Signal Transduction Signal Transduction Old terms Signal transmission




- Slides: 36

Signal Transduction

Signal Transduction (Old terms: Signal transmission or sensory transduction) In this phenomenon a mechanical or chemical stimulus is converted into a specific cellular response. It starts with a signal to a receptor, and ends with a change in cell function or response.

Signal Transduction • Cells respond to their environment by re-organizing their structure, regulating the activity of proteins and altering patterns of gene expression. The stimulus for such responses is known as signal. It may be a small molecule, a macromolecule or a physical agent such as light, temperature, water etc. Signals interact with the responding cell through specific molecules called receptors. • Small molecules often act as diffusible signals. In unicellular organizations diffusible signals may be environmental or may be released from other cells e. g. yeast mating-type pheromones or c. AMP in Dictyostelium (a species of soil-living amoebae). In metazoans (syn: the Animal Kingdom), signals may be released from nearby cells and diffuse over short distances (Paracrine signaling), or they may be released from distant cells and reach their target through vas. system (endocrine signaling). Macromolecular signals are associated with the extracellular matrix or displayed on the surface of neighboring cells (Juxtacrine signaling). A molecular signal that binds to a receptor is called ligand.

Signal Transduction in Prokaryotes • Animal (mammals) possess a well developed nervous system by which they are able to sense and respond to environment. • Many processes such as (1) stimulus detection (2) signal amplification (3) appropriate output responses are present in all cell sensory systems including bacteria. • Many bacterial signaling pathways consist of molecular units called transmitters and receivers. This is called two-component regulatory system. • Bacteria use two component regulatory systems to sense extracellular signals. They sense chemicals in the environment by means of a small familyof cell surface receptors, each involved in the response to a specific group of chemicals called ligands. A protein in the plasma membrane of bacteria binds directly to a ligand or binds to a soluble protein that has already attached to the ligand, in the periplasmic space between the PM and the cell wall. • Upon binding, the membrane protein undergoes a conformational change that passes across the membrane to the cytosolic domain of the receptor protein. This conformational change initiates the signaling pathway that leads to the response.

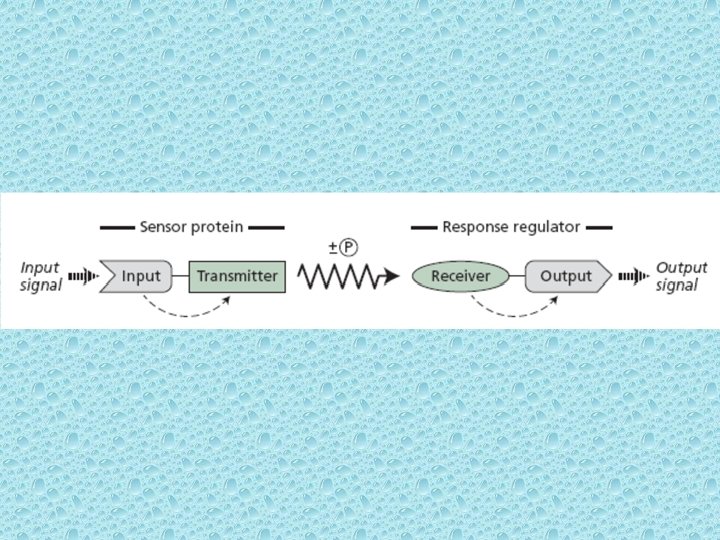
Signaling via bacterial two-component-systems • The sensor protein present on cell wall detects the stimulus via the input domain and transfers the signal to the transmitter domain by means of conformational change (first dashed arrows). The transmitter domain of the sensor then communicates with the response regulator by protein phosphorylation of the receiver domain. Phosphorylation of the receiver domain induces a conformational change (second dashed arrows) that activates the output domain and brings about the cellular response. • For instance, osmoregulation, chemotaxis and sporulation are regulated by two component systems.


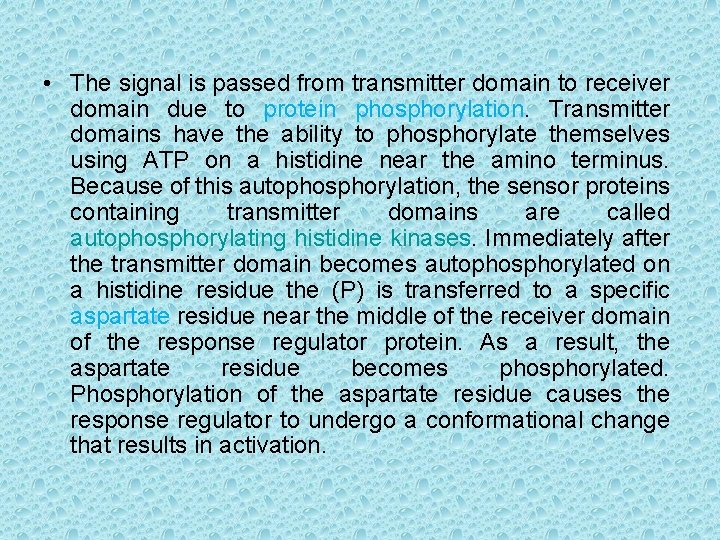
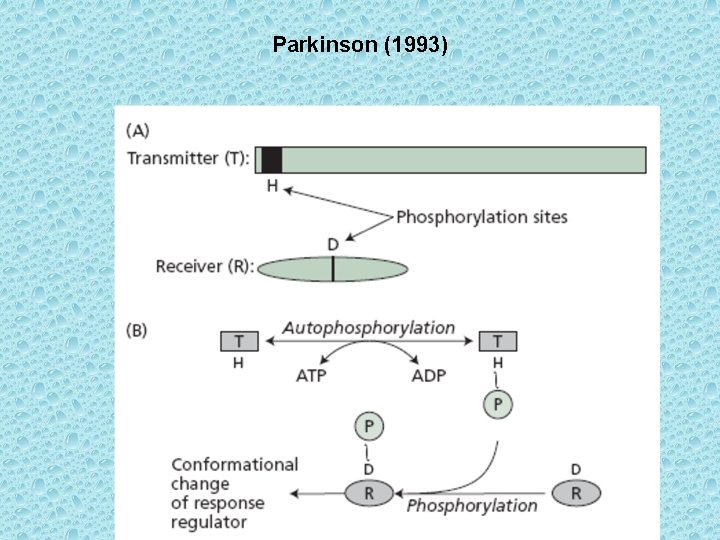
• The signal is passed from transmitter domain to receiver domain due to protein phosphorylation. Transmitter domains have the ability to phosphorylate themselves using ATP on a histidine near the amino terminus. Because of this autophosphorylation, the sensor proteins containing transmitter domains are called autophosphorylating histidine kinases. Immediately after the transmitter domain becomes autophosphorylated on a histidine residue the (P) is transferred to a specific aspartate residue near the middle of the receiver domain of the response regulator protein. As a result, the aspartate residue becomes phosphorylated. Phosphorylation of the aspartate residue causes the response regulator to undergo a conformational change that results in activation.

Parkinson (1993)

Signal Transduction in Eukaryotes • In complex multi-cellular organisms two main systems have been evolved – (i) nervous system – (ii) endocrine system • In contrast, plants lack nervous system but they have evolved hormones as chemical messengers. However, generally plant signal transduction pathways differ to a great extent from those of animals. But nevertheless there are some common signal mechanisms in both animals and plants that we are discussing here.

Hormones • There are two classes of hormones depending on their ability to move across the plasma membrane. – Lipophilic hormones: which diffuse readily across the hydrophobic bilayer of plasma membrane e. g. Androgens, glucocorticoids, estrogens and brassinosteroids. – Water soluble hormones which are unable to enter the cell by their own e. g. antidiuretic hormone (ADH), glucagons, Thyroid Stimulating Hormone (TSH)

• Lipophilic hormones bind mainly to receptors in the cytoplasm or nucleus, whereas water soluble hormones bind to receptors located on the cell surface. • In both cases ligand binding changes the receptor by causing a conformational change. • Some receptors such as steroid hormone receptors can regulate gene expression directly. • But in most cases the receptor initiates one or most sequences of biochemical reactions that connect the stimulus to a cellular response. Such a sequence of reactions is called a signal transduction pathway. • The final or end result of signal transduction pathways is to regulate transcription factors which in turn regulate gene expression.

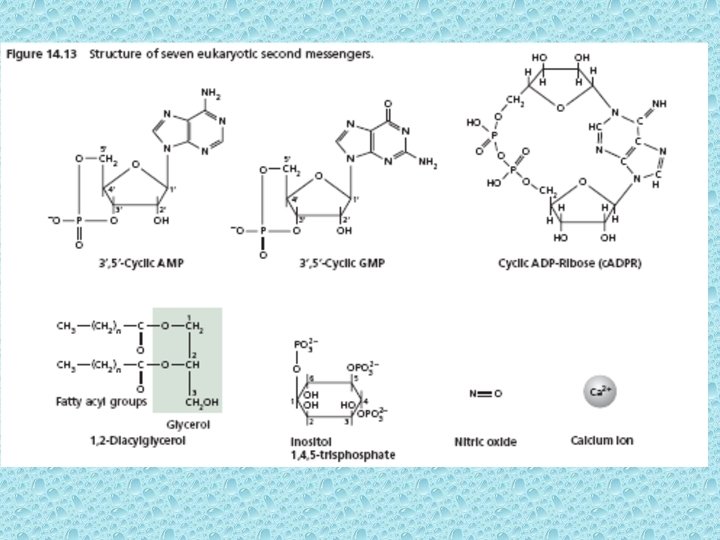
Signal transduction pathways • Signal transduction pathways often involve generation of second messengers. They actually transmit secondary signals inside the cell that greatly amplify the original signal. • Molecules inside the cells that act to transmit signals from a receptor to a target. The term second messenger is used to distinguish them from hormones and other molecules that function outside the cell as “first messengers” in the transmission of biological information. • Most common second messengers are c. AMP, c. GMP, 1, 2 diacylglycerol (DAG), or inositol 1, 4, 5 triphosphate or inositol 4, 5, 6 triphosphate (IP 3) and Ca 2+. • Hormones binding normally causes enhanced levels of one or more of these second messengers resulting in the activation or inactivation of enzymes or regulatory proteins.


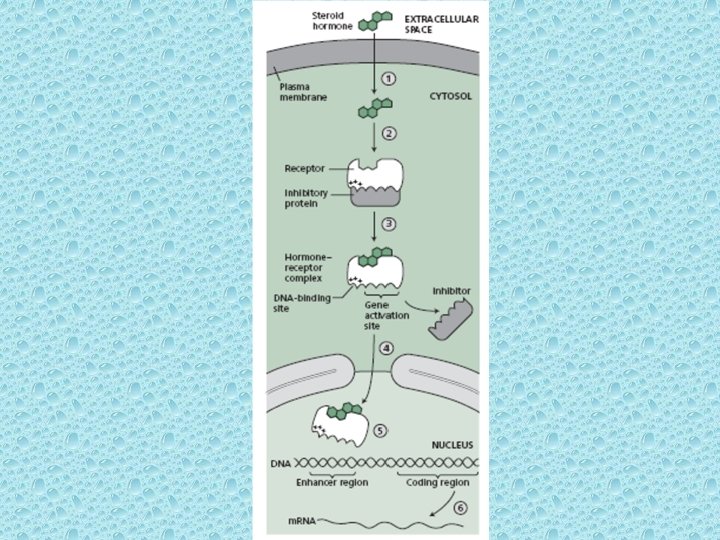
Steroid Receptors can act as transcription factors • Steroid Receptors can act as transcription factors. Almost all the steroid hormones can freely pass through the PM because of their lipophilic/hydrophobic nature and they bind to the intra-cellular receptor proteins. On binding, these proteins function as transcription factors. All such steroid receptor proteins have similar DNA binding domains. Steroid response elements are typically located in enhancer regions of steroid stimulated genes. • Most steroid receptors are localized in the nucleus, where they get anchored to nuclear proteins in an inactive form. When the receptor binds to the steroid, it is released from the inactive anchor protein and becomes activated as a transcription factor. The activated transcription factor then binds to the enhancer and stimulates transcription. • Not all intracellular steroid receptors are localized in the nucleus. The receptor for cortisol (glucocorticoid hormone) differs from the others in that it is located in the cytosol, anchored in an inactive cytosolic protein.


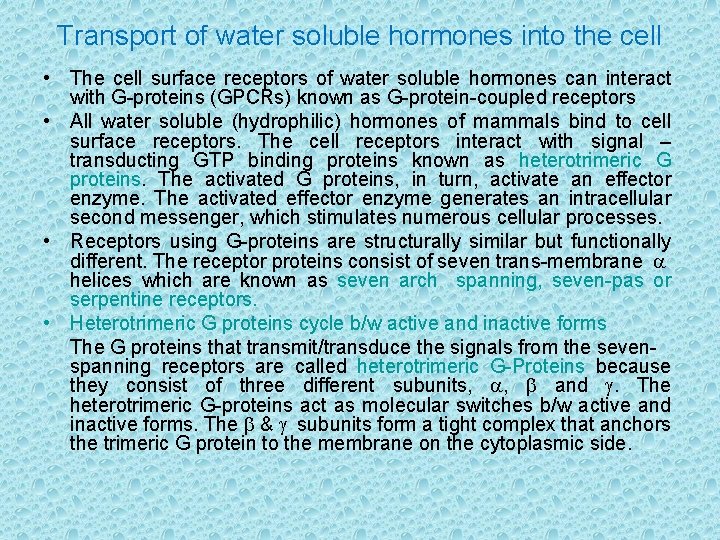
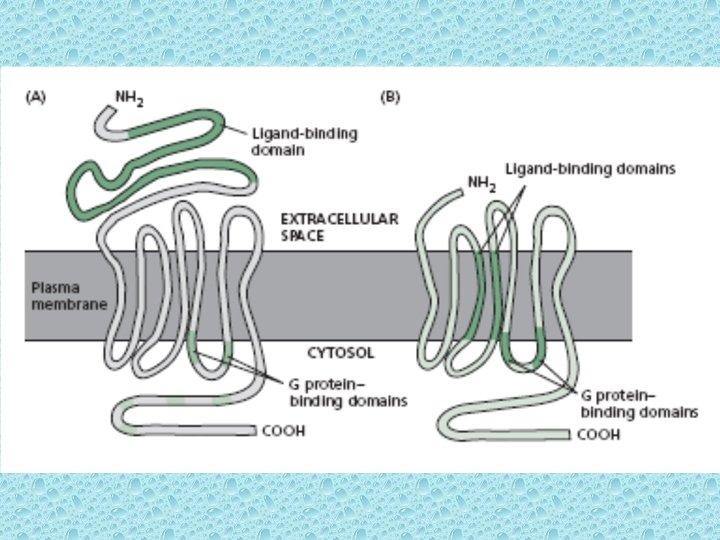
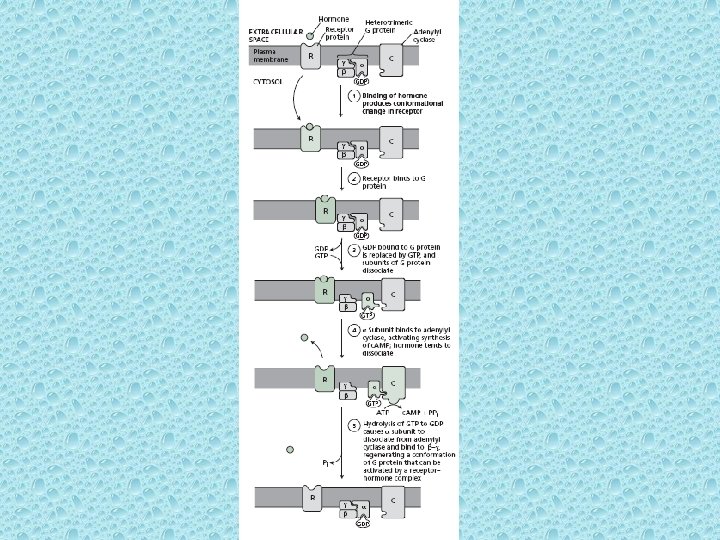
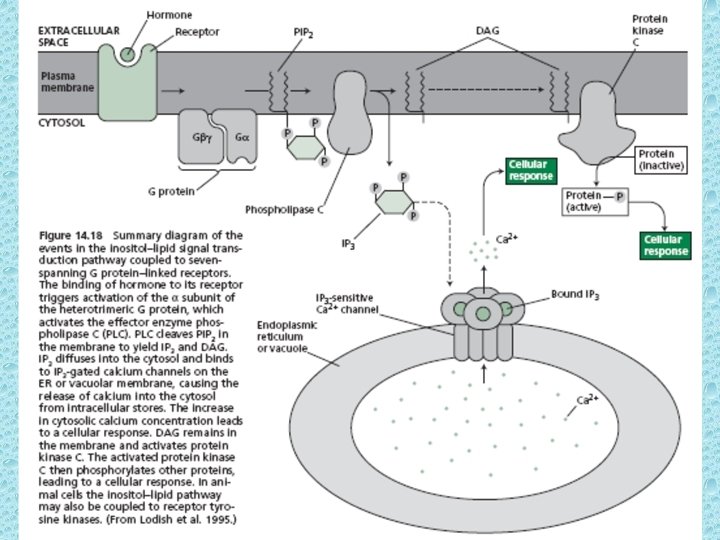
Transport of water soluble hormones into the cell • The cell surface receptors of water soluble hormones can interact with G-proteins (GPCRs) known as G-protein-coupled receptors • All water soluble (hydrophilic) hormones of mammals bind to cell surface receptors. The cell receptors interact with signal – transducting GTP binding proteins known as heterotrimeric G proteins. The activated G proteins, in turn, activate an effector enzyme. The activated effector enzyme generates an intracellular second messenger, which stimulates numerous cellular processes. • Receptors using G-proteins are structurally similar but functionally different. The receptor proteins consist of seven trans-membrane a helices which are known as seven arch spanning, seven-pas or serpentine receptors. • Heterotrimeric G proteins cycle b/w active and inactive forms The G proteins that transmit/transduce the signals from the sevenspanning receptors are called heterotrimeric G-Proteins because they consist of three different subunits, a, b and g. The heterotrimeric G-proteins act as molecular switches b/w active and inactive forms. The b & g subunits form a tight complex that anchors the trimeric G protein to the membrane on the cytoplasmic side.

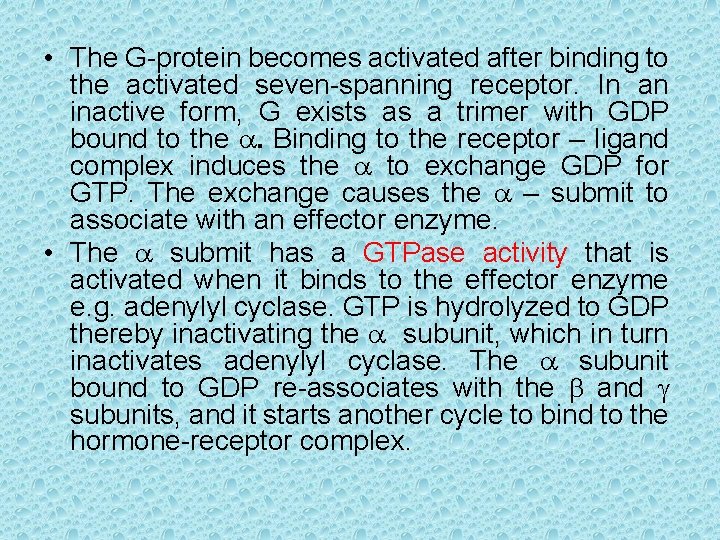
• The G-protein becomes activated after binding to the activated seven-spanning receptor. In an inactive form, G exists as a trimer with GDP bound to the a. Binding to the receptor – ligand complex induces the a to exchange GDP for GTP. The exchange causes the a – submit to associate with an effector enzyme. • The a submit has a GTPase activity that is activated when it binds to the effector enzyme e. g. adenylyl cyclase. GTP is hydrolyzed to GDP thereby inactivating the a subunit, which in turn inactivates adenylyl cyclase. The a subunit bound to GDP re-associates with the b and g subunits, and it starts another cycle to bind to the hormone-receptor complex.

Nobel Prize in Chemistry 2012 • Robert J. Lefkowitz and Brian K. Kobilka won the 2012 Nobel Prize in Chemistry for their research on G-protein-coupled receptors (GPCRs). These receptors allow cells in the body to sense and respond to outside signals and are the target for several commonly prescribed drugs including β-blockers (protection for heart attack), antihistamines and antidepressants.

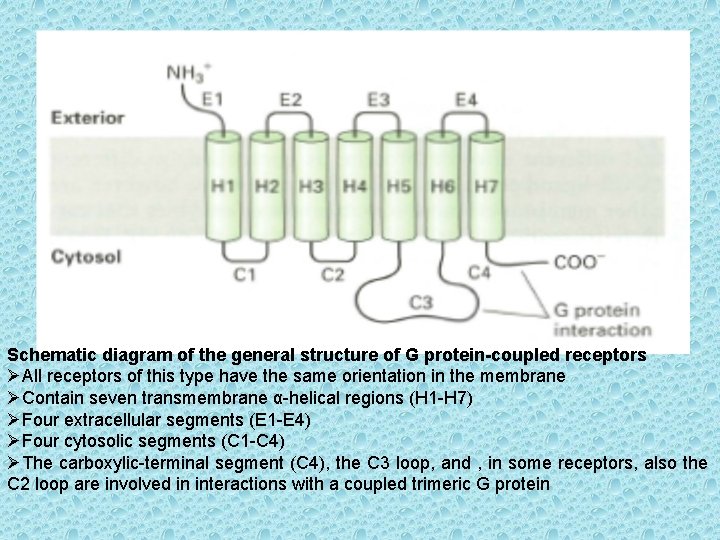
Schematic diagram of the general structure of G protein-coupled receptors ØAll receptors of this type have the same orientation in the membrane ØContain seven transmembrane α-helical regions (H 1 -H 7) ØFour extracellular segments (E 1 -E 4) ØFour cytosolic segments (C 1 -C 4) ØThe carboxylic-terminal segment (C 4), the C 3 loop, and , in some receptors, also the C 2 loop are involved in interactions with a coupled trimeric G protein



Activation of adenylyl cyclase increases the level of cyclic AMP • c. AMP is an important signaling molecule in prokaryotes, animal cells, and plant cells. In higher animals, adenylate cyclase is an integral membrane protein that contains two clusters/groups of six-membrane spanning domains, two of which extending into the cytoplasm as catalytic domains. Activation of adenylyl cyclase by heterotrimeric G proteins increases the conc. of c. AMP in the cell, which is normally maintained at a low level by the action of c. AMP phosphodiesterase which hydrolyzes c. AMP to 5 AMP. Now the evidence is growing to affirm the presence of adenylate cyclase in plants (Gehring 2010).

• Plant-activated bacterial receptor adenylate cyclases modulate epidermal infection in the Sinorhizobium meliloti. Medicago symbiosis (Tian et al. 2012, from France) published in PNAS.

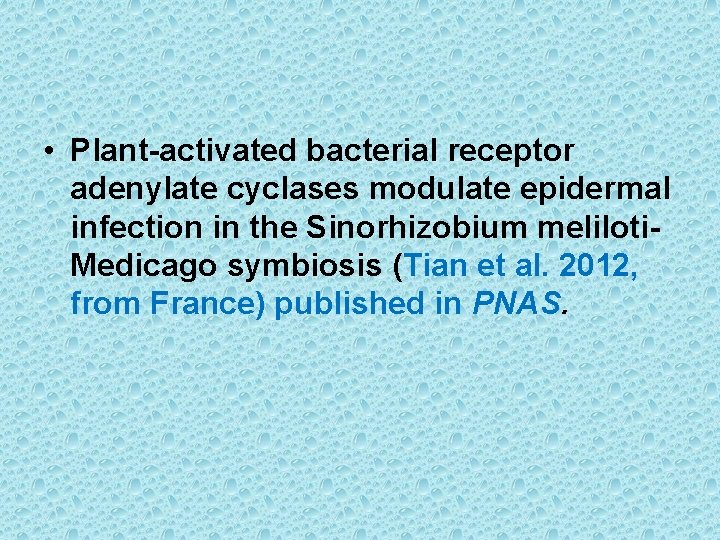
IP 3 - second messenger of water soluble hormones Activation of Phospholipase C initiates the IP 3 pathway Calcium serves as a second messenger for many signaling events in both animals and plants. The concentration of free Ca 2+ in the cytosol is very low (0. 1µM). Ca-ATPases on PM and ER pump calcium out of cell and into the ER lumen, respectively. In plant cells most of the Ca of the cell accumulates in the vacuole. The transport of Ca at tonoplast is carried out by proton pumps via Ca 2+-H+ antiporter. • In animal cells, certain hormones can induce a transient rise in the cytosolic Ca up to 5 u. M. The rise in cytosolic Ca occurs due to opening of intracellular compartments. The coupling of hormone binding to the opening of intra-cellular Ca channels is mediated by another second messenger IP 3.


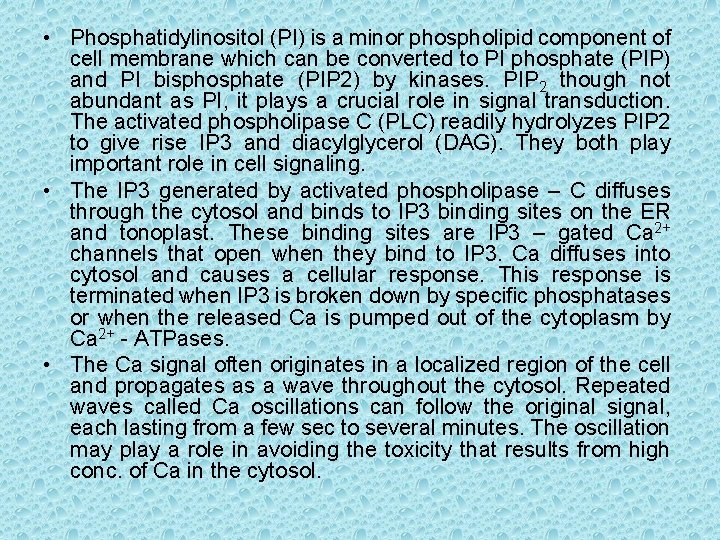
• Phosphatidylinositol (PI) is a minor phospholipid component of cell membrane which can be converted to PI phosphate (PIP) and PI bisphosphate (PIP 2) by kinases. PIP 2 though not abundant as PI, it plays a crucial role in signal transduction. The activated phospholipase C (PLC) readily hydrolyzes PIP 2 to give rise IP 3 and diacylglycerol (DAG). They both play important role in cell signaling. • The IP 3 generated by activated phospholipase – C diffuses through the cytosol and binds to IP 3 binding sites on the ER and tonoplast. These binding sites are IP 3 – gated Ca 2+ channels that open when they bind to IP 3. Ca diffuses into cytosol and causes a cellular response. This response is terminated when IP 3 is broken down by specific phosphatases or when the released Ca is pumped out of the cytoplasm by Ca 2+ - ATPases. • The Ca signal often originates in a localized region of the cell and propagates as a wave throughout the cytosol. Repeated waves called Ca oscillations can follow the original signal, each lasting from a few sec to several minutes. The oscillation may play a role in avoiding the toxicity that results from high conc. of Ca in the cytosol.


Diacylglycerol activates protein kinase C • Breakdown of PIP 2 by phospholipase C produces IP 3 and DAG. IP 3 being hydrophilic diffuses rapidly into the cytoplasm but DAG being a lipid remains in the membrane. In animal cells DAG can come close to protein kinase C (PKC) and activates it. The PKC is located in the cytosol in an inactive form, but upon binding to the Ca 2+ it undergoes conformational change and associates with a PKC receptor protein that transports it to the inner surface of the PM where it encounters DAG. PKC activity has also been detected in plants. G proteins, phospholipase C and various protein kinases have been discovered in plant membranes.

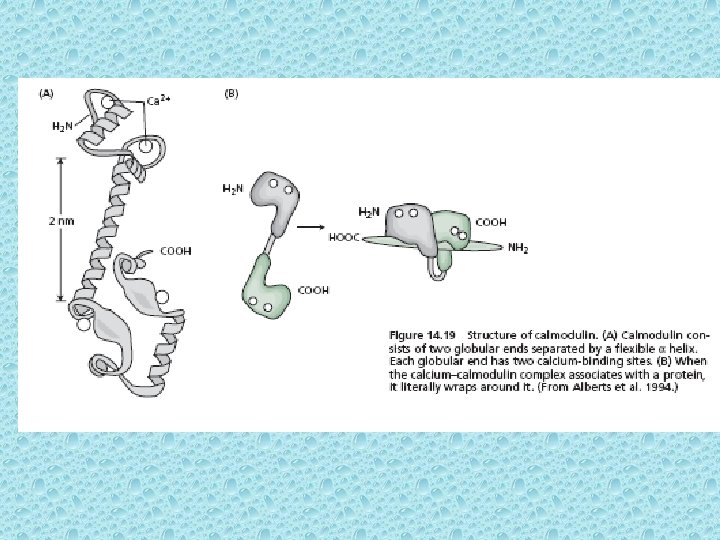
Role of Ca - Calmodulin (Ca-modulated protein (Ca. M)) in activation of Protein kinases -- act as on-off switches • In addition to directly binding of Ca with some proteins, such as channels, most of the effects of Ca are due to its binding to regulatory proteins such as calmodulin (17 k. Da 148 amino acids). Calmodulin widely occurs in eukaryotes both animals and plants as well as now found in prokaryotes (Shemarova and Nesterov, 2005). • There are some other Ca-binding proteins in addition to calmodulin that possess a similar Ca-binding site e. g. in protein parvalbumin (EF hand = E & F are two ά helices) • Each calmodulin molecule binds 4 Ca 2+ ions and changes conformation thereby enabling it to bind activate other proteins. Ca 2+ - calmodulin can stimulate some enzymes directly such as PM Ca 2+ -ATPase which pumps Ca 2+ out of the cell. In addition, most of the effects of Ca are brought about by activation of Ca 2+ - calmodulin dependent protein kinases (Ca. M kinases). The Ca. M kinases phosphorylate serine or threonine residues of their target enzymes, causing enzyme activation.

Enzymes regulated by Ca or Calmodulin • • Adenylyl cyclase Ca – dependent protein kinase Ca 2+ Mg 2+ - ATPase NAD Kinase Phospholipase kinase PA carboxylase PA dehydrogenase PA kinase


Some water soluble hormones bind to Receptor Tyrosine Kinases (RTK) • • Over 50 RTKs have been characterized. They are divided into 14 families based on structure. 58 RTKs have been characterized in man. In animal cells, RTKs are the important class of cell surface receptors. The RTK possess a hormone ligand-binding domain, a trans-membrane domain, and catalytic domain (in cytosol). Since the trans-membrane domain consists of a single ά helix, the hormone cannot transmit a signal directly to the cytosolic side. However, binding of the hormone to the receptor induces dimerization of adjacent receptors which allows the two catalytic domains to come into contact and autophosphorylate the tyrosine residues. Similar to RTKs, in plants Leucine-rich repeat receptor kinases (LRR-RKs) comprise the largest subfamily of transmembrane receptor-like kinases in plants, with over 200 members in Arabidopsis. LRR-RKs regulate a wide variety of developmental and defense-related processes including cell proliferation, stem cell maintenance, hormone perception, host-specific as well as non-host-specific defense response, wounding response, and symbiosis. However, true RTKs have been recently discovered in some fungi. On auto-phosphorylation, the catalytic site of the RTKs binds to different cytosolic signaling proteins. After binding to the RTK, the inactive signaling protein is itself phosphorylated on specific tyrosine residues. Some transcription factors are activated like this. However, the signaling initiated by RTKs begins with the small monomeric (only ά unit) G proteins called as Ras.

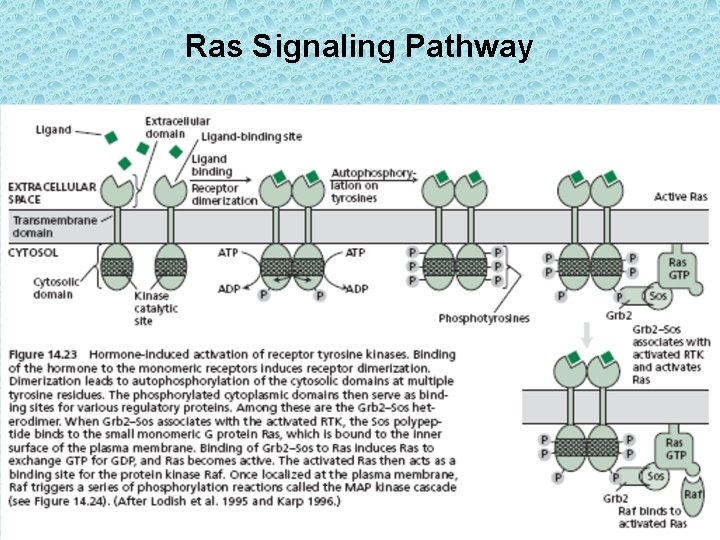
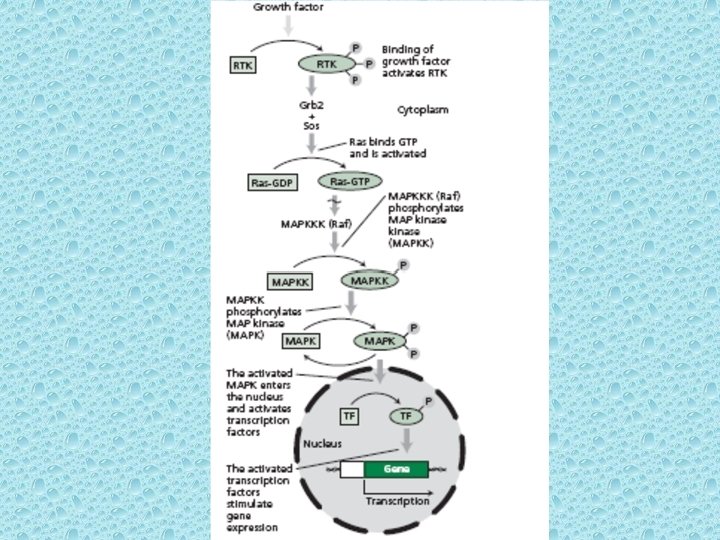
• There are three common monomeric G proteins: Ras, Rab and Rho/Rac. All belong to Ras super-family of monomeric GTPases. Rho and Rac relay signals from surface receptor to the actin cytoskeleton, whereas members of the Rab family of GTPases are involved in regulating intracellular membrane vesicle traffic. Ras proteins transmit signals from RTKs to the nucleus. Ras is a G protein that cycles b/w an inactive GDP – binding form and an active GTP – binding form. Ras can also act as GTPase to hydrolyze bound GTP to GDP thereby terminating the response. • The crystal structure of Arabidopsis thaliana RAC 7/ROP 9 has been determined: The first RAS superfamily GTPase from the plant kingdom (Sormo et al. , 2006; Sormo et al. 2011 Trends Plant Sci) •

• Binding of hormone to the RTK induces dimerization followed by auto-phosphorylation of the catalytic domain. Autophosphorylation of the receptor causes binding to the Grb 2 protein which is tightly bound to another protein known as Sos. The Grb–Sos complex then attaches to the RTK. Upon binding to Sos, Ras exchanges GDP with GTP and Ras then becomes active. • The activated Ras is then able to bind to another soluble serine/threonine kinase known as Raf. Upon binding to Ras, Raf becomes active and initiates a chain of phosphorylation reactions called the MAPK cascade. In plants the ethylene receptors pass signals to a protein kinase of Raf family. • MAPK (mitrogen – activated protein kinase) cascade refers to a series of protein kinases that phosphorylate each other in a specific sequence. Ultimately, the phosphorylated MAPK enters the nucleus and activates transcription factors. The activated transcription factors then stimulate gene expression.

Ras Signaling Pathway
