combustion Analysis

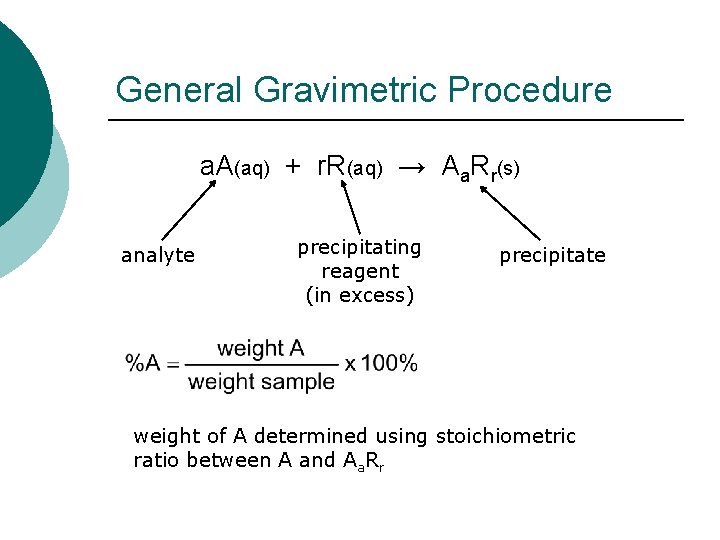
General Gravimetric Procedure a. A(aq) + r. R(aq) → Aa. Rr(s) analyte precipitating reagent (in excess) precipitate weight of A determined using stoichiometric ratio between A and Aa. Rr

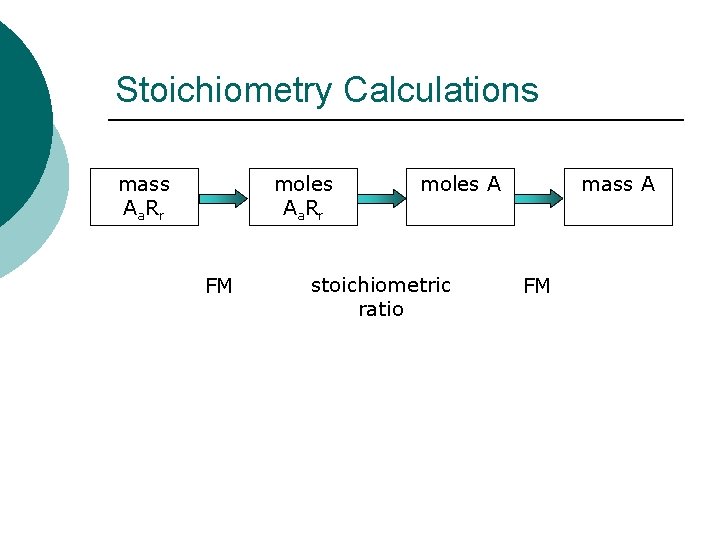
Stoichiometry Calculations mass Aa. Rr moles Aa. Rr FM moles A stoichiometric ratio mass A FM

EXAMPLE, p. 145: A Simple Gravimetric Calculation A 10. 00 -m. L solution containing Cl- was treated with excess Ag. NO 3 to precipitate 0. 4368 -g of Ag. Cl (FM = 143. 321). What was the molarity of Cl- in the unknown?


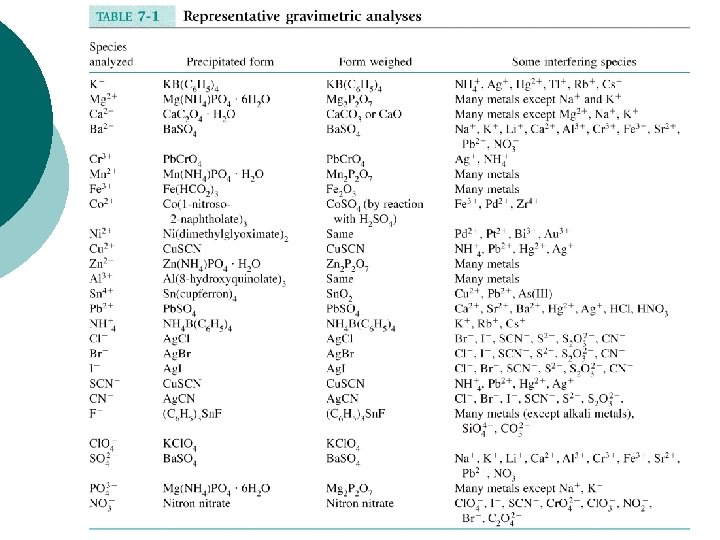
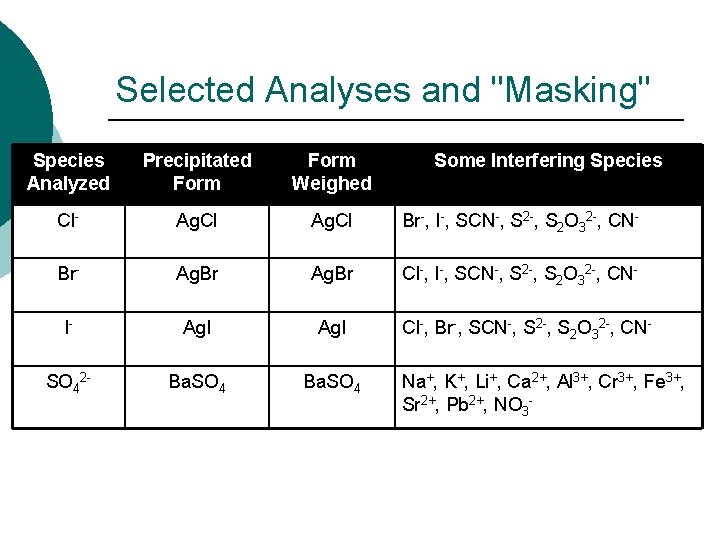
Selected Analyses and "Masking" Species Analyzed Precipitated Form Weighed Some Interfering Species Cl- Ag. Cl Br-, I-, SCN-, S 2 O 32 -, CN- Br- Ag. Br Cl-, I-, SCN-, S 2 O 32 -, CN- I- Ag. I SO 42 - Ba. SO 4 Cl-, Br-, SCN-, S 2 O 32 -, CNNa+, K+, Li+, Ca 2+, Al 3+, Cr 3+, Fe 3+, Sr 2+, Pb 2+, NO 3 -

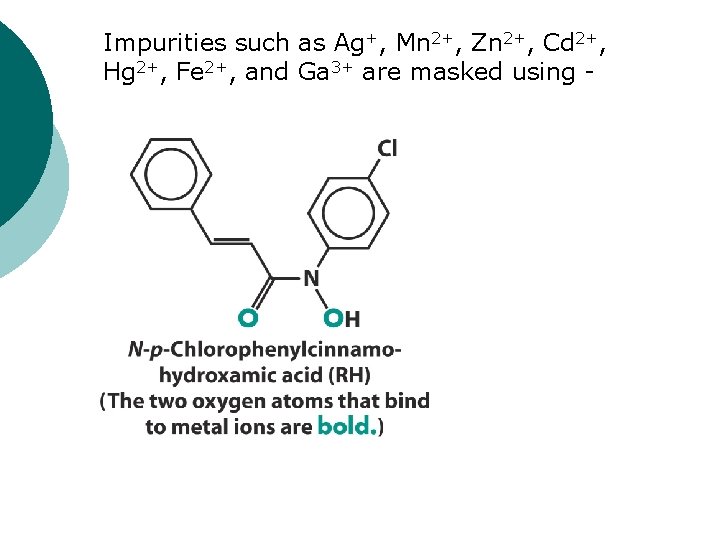
Impurities such as Ag+, Mn 2+, Zn 2+, Cd 2+, Hg 2+, Fe 2+, and Ga 3+ are masked using -



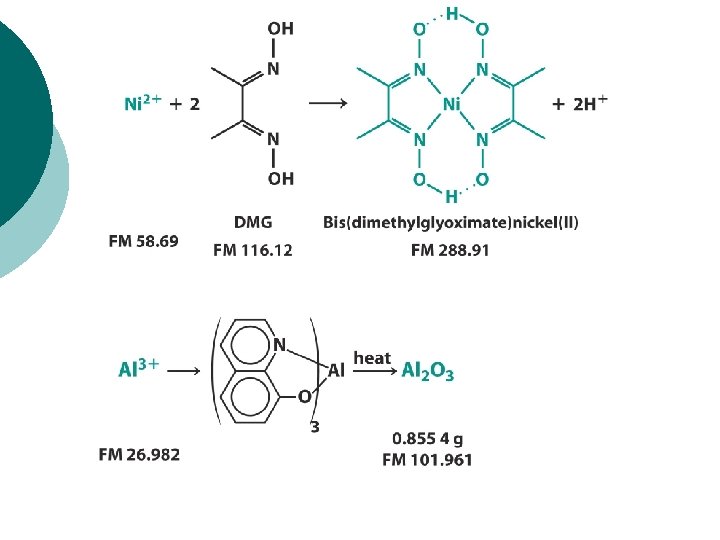
EXAMPLE, p. 156: When the Stoichiometry is Not 1: 1 Solid residue weighing 8. 4448 -g from an aluminum refining process was dissolved in acid, treated with 8 hydroxyquinoline, and ignited to give Al 2 O 3 weighing 0. 8554 -g. Find the weight precent of Al in the original mixture.

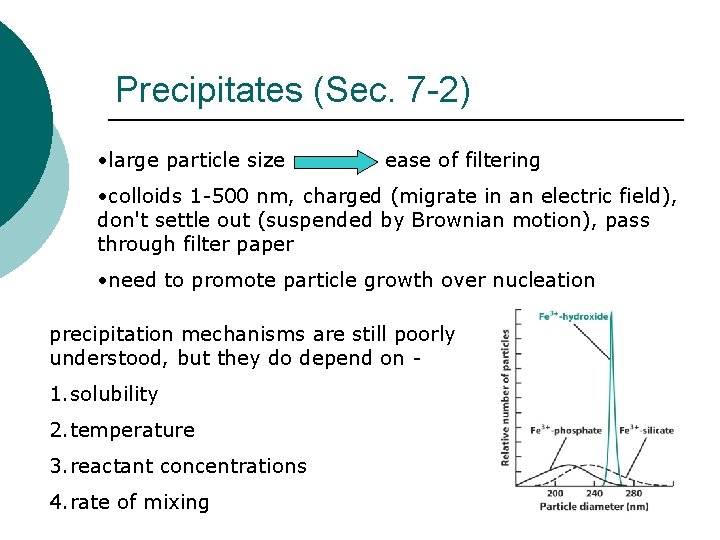
Precipitates (Sec. 7 -2) • large particle size ease of filtering • colloids 1 -500 nm, charged (migrate in an electric field), don't settle out (suspended by Brownian motion), pass through filter paper • need to promote particle growth over nucleation precipitation mechanisms are still poorly understood, but they do depend on - 1. solubility 2. temperature 3. reactant concentrations 4. rate of mixing

Relative Supersaturation (RS) • Two competing mechanisms: nucleation vs. particle growth • supersaturated solutions result in nucleation (the formation of many small colloids) Q = supersaturated concentration S = equilibrium concentration Q RS S 0 2 S 1 3 S 2

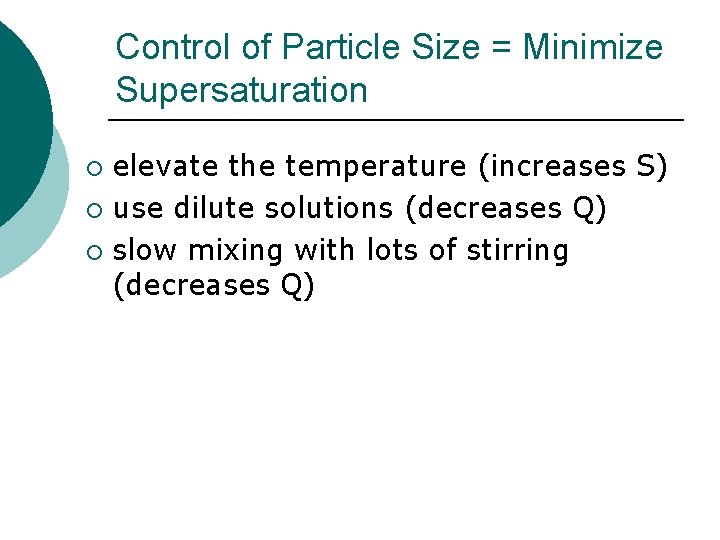
Control of Particle Size = Minimize Supersaturation elevate the temperature (increases S) ¡ use dilute solutions (decreases Q) ¡ slow mixing with lots of stirring (decreases Q) ¡

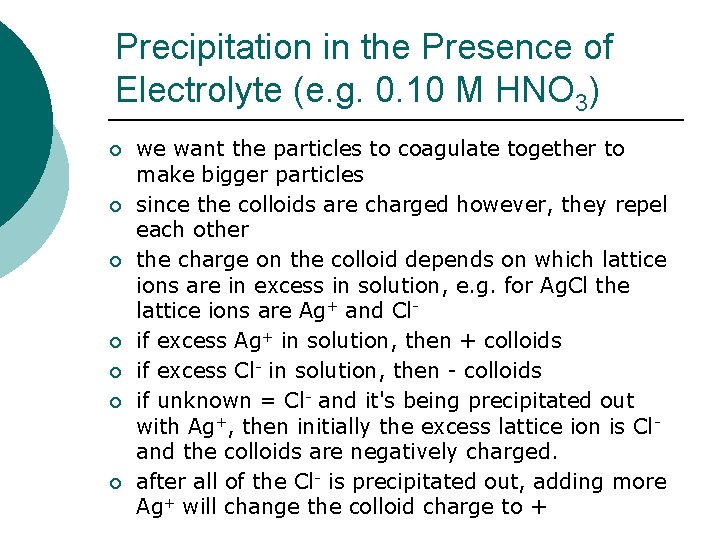
Precipitation in the Presence of Electrolyte (e. g. 0. 10 M HNO 3) ¡ ¡ ¡ ¡ we want the particles to coagulate together to make bigger particles since the colloids are charged however, they repel each other the charge on the colloid depends on which lattice ions are in excess in solution, e. g. for Ag. Cl the lattice ions are Ag+ and Clif excess Ag+ in solution, then + colloids if excess Cl- in solution, then - colloids if unknown = Cl- and it's being precipitated out with Ag+, then initially the excess lattice ion is Cland the colloids are negatively charged. after all of the Cl- is precipitated out, adding more Ag+ will change the colloid charge to +

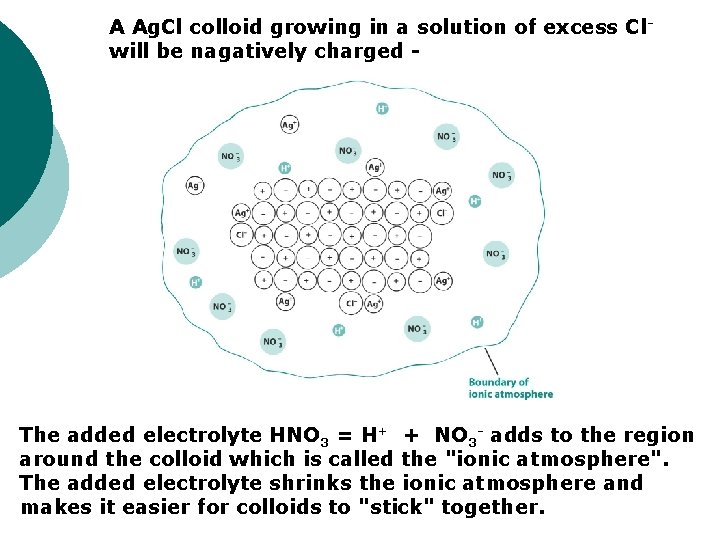
A Ag. Cl colloid growing in a solution of excess Clwill be nagatively charged - The added electrolyte HNO 3 = H+ + NO 3 - adds to the region around the colloid which is called the "ionic atmosphere". The added electrolyte shrinks the ionic atmosphere and makes it easier for colloids to "stick" together.

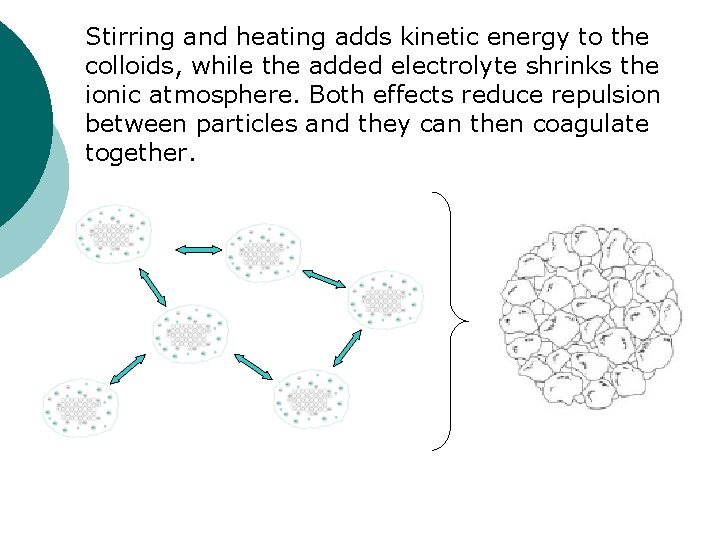
Stirring and heating adds kinetic energy to the colloids, while the added electrolyte shrinks the ionic atmosphere. Both effects reduce repulsion between particles and they can then coagulate together.

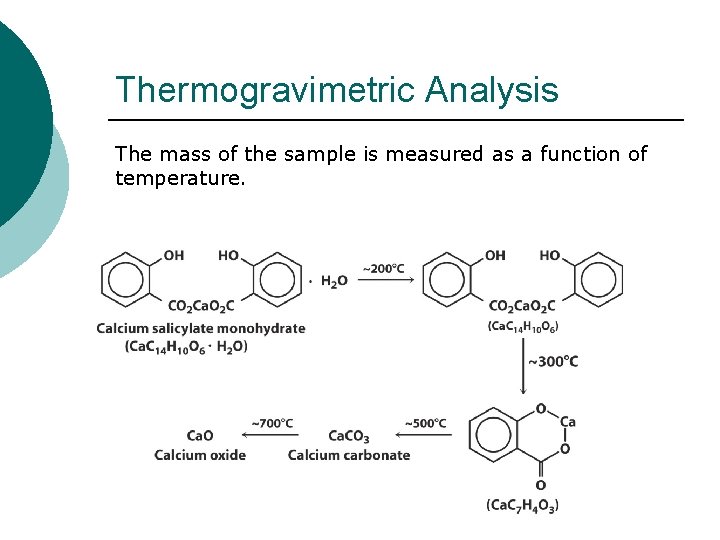
Thermogravimetric Analysis The mass of the sample is measured as a function of temperature.

For this gravimetric analysis, the precipitate must be heated to at least 800 o. C in order to drive off all the water, volatilize the HNO 3, and reduce the sample to Ca. O. = "heating to constant weight"