CH 1012 LECTURE 1 CH 1012 MOLECULAR CHEMISTRY






- Slides: 18

CH 1012 LECTURE 1 CH 1012 MOLECULAR CHEMISTRY · 12 Modules (Learn. JCU CH 1012) Computer Labs 20 hr access • Lectures: 33 (+ 2 revision) • Tests: 2 Tutorials: 10 • Practicals: 9 Room E 1. 113 • Mike Liddell Room E 1. 102 B 12 -1 pm • Ph: 4042 1275 michael. liddell@jcu. edu. au • WEB : http: //learnjcu. edu. au • Notices : Learn-JCU • Module lockups: robert. thomas@jcu. edu. au L 1 / 1

CH 1012 WHAT IS TO COME - CH 1012 • Inorganic: Periodicity groups 1 A - 4 A, coordination chemistry, stereochemistry, ligands, macrocycles, crystal field theory. • Physical: Phase equilibria (distillation), colloids & properties, molecular orbital theory, spectroscopy, metallurgy, new materials (alloys, ceramics, polymers). • Organic: Purification and characterisation of organic compounds. IR, UV/Vis and NMR Spectroscopy. Common reaction types radical, SN 1, SN 2, addition, elimination … Reactions of alkanes, alkenes, aromatics, aldehydes, ketones, and carboxylic acids. L 1 / 2

CH 1012 ASSESSMENT • Examination (2 hr. ) June 42% • Laboratory (3 hr. x 9 wks. ) 20% - attendance > 80% required to pass - medical cert. needed if absent • Tutorials & Tests (1 hr. x 11 wks. ) 33% - 2 x tests (calculator) = 28% • Learn. JCU Modules (12) 5% - complete each with a total mark > 50% required to pass • Invigilated components : The final exam and test dates are fixed. You cannot sit at another time (unless medically ill etc) N. B. flying somewhere is not an excuse. 3

CH 1012 PURCHASES • Texts: • 1) Brown, Le May, Bursten: Chemistry. 10 e 2) Mc. Murray: Fund. of Organic Chemistry + Harcourt Organic CD-ROM. 6 e 1) $ 121 2) $114 • • Faculty Office CH 1012 Lecture Notes CH 1012 Laboratory Notes CH 1012 Report Book CH 1012 Book of Readings » $ 18 $5 $4 $3 • Pay at the Cashier in A 1 and collect your books from the faculty office E 1. 102 N. *Book of Readings printed after Week 1. 4

CH 1012 LABORATORY PURCHASES • Laboratory Breakage Deposit Fac. office = $ 20 Bring your receipt to the first practical Most of it is refunded ($2 labcoat isn’t) Refunds must be collected < 4 weeks after week 13 of the semester. • Laboratory coat Provided • Safety glasses Provided • Footwear No sandals or thongs or barefeet. 5

CH 1012 COURSE CONTENT • Lectures: The main means of introducing material. A lot of areas are only covered in the lectures and so you will need to attend. • Learn. JCU modules: Computer learning & assessment is used in CH 1012. The quiz pages in the modules are assessed. By completing all of them and having a total score >50% you get your 5% assessment. • Tutorials / Tests: to give you some feedback on how you are going. • Practicals: relevant experimentation. • Reading: you need to read around what is in the lectures and modules to do well. L 1 / 6

CH 1012 RESERVE AND MODULES Closed Reserve in the Library: • Copies of Brown, Le May, Bursten and Mc. Murray. • Copies of Brown-Le May Partial Solutions guide & Mc. Murray Study guide which give worked answers & further study practice. It is expected you find these Solutions guides and get used to using them. • Book of Readings. Module lock-ups: e-mail robert. thomas@jcu. edu. au - let him know specifically the Module and screen that is involved (eg. M 1/P 5). L 1 / 7

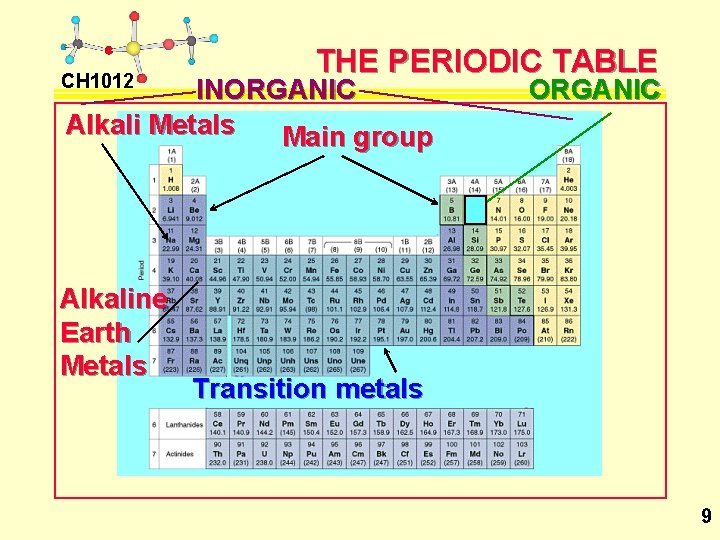
CH 1012 INORGANIC CHEMISTRY Inorganic chemistry – the chemistry of compounds that are not organic in nature. (Organic compounds have C as the basic structural element & do not contain metals. ) • Inorganic chemistry is generally split up into the following divisions: 1) Main group chemistry 2) Transition metal chemistry 3) Lanthanide-actinide chemistry We will look at divisions 1) & 2), and a general sub-grouping called organometallic chemistry. L 1 / 8

CH 1012 THE PERIODIC TABLE INORGANIC Alkali Metals Main group Alkaline Earth Metals ORGANIC Transition metals 9

CH 1012 MATERIALS Types of material Element: C(s) - pure, one atom type graphite, diamond, C 60 allotropes - different forms • not a common form for most elements • Noble gases, N 2, O 2, S 8, Cu, Ag, Au, Pt Compound: C 2 H 6 O 1 - pure, >one atom type eg. Si. O 2, Al(OH)3, Fe 2 O 3 isomers • the most common form of most elements • pure elements and compounds are present as distinct phases and are held together by specific types of bonding. 10

CH 1012 PHASES Phases (states of matter) Gas - particles are very mobile (km / s) - large average interparticle distance - fills container, no surface, low r eg. H 2 = homonuclear diatomic Liquid - particles are mobile (mm / s) - small interparticle distance - fills container, forms a surface Solid - particles non mobile and in contact - fixed shape, high r Amorphous - Si. O 2 - glass Crystalline - Si. O 2 - quartz 11

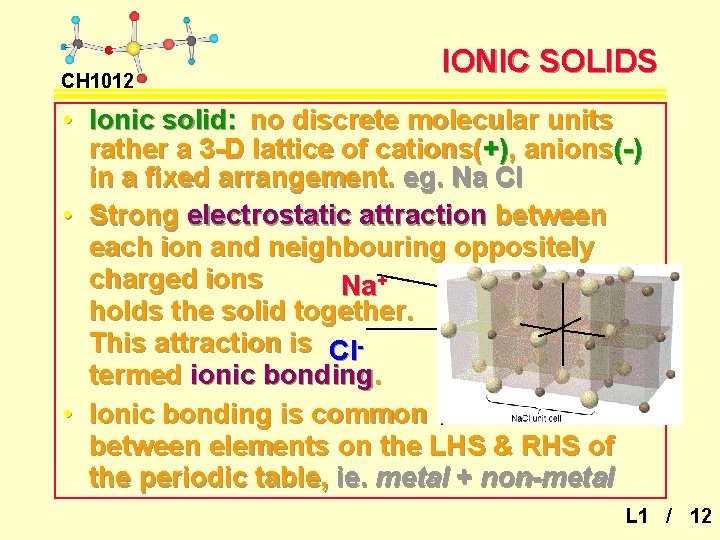
CH 1012 IONIC SOLIDS • Ionic solid: no discrete molecular units rather a 3 -D lattice of cations(+), anions(-) in a fixed arrangement. eg. Na Cl • Strong electrostatic attraction between each ion and neighbouring oppositely charged ions Na+ holds the solid together. This attraction is Cltermed ionic bonding. • Ionic bonding is common between elements on the LHS & RHS of the periodic table, ie. metal + non-metal L 1 / 12

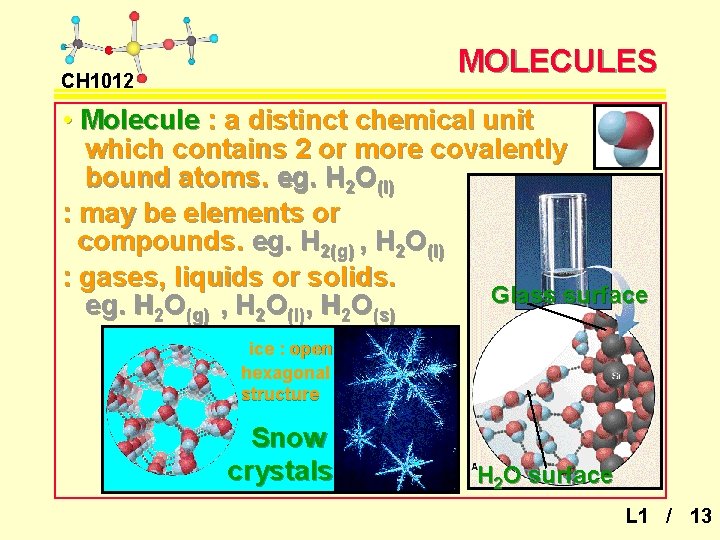
MOLECULES CH 1012 • Molecule : a distinct chemical unit which contains 2 or more covalently bound atoms. eg. H 2 O(l) : may be elements or compounds. eg. H 2(g) , H 2 O(l) : gases, liquids or solids. Glass surface eg. H 2 O(g) , H 2 O(l), H 2 O(s) ice : open hexagonal structure Snow crystals H 2 O surface L 1 / 13

CH 1012 COVALENT BOND Covalent bonding - non-metal + non-metal • sharing e- pair(s) between atoms so that the resulting molecule is more stable than the eg. Cl 2 constituent atoms A covalent bond = a physically observable constant interatomic distance and increased interatomic electron density. eg. Cl-Cl Å (Å = 10 -10 m) 14

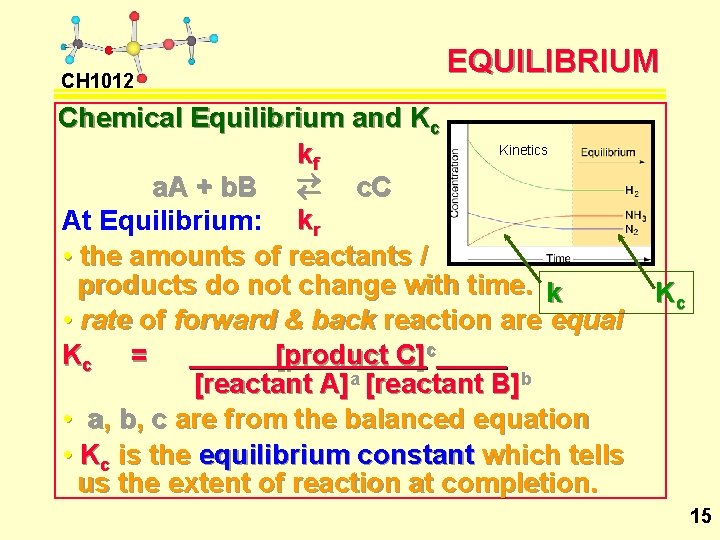
CH 1012 EQUILIBRIUM Chemical Equilibrium and Kc Kinetics kf a. A + b. B ® ¬ c. C At Equilibrium: kr • the amounts of reactants / products do not change with time. k • rate of forward & back reaction are equal Kc = [product C]c [reactant A]a [reactant B]b • a, b, c are from the balanced equation • Kc is the equilibrium constant which tells us the extent of reaction at completion. Kc 15

CH 1012 LE CHATELIER Le Chatelier's principle When a chemical system at equilibrium is disturbed then it regains equilibrium by undergoing a net reaction that reduces the effect of the disturbance to try to restore K. a. A + b B ® ¬ c. C + d D • addition of reactant A to the LHS promotes the forward reaction LHS ® RHS • addition of product C to the RHS promotes the reverse reaction LHS ¬ RHS 16

CH 1012 • • • FOR THIS WEEK You will need to complete the first module Make as many purchases as you can. Class representative ? 17

CH 1012 WORK AHEAD • Module to Cover this week 1. Bonding, Equilibria and Colloids Related sections in text Brown/Lemay/Bursten A. Intro to Chemistry, Atoms, Periodic Table P 1 - 7 , 49 -52 B. Units & Significant figures, conversions P 14 - 29 C. Atomic structure P 43 - 46, 224 -238 D. Molecules, Compounds, Ionic Cmpds P 52 - 60 E. “Lewis Dot” Formulations P 302, 310 F. Bonding and Ionic compounds P 303 – 305 G. Colloids P 558 – 563 H. Equilibrium P 629 - 631 I. Equilibrium constant K P 632 - 634 J. Le Chatelier’s principle P 649 - 654 K. Kp, Kc calculations P 643 – 645 * See also Book of Readings, Everett for extra material on Colloids. 18