CHAPTER 15 Cell Signaling and Signal Transduction Communication

![The variety of processes that can be affected by changes in [c. AMP] The variety of processes that can be affected by changes in [c. AMP]](https://slidetodoc.com/presentation_image_h2/998598966cb211069085c8fd12ff6914/image-33.jpg)








- Slides: 87

CHAPTER 15 Cell Signaling and Signal Transduction: Communication Between Cells

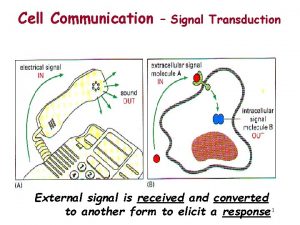
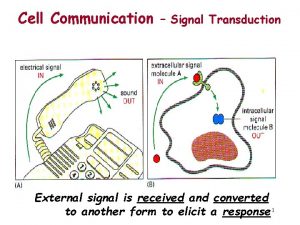
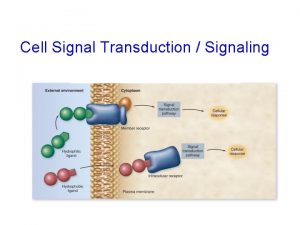
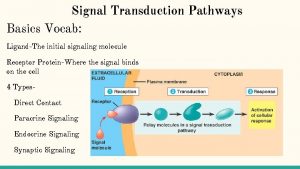
Introduction • Cells must respond adequately to external stimuli to survive. • Cells respond to stimuli via cell signaling. • Some signal molecules enter cells; others bind to cell-surface receptors.

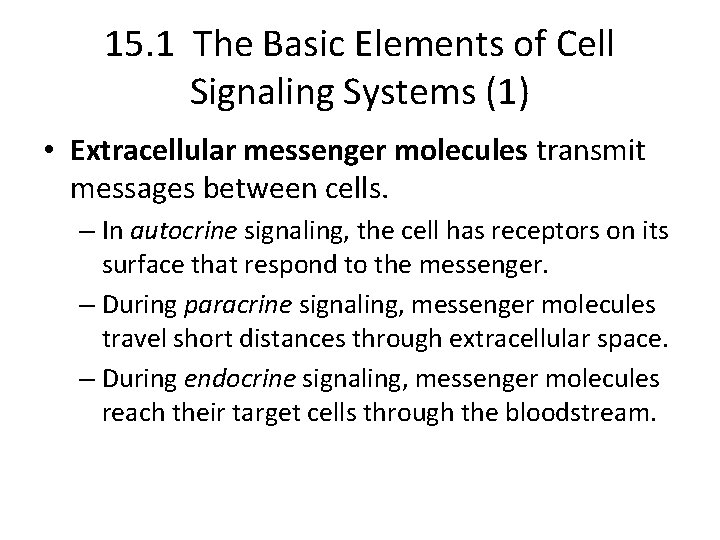
15. 1 The Basic Elements of Cell Signaling Systems (1) • Extracellular messenger molecules transmit messages between cells. – In autocrine signaling, the cell has receptors on its surface that respond to the messenger. – During paracrine signaling, messenger molecules travel short distances through extracellular space. – During endocrine signaling, messenger molecules reach their target cells through the bloodstream.

Types of intercelular signaling

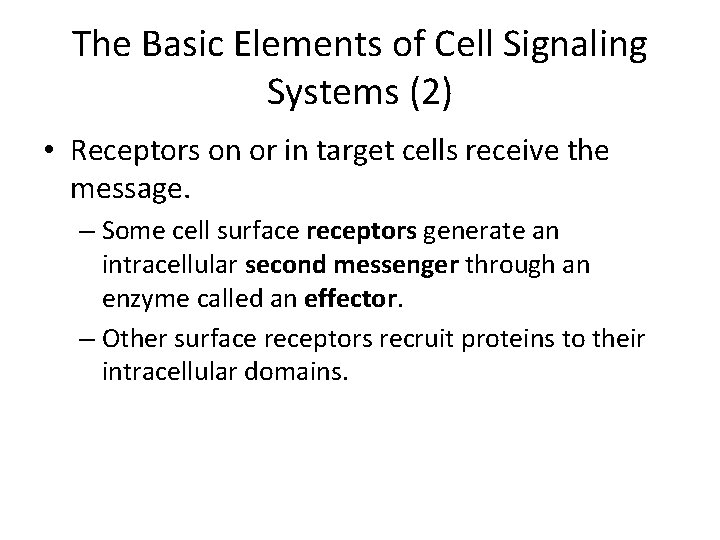
The Basic Elements of Cell Signaling Systems (2) • Receptors on or in target cells receive the message. – Some cell surface receptors generate an intracellular second messenger through an enzyme called an effector. – Other surface receptors recruit proteins to their intracellular domains.

Overview of signaling pathways

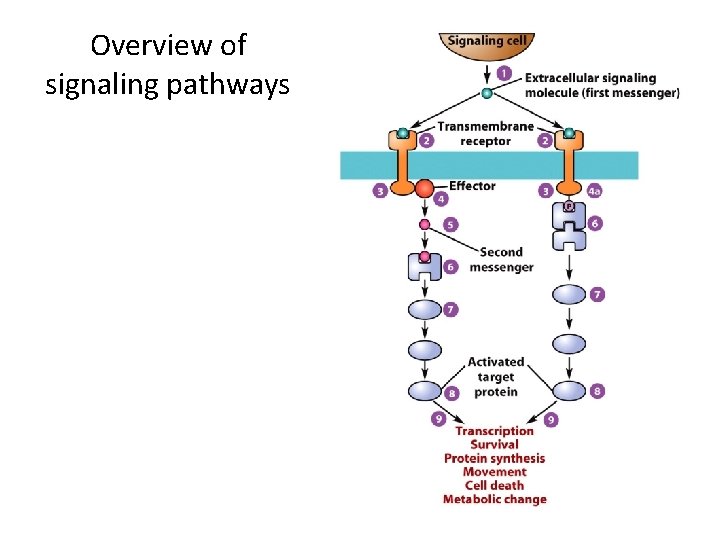
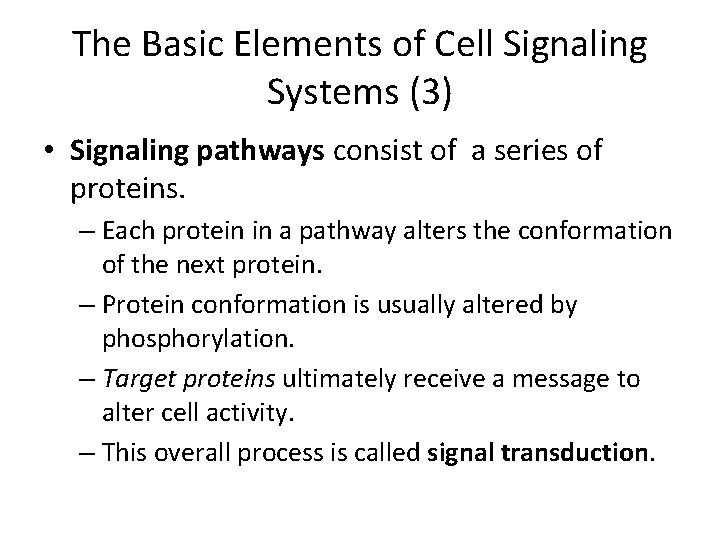
The Basic Elements of Cell Signaling Systems (3) • Signaling pathways consist of a series of proteins. – Each protein in a pathway alters the conformation of the next protein. – Protein conformation is usually altered by phosphorylation. – Target proteins ultimately receive a message to alter cell activity. – This overall process is called signal transduction.

A signal transduction pathway

15. 2 A Survey of Extracellular Messengers and Their Receptors (1) • Extracellular messengers include: – Small molecules such as amino acids and their derivatives. – Gases such as NO and CO – Steroids – Eicosanoids, which are lipids derived from fatty acids. – Various peptides and proteins

A Survey of Extracellular Messengers and Their Receptors (2) • Receptor types include: – G-protein coupled receptors (GPCRs) – Receptor protein-tyrosine kinases (RTKs) – Ligand gated channels – Steroid hormone receptors – Specific receptors such as B-and T-cell receptors

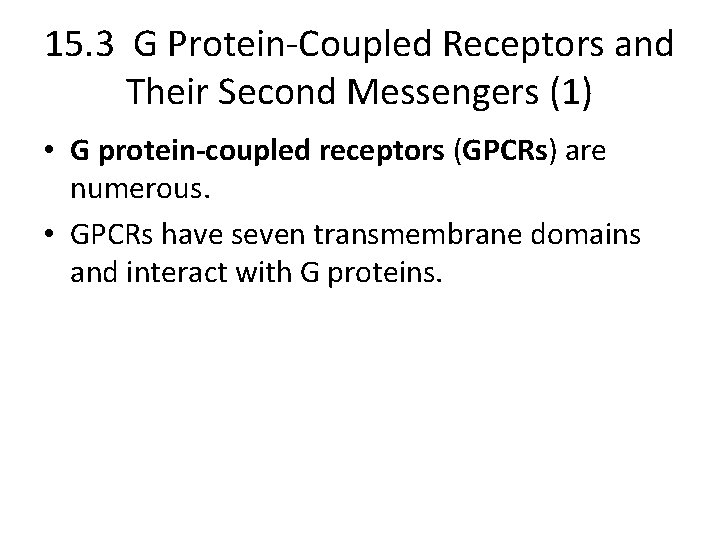
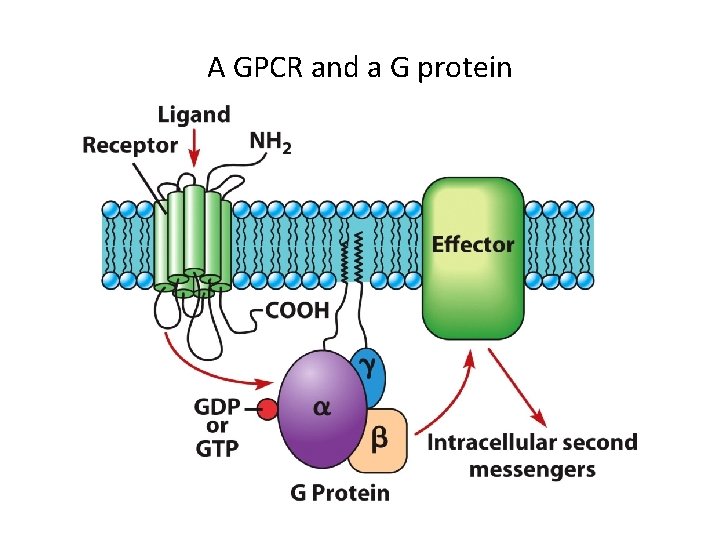
15. 3 G Protein-Coupled Receptors and Their Second Messengers (1) • G protein-coupled receptors (GPCRs) are numerous. • GPCRs have seven transmembrane domains and interact with G proteins.

A GPCR and a G protein

A GPCR and a G protein

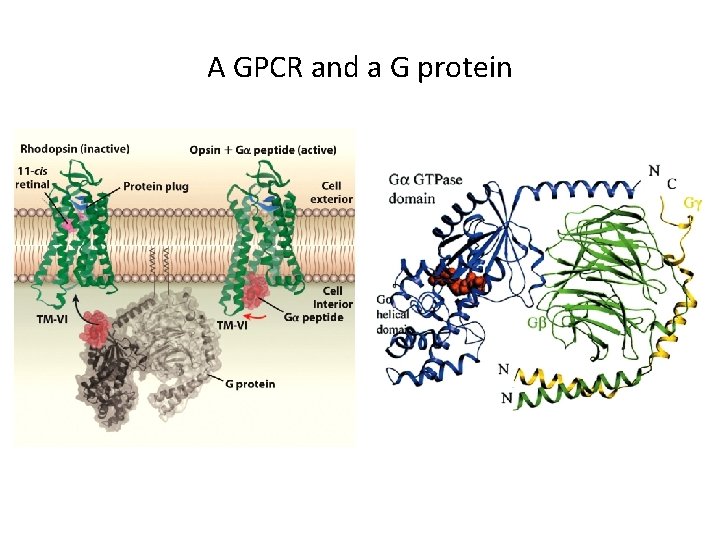
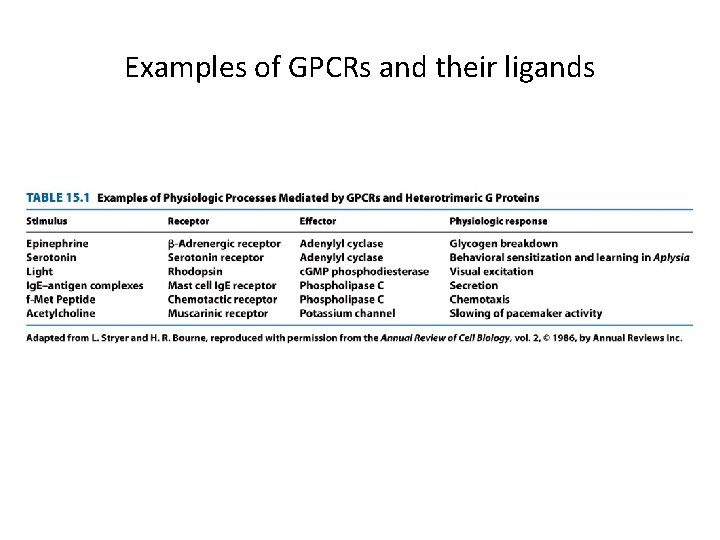
Examples of GPCRs and their ligands

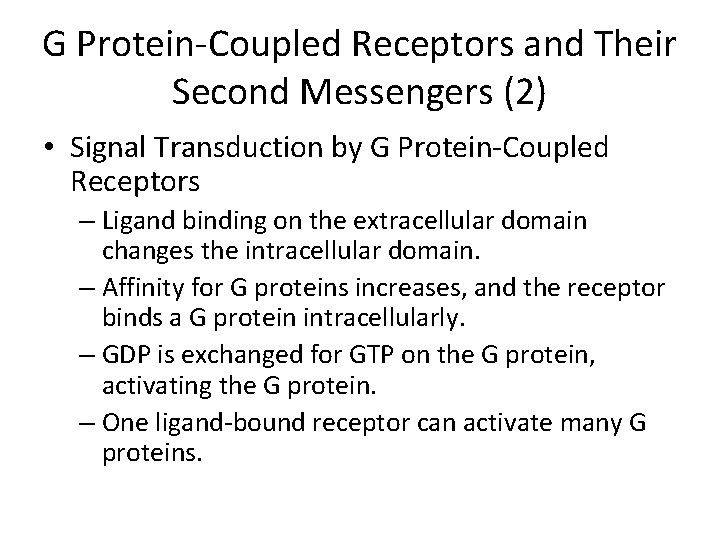
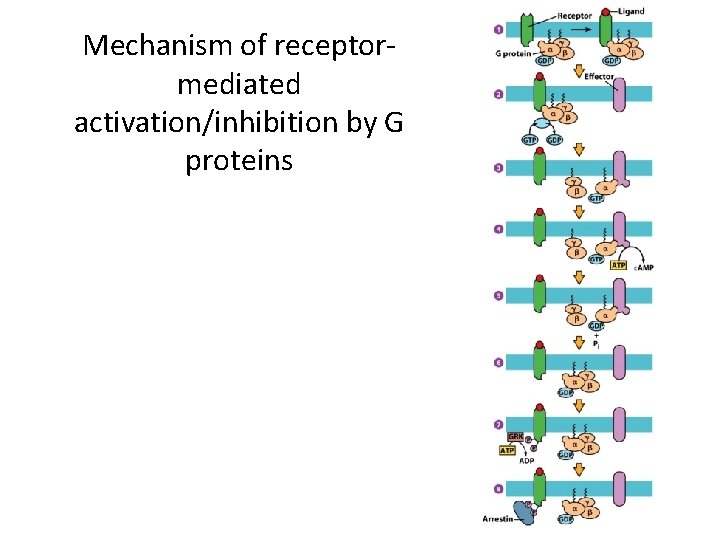
G Protein-Coupled Receptors and Their Second Messengers (2) • Signal Transduction by G Protein-Coupled Receptors – Ligand binding on the extracellular domain changes the intracellular domain. – Affinity for G proteins increases, and the receptor binds a G protein intracellularly. – GDP is exchanged for GTP on the G protein, activating the G protein. – One ligand-bound receptor can activate many G proteins.

Mechanism of receptormediated activation/inhibition by G proteins

G Protein-Coupled Receptors and Their Second Messengers (3) • Termination of the Response – Desensitization – by blocking active receptors from turning on additional G proteins. – G protein-coupled receptor kinase (GRK) activates a GPCR via phosphorylation. – Proteins called arrestins compete with G proteins to bind GPCRs. – Termination of the response is accelerated by regulators of G protein signaling (RGSs).

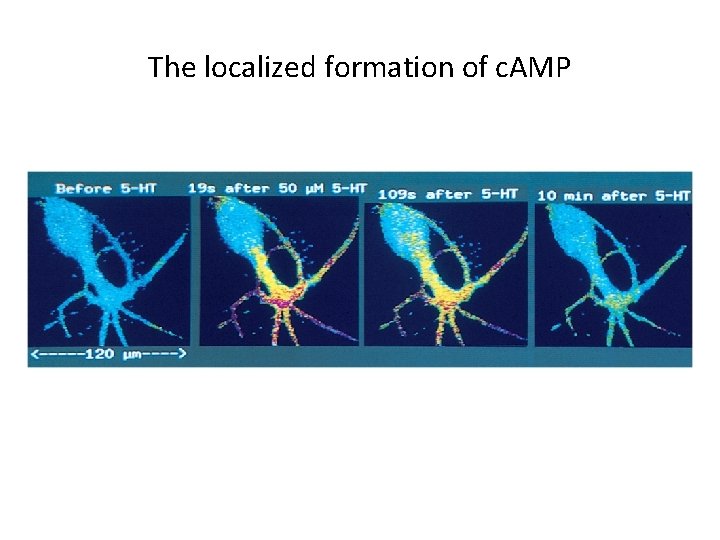
G Protein-Coupled Receptors and Their Second Messengers (4) • Bacterial Toxins, such as cholera toxin and pertussis virulence factors, target GPCRs and G proteins. • Second Messengers – The Discovery of Cyclic AMP • It is a second messenger, which is released into the cytoplasm after binding of a ligand. • Second messengers amplify the response to a single extracellular ligand.

The localized formation of c. AMP

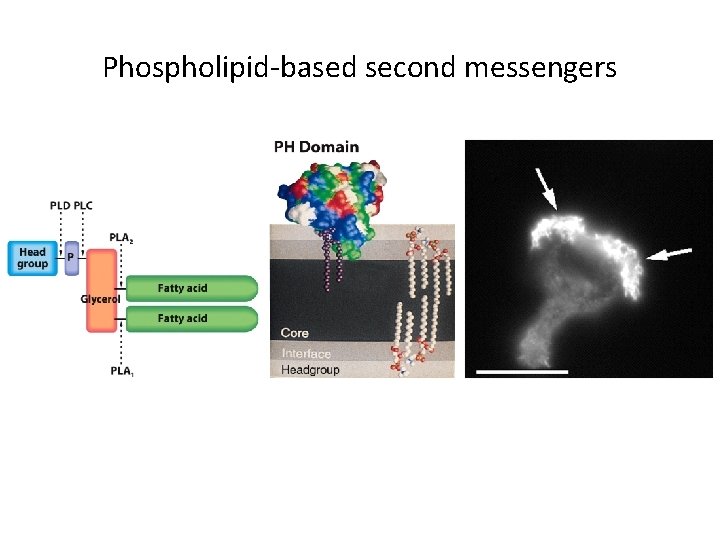
G Protein-Coupled Receptors and Their Second Messengers (5) • Phosphatidylinositol-Derived Second Messengers – Some phospholipids of cell membranes are converted into second messengers by activated phospholipases. • Phosphatidylinositol Phosphorylation – Phosphoinositides (PI) are derivatives of phosphatodylinositol.

Phospholipid-based second messengers

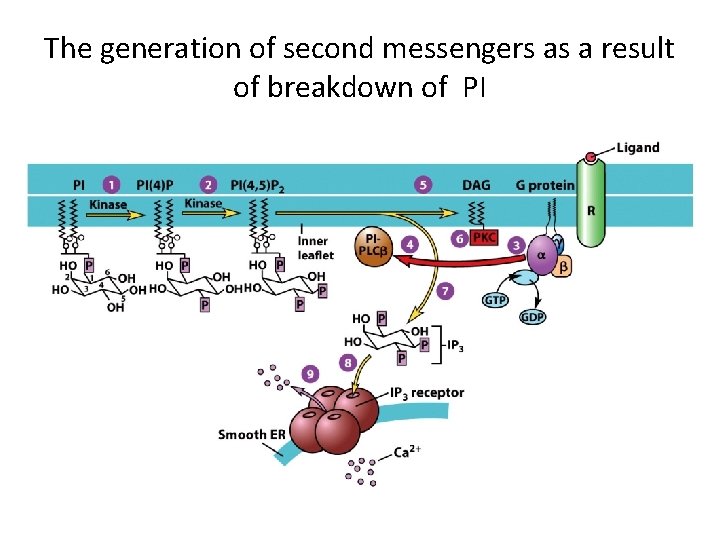
G Protein-Coupled Receptors and Their Second Messengers (6) • Phosphatidylinositol-specific phospholipase C produces second messengers derived from phosphatidylinositol-inositol triphosphate (IP 3) and diacylglycerol (DAG). • DAG activates protein kinase C, which phosphorylates serine and threonine residues on target proteins. • The phosphorylated phosphoinositides form lipid-binding domains called PH domains.

The generation of second messengers as a result of breakdown of PI

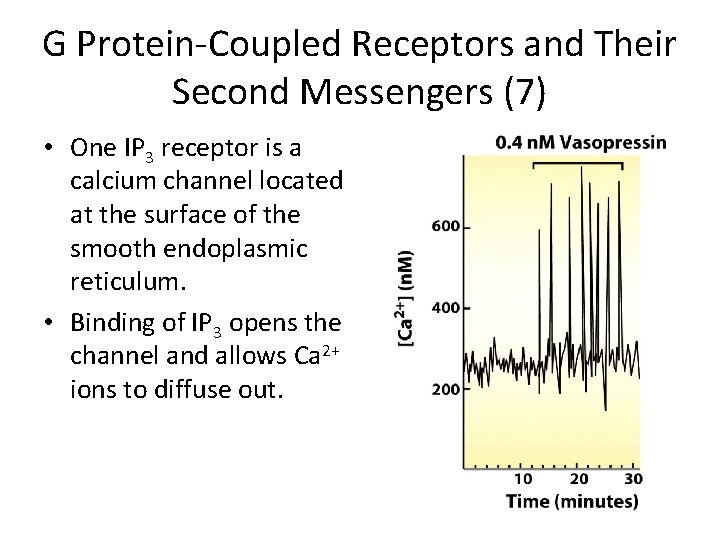
G Protein-Coupled Receptors and Their Second Messengers (7) • One IP 3 receptor is a calcium channel located at the surface of the smooth endoplasmic reticulum. • Binding of IP 3 opens the channel and allows Ca 2+ ions to diffuse out.

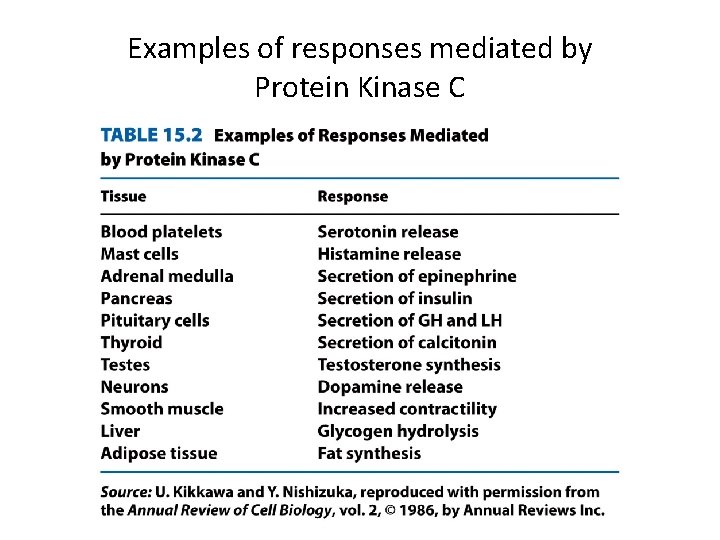
Examples of responses mediated by Protein Kinase C

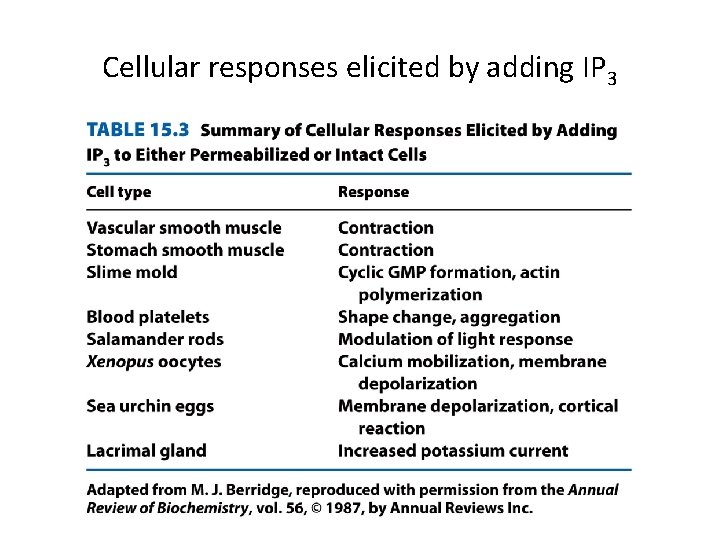
Cellular responses elicited by adding IP 3

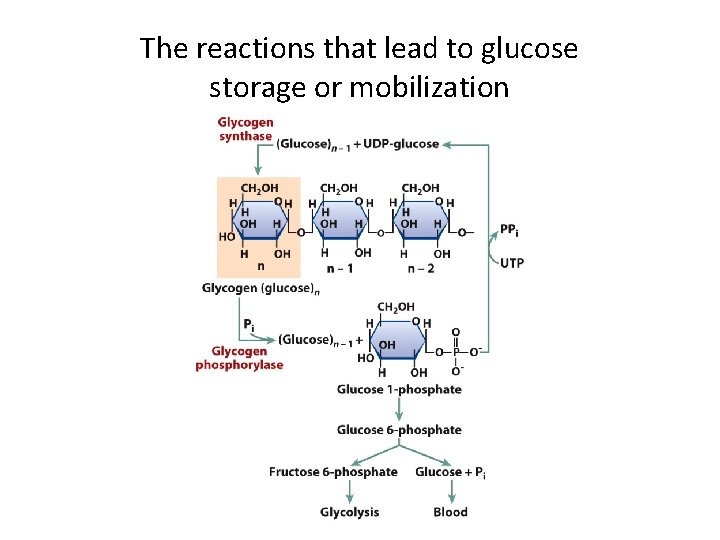
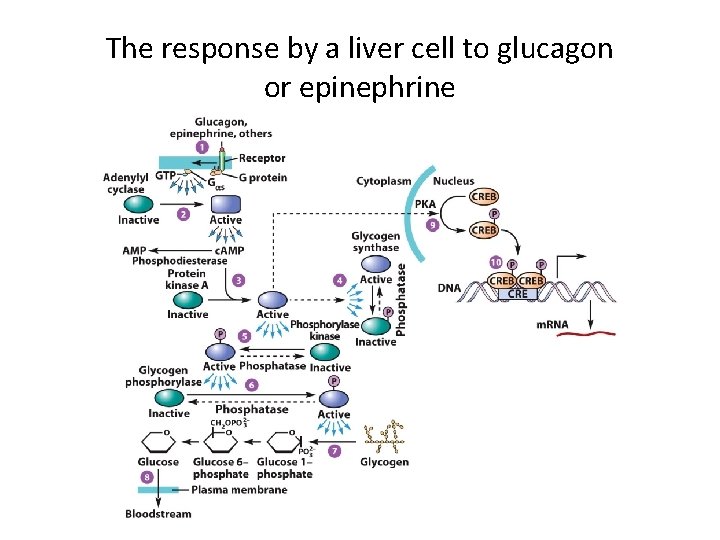
G Protein-Coupled Receptors and Their Second Messengers (8) • Regulation of Blood Glucose Levels – Different stimuli acting on the same target cell may induce the same response. • Glucagon and epinephrine bind to different receptors on the same cell. • Both hormones stimulate glucoses breakdown and inhibit its synthesis. • c. AMP is activated by the G protein of both hormone receptors – Responses are amplified by signal cascades.

The reactions that lead to glucose storage or mobilization

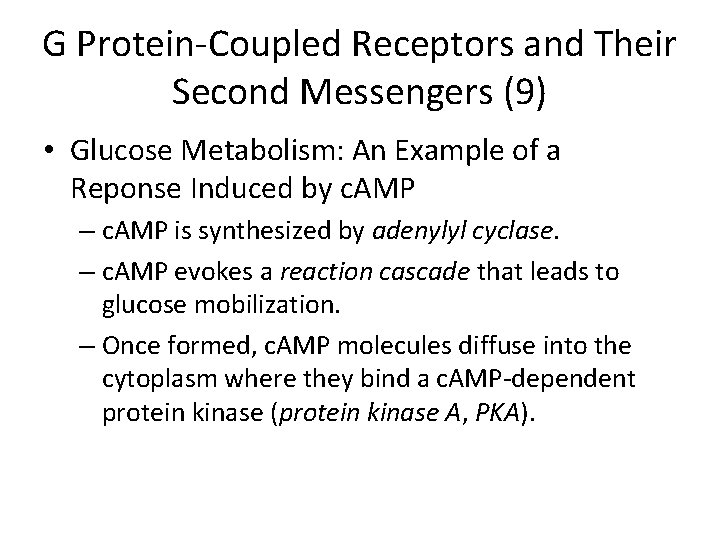
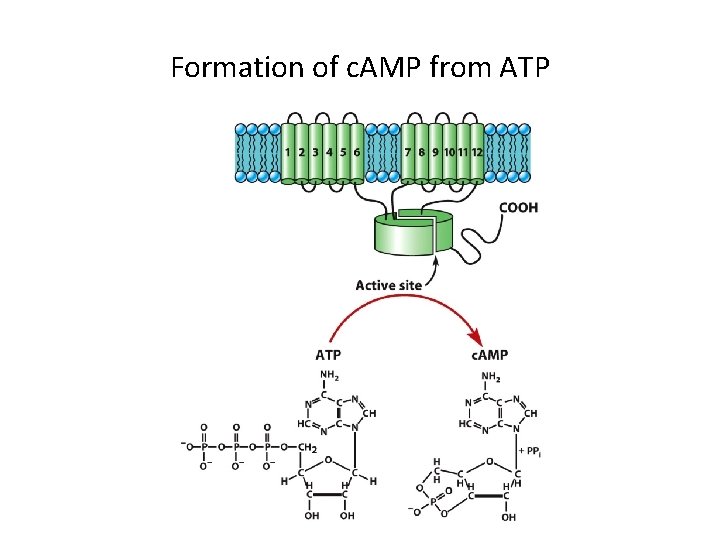
G Protein-Coupled Receptors and Their Second Messengers (9) • Glucose Metabolism: An Example of a Reponse Induced by c. AMP – c. AMP is synthesized by adenylyl cyclase. – c. AMP evokes a reaction cascade that leads to glucose mobilization. – Once formed, c. AMP molecules diffuse into the cytoplasm where they bind a c. AMP-dependent protein kinase (protein kinase A, PKA).

Formation of c. AMP from ATP

The response by a liver cell to glucagon or epinephrine

G Protein-Coupled Receptors and Their Second Messengers (10) • Other Aspects of c. AMP Signal Transduction Pathways – Some PKA molecules phosphorylate nuclear proteins. – Phosphorylated transcription factors regulate gene expression. – Phosphatases halt the reaction cascade. – c. AMP is produced as long as the external stimulus is present.
![The variety of processes that can be affected by changes in c AMP The variety of processes that can be affected by changes in [c. AMP]](https://slidetodoc.com/presentation_image_h2/998598966cb211069085c8fd12ff6914/image-33.jpg)
The variety of processes that can be affected by changes in [c. AMP]

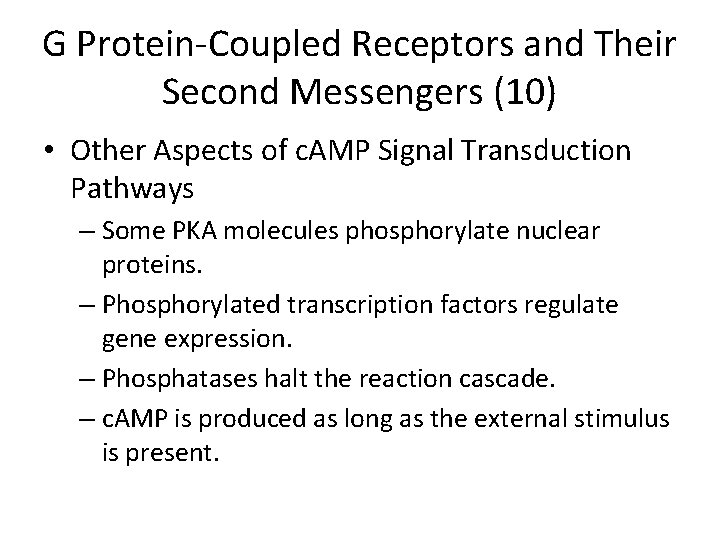
Examples of hormone-induced responses mediated by c. AMP

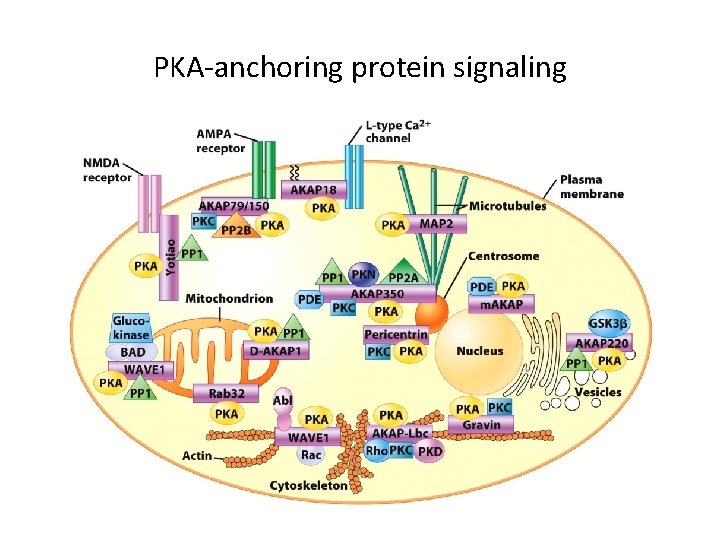
PKA-anchoring protein signaling

G Protein-Coupled Receptors and Their Second Messengers (11) • The Role of GPCRs in Sensory Perception – Rhodopsin is a photosensitive protein for blackand-white vision that is also a GPCR. – Several color receptors are GPCRs. – Odorant receptors in the nose are GPCRs. – Taste receptors for bitter and some sweet flavors are GPCRs.

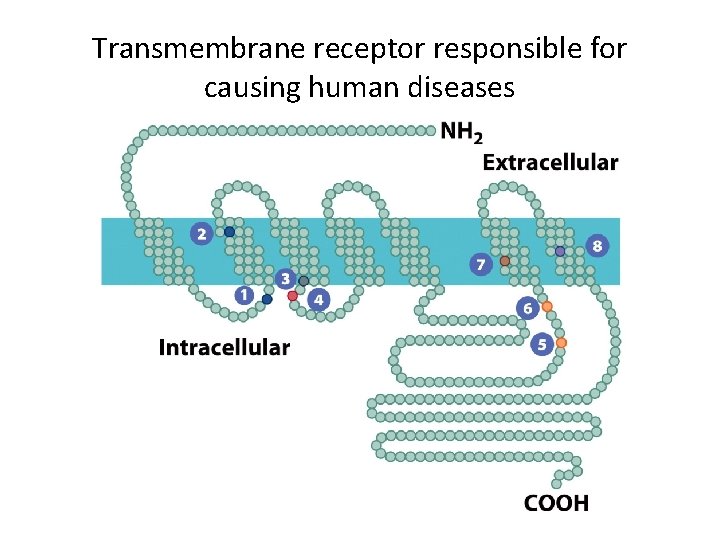
The Human Perspective: Disorders Associated with G Protein-Coupled Receptors (1) • Several disorders are caused by defects in receptors or G proteins. • Loss of function mutations result in nonfunctional signal pathways. – Retinitis pigmentosa, a progressive degeneration of the retina, can be caused my mutations in rhodopsin’s ability to activate a G protein.

Transmembrane receptor responsible for causing human diseases

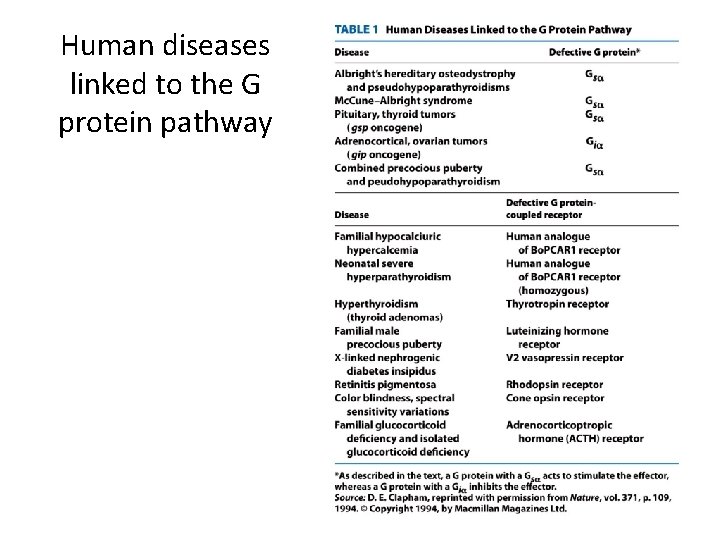
Human diseases linked to the G protein pathway

The Human Perspective: Disorders Associated with G Protein-Coupled Receptors (2) • Gain of function mutations may create a constitutively activated G protein. – Some benign thyroid tumors are caused by a mutation in a receptor. • Certain polymorphisms in G protein-related genes may result in an increased susceptibility to asthma or high blood pressure, as well as decreased susceptibility to HIV.

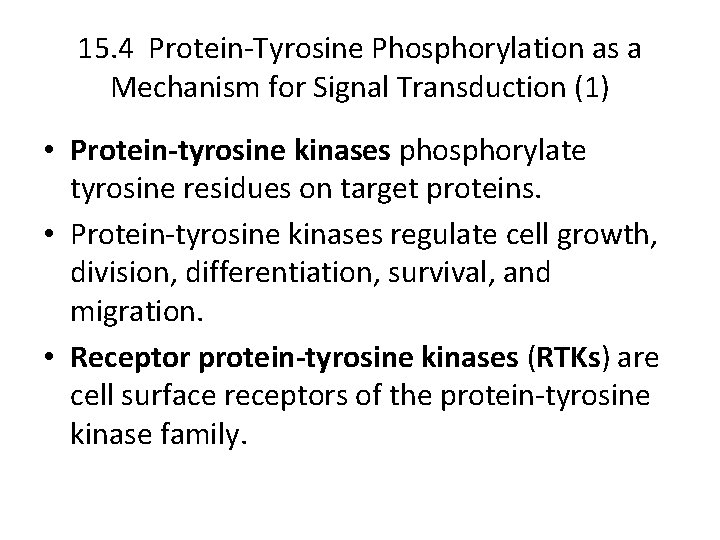
15. 4 Protein-Tyrosine Phosphorylation as a Mechanism for Signal Transduction (1) • Protein-tyrosine kinases phosphorylate tyrosine residues on target proteins. • Protein-tyrosine kinases regulate cell growth, division, differentiation, survival, and migration. • Receptor protein-tyrosine kinases (RTKs) are cell surface receptors of the protein-tyrosine kinase family.

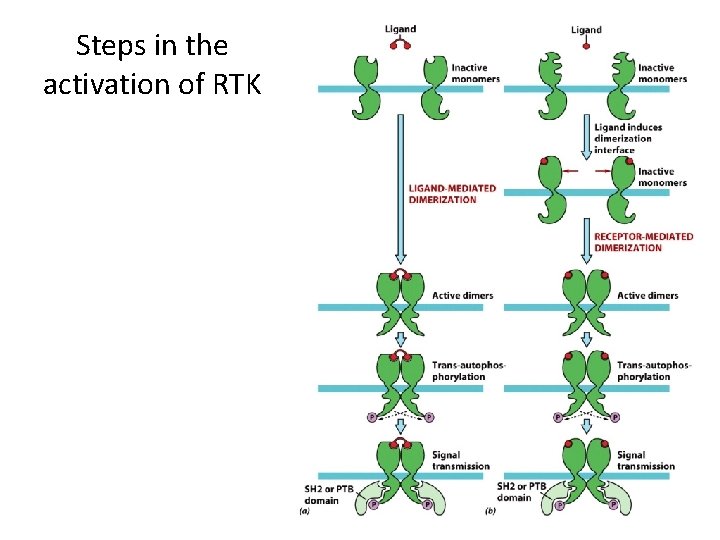
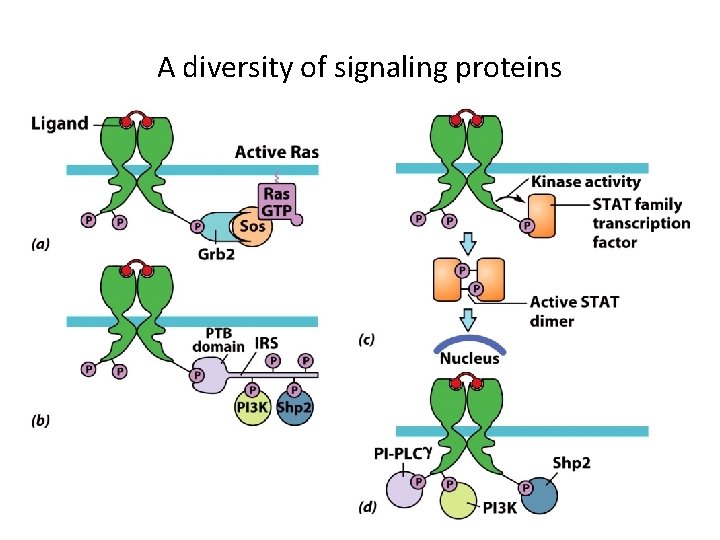
Protein-Tyrosine Phosphorylation as a Mechanism for Signal Transduction (2) • Receptor Dimerization – Results from ligand binding. – Protein kinase activity is activated. • Tyrosine kinase phosphorylates another subunit of the receptor (autophosphorylation). • RTKs phosphorylate tyrosines within phosphotyrosine motifs.

Steps in the activation of RTK

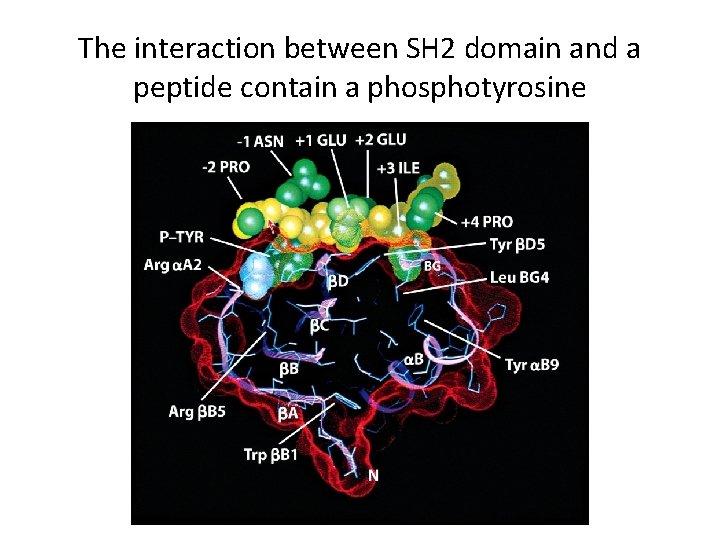
Protein-Tyrosine Phosphorylation as a Mechanism for Signal Transduction (3) • Phosphotyrosine-Dependent Protein-Protein Interactions – Phosphorylated tyrosines bind effector proteins that have SH 2 domains and PTB domains. – SH 2 and PTB domain proteins include: • Adaptor proteins that bind other proteins. • Docking proteins that supply receptors with other tyrosine phosphorylation sites. • Signaling enzymes (kinases) that lead to changes in cell. • Transcription factors

The interaction between SH 2 domain and a peptide contain a phosphotyrosine

A diversity of signaling proteins

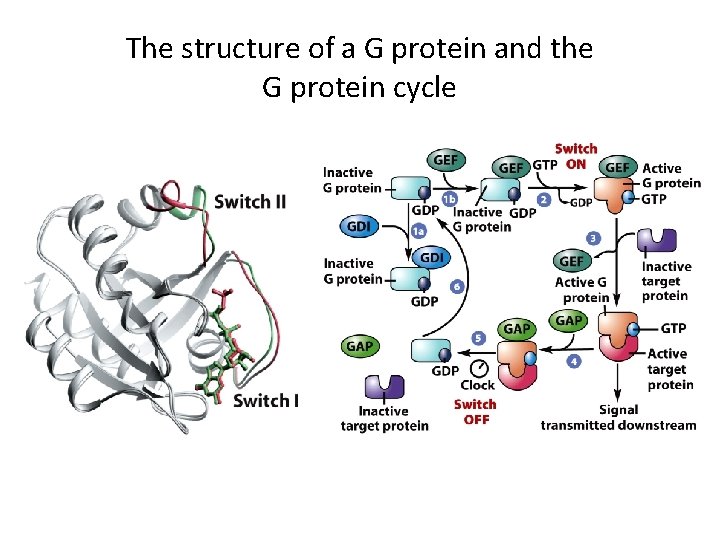
Protein-Tyrosine Phosphorylation as a Mechanism for Signal Transduction (4) • The Ras-MAP Kinase Pathway – Ras is a G protein embedded in the membrane by a lipid group. – Ras is active when bound to GTP and inactive when bound to GDP.

The structure of a G protein and the G protein cycle

Protein-Tyrosine Phosphorylation as a Mechanism for Signal Transduction (5) • Ras-MAP kinase pathway (continued) – Accessory proteins play a role: • GTPase-activating proteins (GAPs) shorten the active time of Ras. • Guanine nucleotide-exchange factors (GEFs) stimulate the exchange of GDP for GTP. • Guanine nucleotide-dissociation inhibitors (GDIs) inhibit release of GDP.

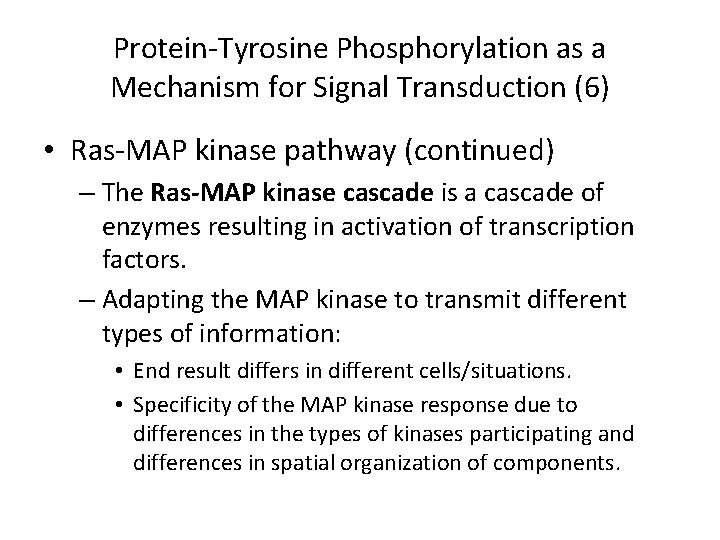
Protein-Tyrosine Phosphorylation as a Mechanism for Signal Transduction (6) • Ras-MAP kinase pathway (continued) – The Ras-MAP kinase cascade is a cascade of enzymes resulting in activation of transcription factors. – Adapting the MAP kinase to transmit different types of information: • End result differs in different cells/situations. • Specificity of the MAP kinase response due to differences in the types of kinases participating and differences in spatial organization of components.

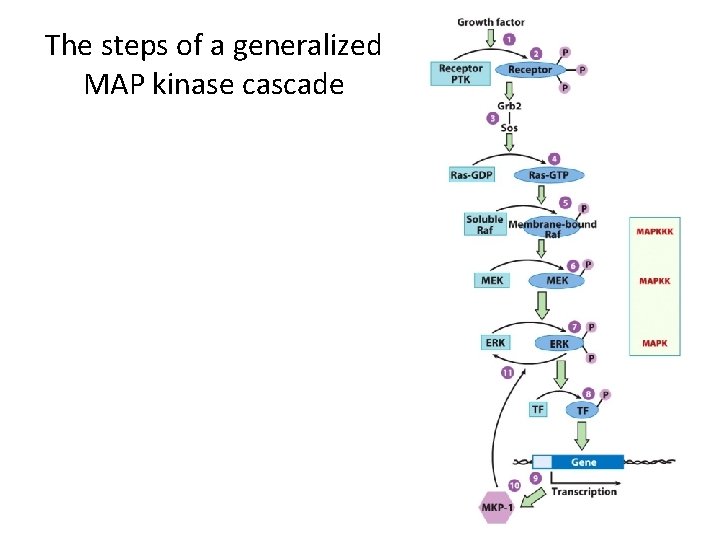
The steps of a generalized MAP kinase cascade

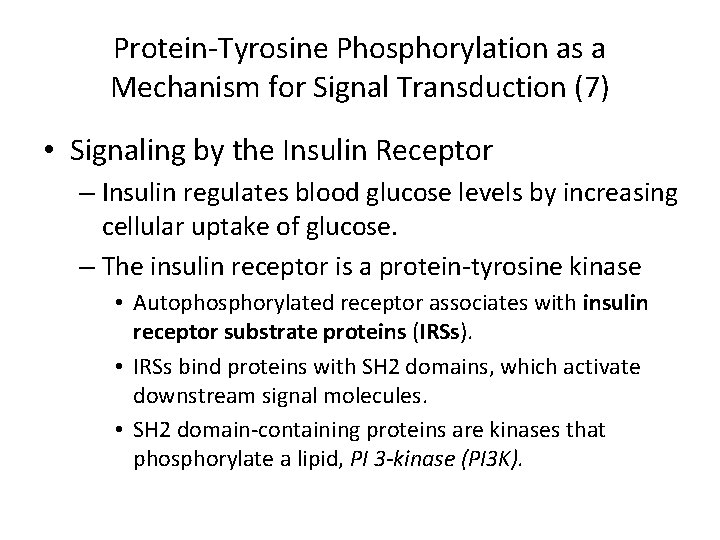
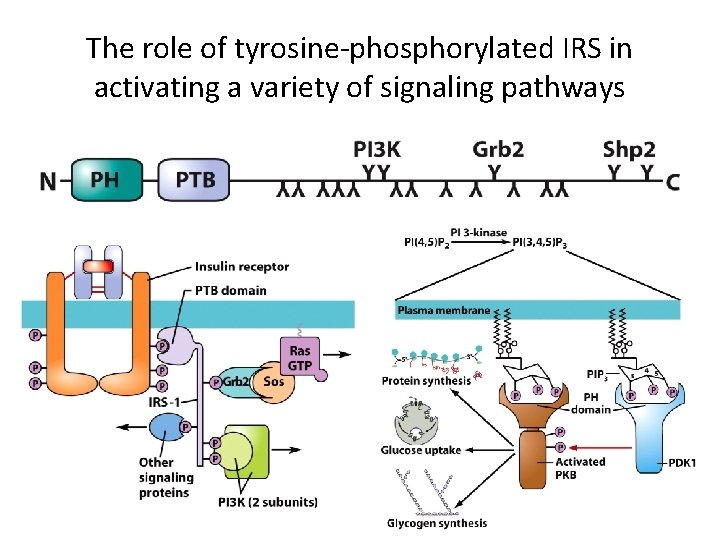
Protein-Tyrosine Phosphorylation as a Mechanism for Signal Transduction (7) • Signaling by the Insulin Receptor – Insulin regulates blood glucose levels by increasing cellular uptake of glucose. – The insulin receptor is a protein-tyrosine kinase • Autophosphorylated receptor associates with insulin receptor substrate proteins (IRSs). • IRSs bind proteins with SH 2 domains, which activate downstream signal molecules. • SH 2 domain-containing proteins are kinases that phosphorylate a lipid, PI 3 -kinase (PI 3 K).

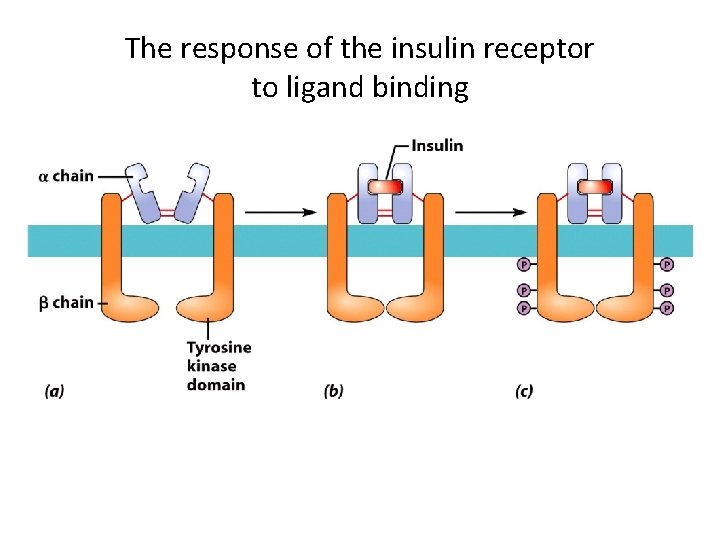
The response of the insulin receptor to ligand binding

The role of tyrosine-phosphorylated IRS in activating a variety of signaling pathways

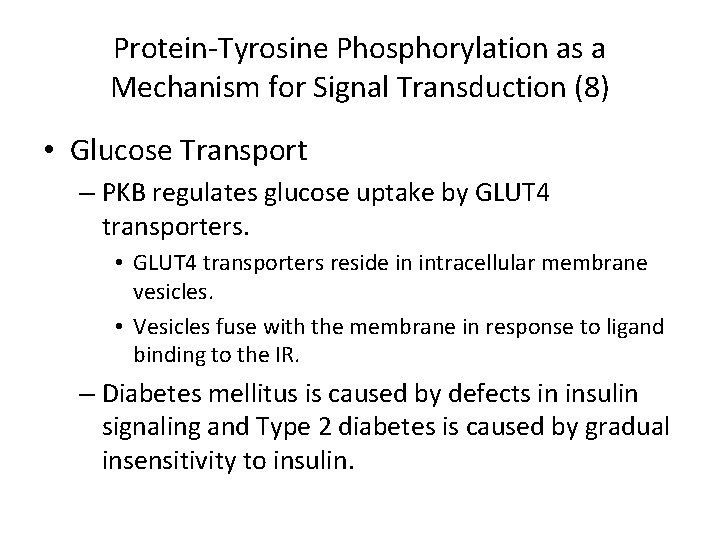
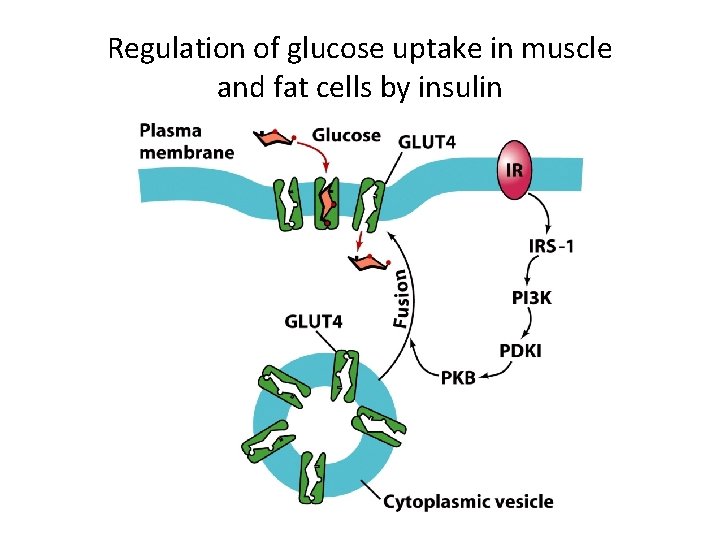
Protein-Tyrosine Phosphorylation as a Mechanism for Signal Transduction (8) • Glucose Transport – PKB regulates glucose uptake by GLUT 4 transporters. • GLUT 4 transporters reside in intracellular membrane vesicles. • Vesicles fuse with the membrane in response to ligand binding to the IR. – Diabetes mellitus is caused by defects in insulin signaling and Type 2 diabetes is caused by gradual insensitivity to insulin.

Regulation of glucose uptake in muscle and fat cells by insulin

Protein-Tyrosine Phosphorylation as a Mechanism for Signal Transduction (9) • Insulin Signaling and Lifespan – Several studies demonstrate that the lifespan can be increased by decreasing the level of insulin. – Human who live along life show high insulin sensitivity. – Laboratory studies show that calorie restriction leads to decreased insulin levels and increased insulin sensitivity.

Protein-Tyrosine Phosphorylation as a Mechanism for Signal Transduction (10) • Signaling Pathways in Plants – Plants lack cyclic nucleotides and RTKs. – Plants have protein kinases that phosphorylate histidine residues. • The downstream cascade is similar to MAP kinase cascade. • The target of the cascade is usually transcription factors.

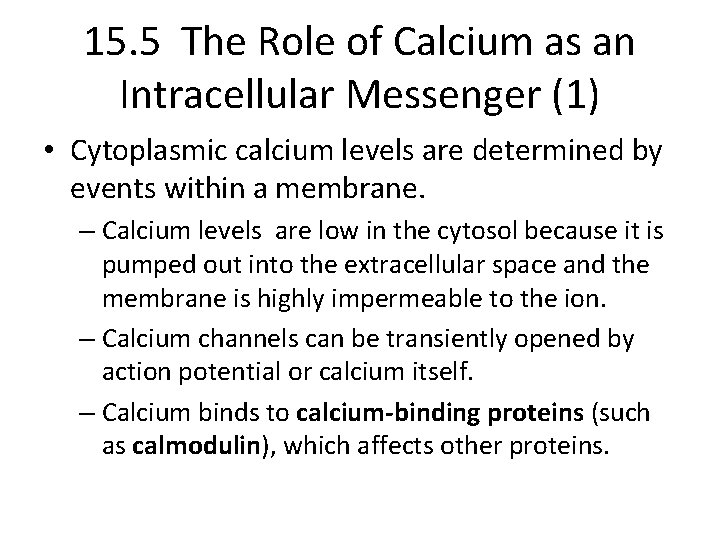
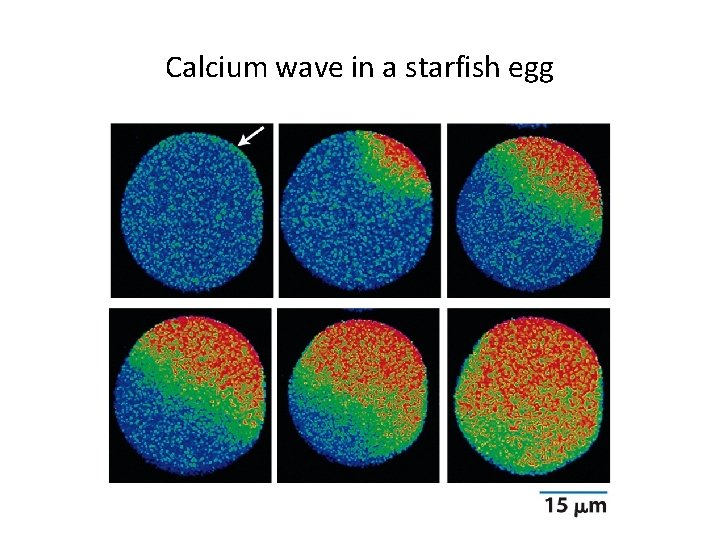
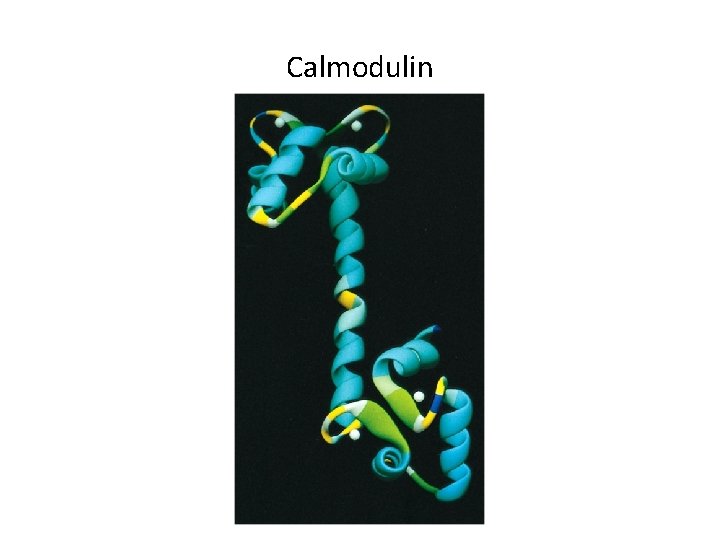
15. 5 The Role of Calcium as an Intracellular Messenger (1) • Cytoplasmic calcium levels are determined by events within a membrane. – Calcium levels are low in the cytosol because it is pumped out into the extracellular space and the membrane is highly impermeable to the ion. – Calcium channels can be transiently opened by action potential or calcium itself. – Calcium binds to calcium-binding proteins (such as calmodulin), which affects other proteins.

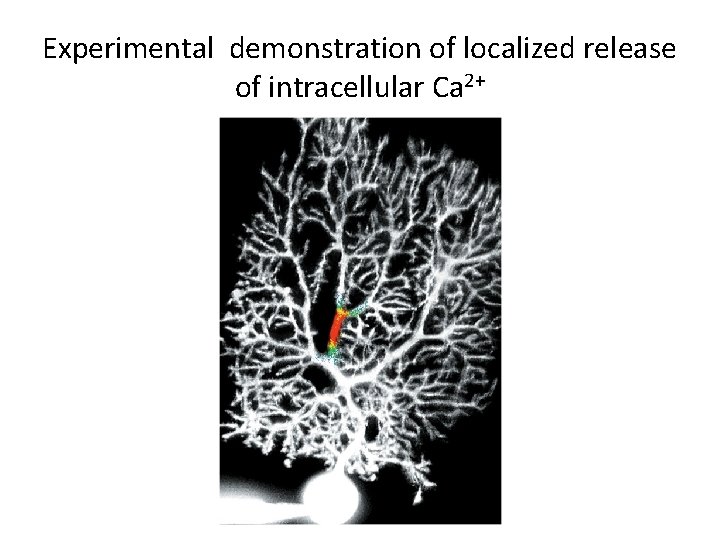
Experimental demonstration of localized release of intracellular Ca 2+

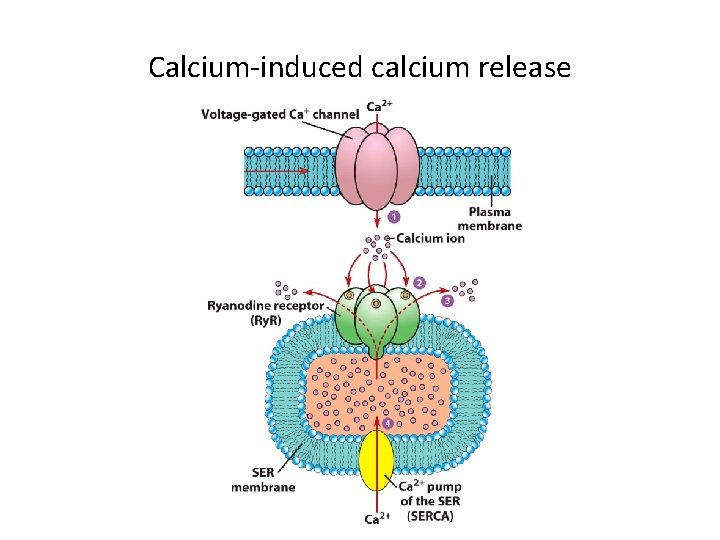
Calcium-induced calcium release

Calcium wave in a starfish egg

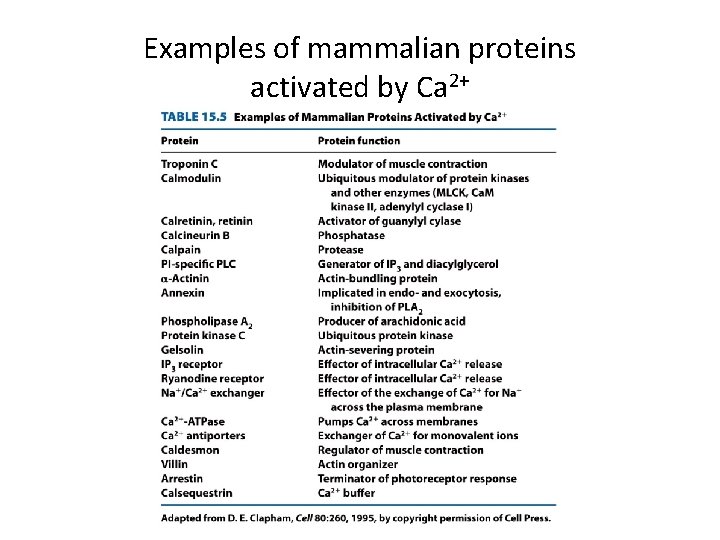
Examples of mammalian proteins activated by Ca 2+

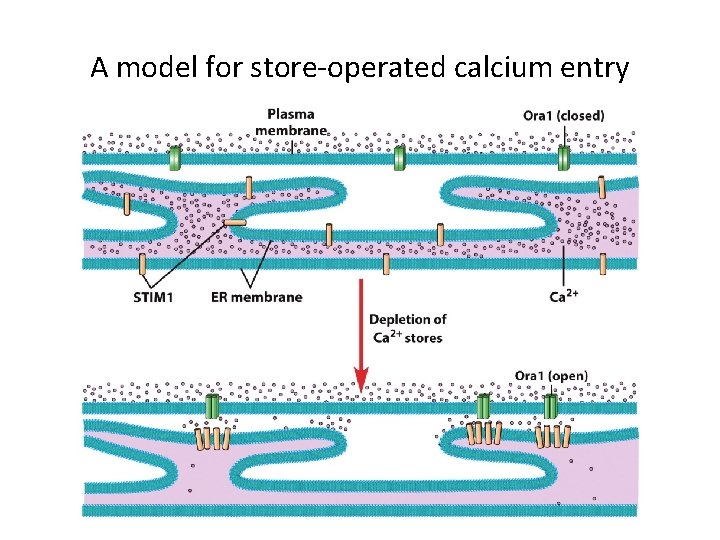
The Role of Calcium as an Intracellular Messenger (2) • Recent research indicates a phenomenon called store-operated calcium entry (SOCE). • During SOCE the depleted calcium levels trigger a response that lead to opening of calcium channels. • The mechanism responsible for SOCE is a signaling system between the ER and plasma membrane.

A model for store-operated calcium entry

Calmodulin

The Role of Calcium as an Intracellular Messenger (3) • Regulating Calcium Concentrations in Plant Cells – Cytosolic calcium changes in response to several stimuli, including light, pressure, gravity, and hormones. – Calcium signaling aids in decreasing turgor pressure in guard cells.

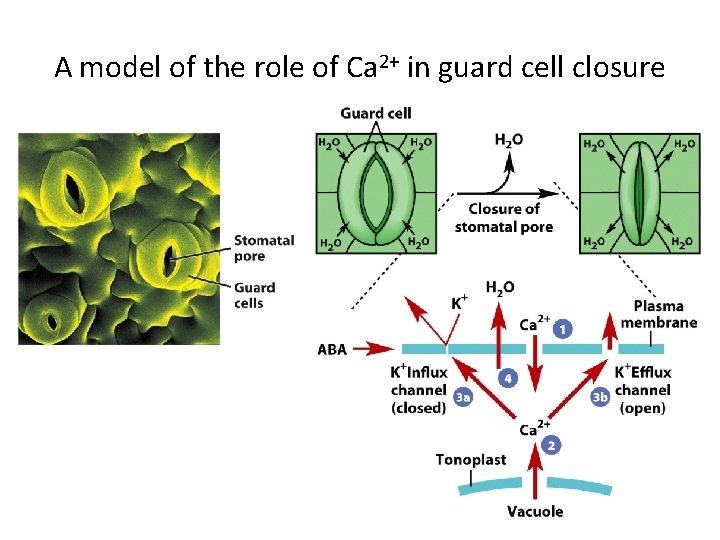
A model of the role of Ca 2+ in guard cell closure

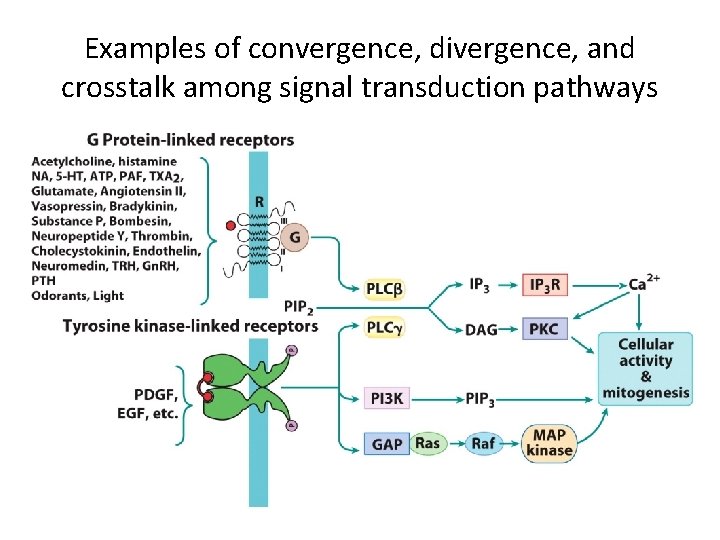
15. 6 Convergence, Divergence and Crosstalk Among Different Signaling Pathways (1) • Signaling pathways can converge , diverge, and crosstalk as follows: – Signals form unrelated receptors can converge to activate a common effector. – Identical signals can diverge to activate a variety of effectors. – Signals can be passed back and forth between pathways as a result of crosstalk.

Examples of convergence, divergence, and crosstalk among signal transduction pathways

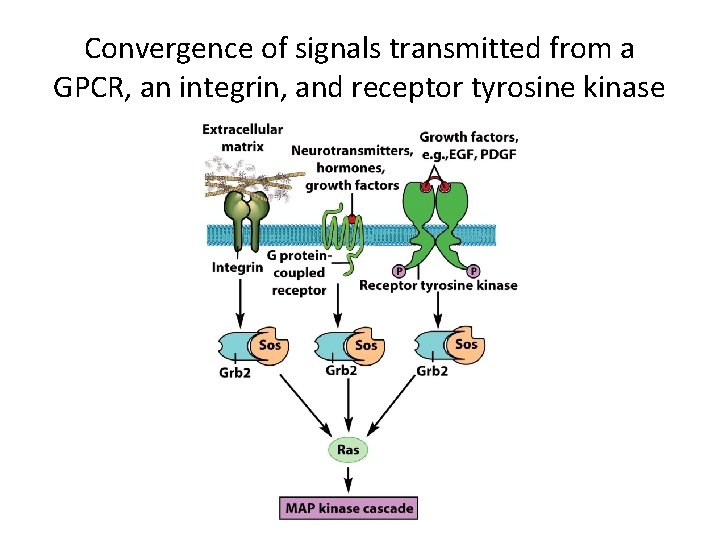
Convergence, Divergence and Crosstalk Among Different Signaling Pathways (2) • Convergence – GPCRs, receptor tyrosine kinases, and integrins bind to different ligands but they all can lead to a docking site for Gbr 2.

Convergence of signals transmitted from a GPCR, an integrin, and receptor tyrosine kinase

Convergence, Divergence and Crosstalk Among Different Signaling Pathways (3) • Divergence – all of the examples of signal transduction so far are evidence of divergence of how a single stimulus sends signals along a variety of different pathways.

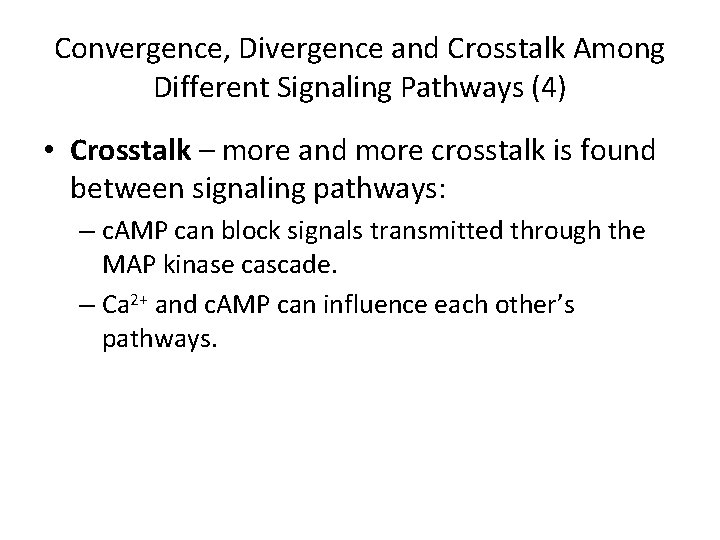
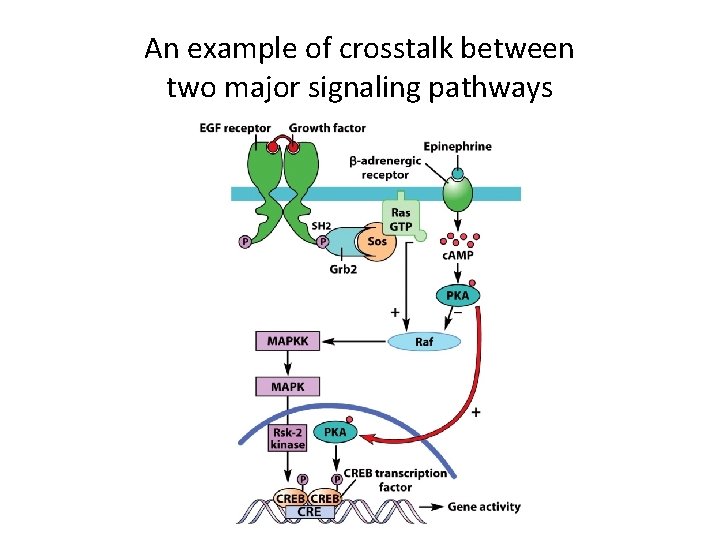
Convergence, Divergence and Crosstalk Among Different Signaling Pathways (4) • Crosstalk – more and more crosstalk is found between signaling pathways: – c. AMP can block signals transmitted through the MAP kinase cascade. – Ca 2+ and c. AMP can influence each other’s pathways.

An example of crosstalk between two major signaling pathways

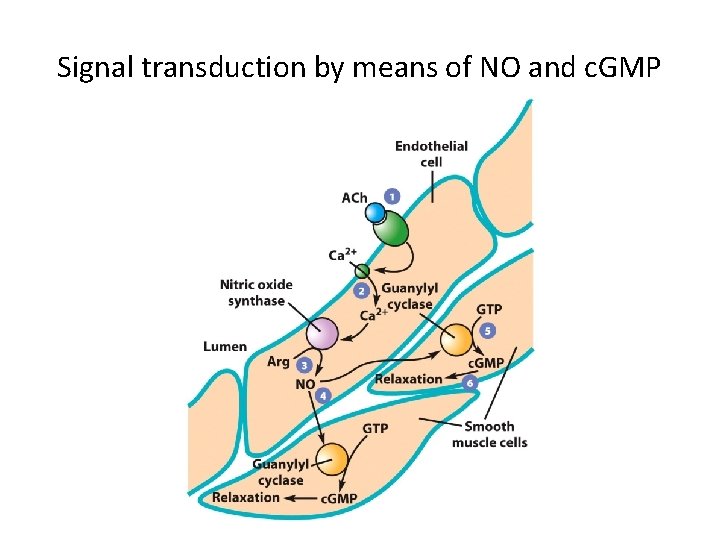
15. 7 The Role of NO as an Intracellular Pathway • Nitric oxide (NO) is both an extracellular and intercellular messenger with a variety of functions. • NO is produced by nitric oxide synthase. – NO stimulates guanylyl cyclase, making c. GMP. – c. GMP decreases cytosolic calcium and relaxes smooth muscle. – NO also plays a role in male arousal.

Signal transduction by means of NO and c. GMP

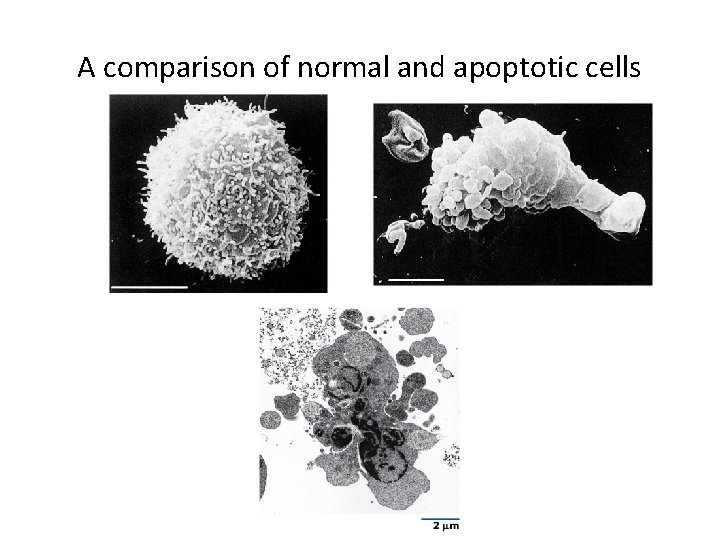
15. 8 Apoptosis (Programmed Cell Death) (1) • Apoptosis is an ordered process involving cell shrinkage, loss of adhesion to other cells, dissection of chromatin, and engulfment by pahgocytosis.

A comparison of normal and apoptotic cells

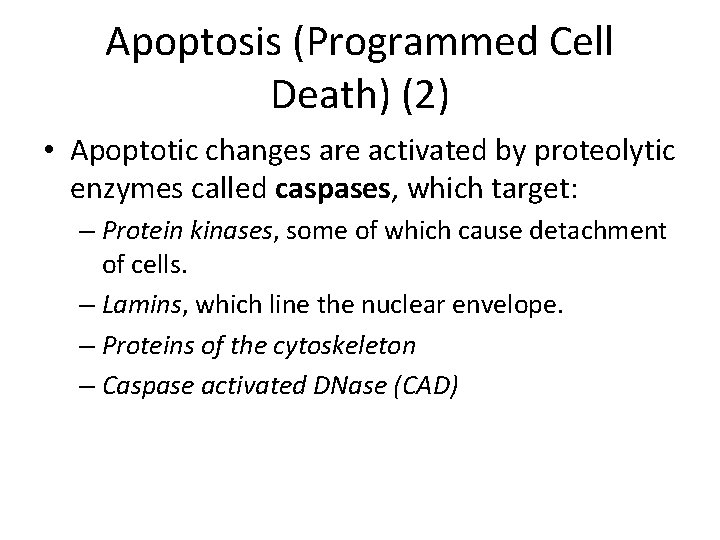
Apoptosis (Programmed Cell Death) (2) • Apoptotic changes are activated by proteolytic enzymes called caspases, which target: – Protein kinases, some of which cause detachment of cells. – Lamins, which line the nuclear envelope. – Proteins of the cytoskeleton – Caspase activated DNase (CAD)

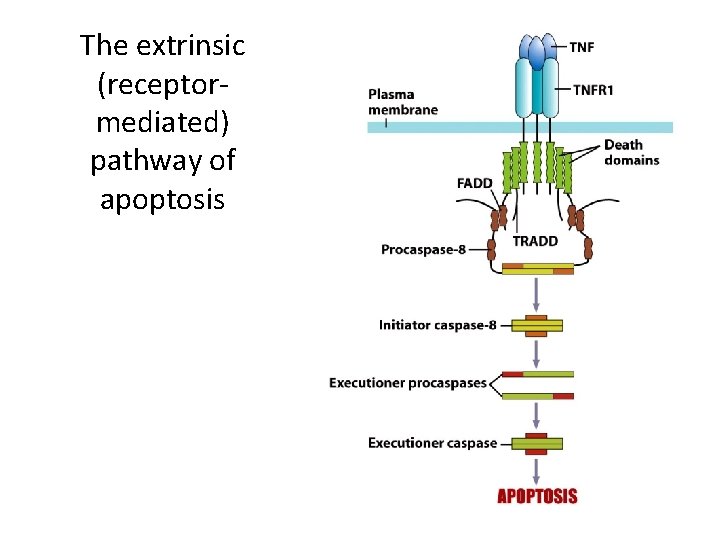
Apoptosis (Programmed Cell Death) (3) • The Extrinsic Pathway of Apoptosis – It is initiated by external stimuli: • Tumor necrosis factor (TNF) is detected by a TNF cell surface receptor. • Bound TNF receptors recruit “procaspases” to the intracellular domain of the receptor. • Procaspases convert other procaspases to caspases. • Caspases activate executioner caspases, leading to apoptosis.

The extrinsic (receptormediated) pathway of apoptosis

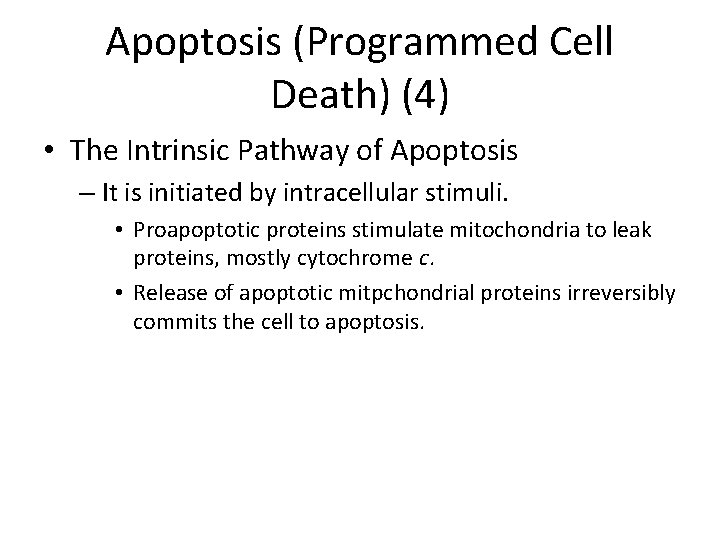
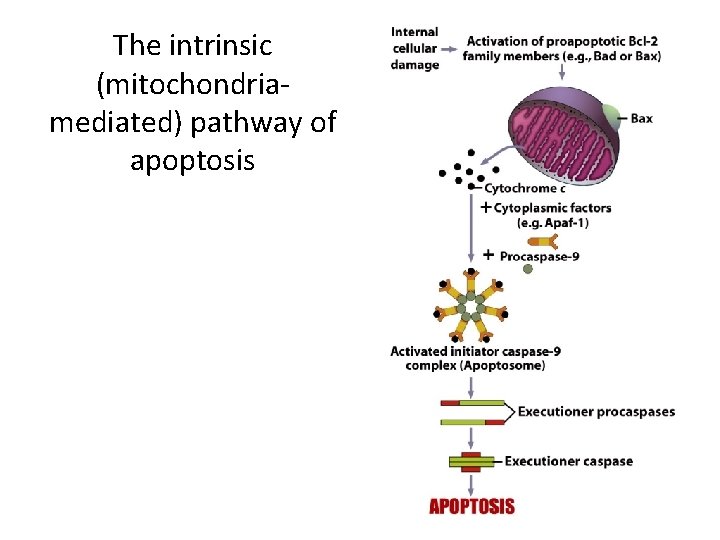
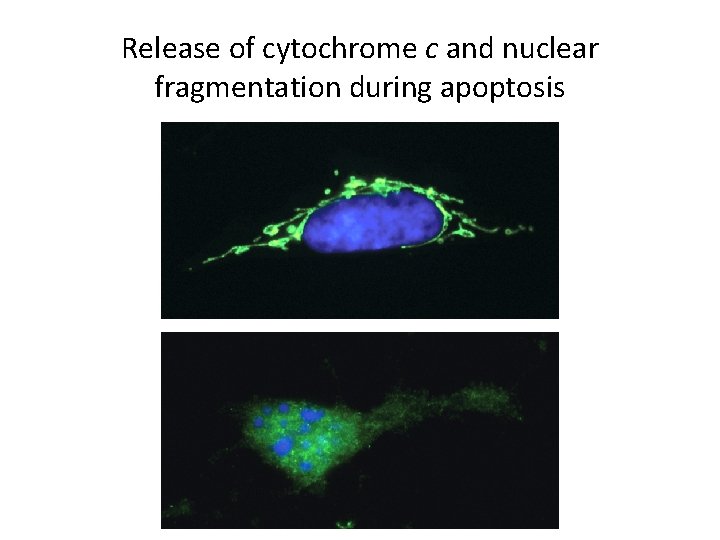
Apoptosis (Programmed Cell Death) (4) • The Intrinsic Pathway of Apoptosis – It is initiated by intracellular stimuli. • Proapoptotic proteins stimulate mitochondria to leak proteins, mostly cytochrome c. • Release of apoptotic mitpchondrial proteins irreversibly commits the cell to apoptosis.

The intrinsic (mitochondriamediated) pathway of apoptosis

Release of cytochrome c and nuclear fragmentation during apoptosis

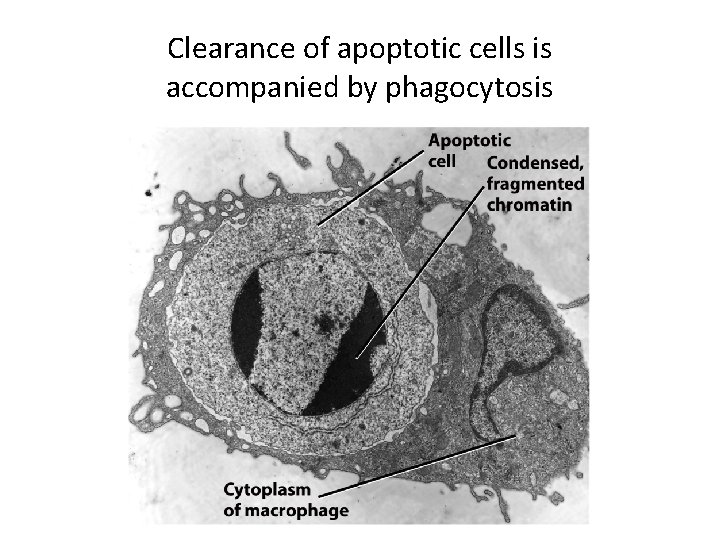
Apoptosis (Programmed Cell Death) (5) • Antiapoptotic proteins promote survival. • Cell fate depends on the balance between proand anti-apoptotic signals. • Apoptotic cell death occurs without spilling cellular contents to prevent inflammation. • Apoptotic cells are cleared by phagocytosis.

Clearance of apoptotic cells is accompanied by phagocytosis
 Cell signal transduction
Cell signal transduction Pogil cellular communication
Pogil cellular communication Dot
Dot Essential cell biology chapter 3 quiz
Essential cell biology chapter 3 quiz Signal transduction
Signal transduction Signal transduction
Signal transduction Cell communication types
Cell communication types Chemical signalling
Chemical signalling 3 stages of cell communication
3 stages of cell communication Exocrine cell signaling
Exocrine cell signaling Cell signaling
Cell signaling Cell signaling overview
Cell signaling overview Pdi cell signaling
Pdi cell signaling Chapter 48 neurons synapses and signaling
Chapter 48 neurons synapses and signaling Chapter 48 neurons synapses and signaling
Chapter 48 neurons synapses and signaling Baseband signal and bandpass signal
Baseband signal and bandpass signal Baseband signal and bandpass signal
Baseband signal and bandpass signal Product of two odd signal is
Product of two odd signal is Textual aspects of lexical competence
Textual aspects of lexical competence Register and signaling vocabulary
Register and signaling vocabulary Autocrine and juxtacrine signaling
Autocrine and juxtacrine signaling Juxtacrine communication
Juxtacrine communication Photoreceptor transduction
Photoreceptor transduction Tonic receptors
Tonic receptors Transduction in the ear
Transduction in the ear Where does transduction occur in the ear
Where does transduction occur in the ear Place theory
Place theory Generalized transduction
Generalized transduction Superior colliculus
Superior colliculus Transduction psychology
Transduction psychology Transduction psychology
Transduction psychology Olfactory transduction
Olfactory transduction Olfactory transduction
Olfactory transduction Olfactory transduction
Olfactory transduction What are phytochromes
What are phytochromes Transduction psychology
Transduction psychology Olfactory transduction
Olfactory transduction Transductant
Transductant Chapter 4 cell theory and cell study
Chapter 4 cell theory and cell study Digital signal as a composite analog signal
Digital signal as a composite analog signal Signal phrasing
Signal phrasing Vehicle ground guide hand signals
Vehicle ground guide hand signals Ligand signaling molecule
Ligand signaling molecule Signaling system 7
Signaling system 7 Chemical signaling
Chemical signaling Paracrine signaling
Paracrine signaling Chemical signaling
Chemical signaling Chemical signaling
Chemical signaling Embryonic and growth industries
Embryonic and growth industries The process of intentionally or unintentionally signaling
The process of intentionally or unintentionally signaling Scp 3710
Scp 3710 Use visual signaling techniques
Use visual signaling techniques Cell coverage for signal and traffic
Cell coverage for signal and traffic Chapter 11 cell communication
Chapter 11 cell communication Advantages and disadvantages of diaphragm cell process
Advantages and disadvantages of diaphragm cell process Prokaryotic and eukaryotic cells
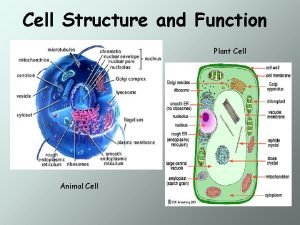
Prokaryotic and eukaryotic cells Difference of animal cell and plant cell
Difference of animal cell and plant cell Tonoplast
Tonoplast Structure of a animal cell
Structure of a animal cell Lead acid battery primary or secondary
Lead acid battery primary or secondary Difference between plant cell and bacterial cell
Difference between plant cell and bacterial cell Events of the cell cycle
Events of the cell cycle Life
Life Idealized animal cell and plant cell
Idealized animal cell and plant cell Walker cell and hadley cell
Walker cell and hadley cell Cell cycle and cell division
Cell cycle and cell division Animal cells and plant cells venn diagram
Animal cells and plant cells venn diagram Phases of cell cycle
Phases of cell cycle Electrolytic cell
Electrolytic cell Animal cell and plant cell
Animal cell and plant cell Noise is added to a signal in a communication system *
Noise is added to a signal in a communication system * Signal space analysis in digital communication
Signal space analysis in digital communication Data encoding techniques
Data encoding techniques Cell city analogy
Cell city analogy Prokaryotic vs eukaryotic cell
Prokaryotic vs eukaryotic cell Cathode and anode half reactions
Cathode and anode half reactions Dry cell vs wet cell
Dry cell vs wet cell Cell wall function
Cell wall function Cell wall cell membrane
Cell wall cell membrane Cell line vs cell strain
Cell line vs cell strain Cell line vs cell strain
Cell line vs cell strain Cell city project
Cell city project Cell-cell junction
Cell-cell junction Cell-cell junction
Cell-cell junction What cell organelle is like lysol spray cleaning the cell
What cell organelle is like lysol spray cleaning the cell Carbohydrate side chain
Carbohydrate side chain Parts of a cell graphic organizer
Parts of a cell graphic organizer Prokaryotic v. eukaryotic cells
Prokaryotic v. eukaryotic cells